Influence of imidazolium-based ionic liquid electrolytes on the performance of nano-structured MnO2 hollow spheres as electrochemical supercapacitor†
Abstract
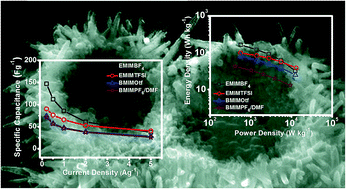
Use of room temperature ionic liquids (ILs) as electrolytes with a wider potential window offers scope for developing high energy density supercapacitors for efficient energy storage. In this work, a comparative study on the performance of nanostructured MnO2 hollow spheres as electrochemical supercapacitor was made by fabricating activated carbon (AC)//MnO2 asymmetric supercapacitor cells using imidazolium-based ILs as electrolytes. Mesoporous MnO2 hollow spheres were synthesized through a simple low temperature (80 °C) solvothermal method by reduction of KMnO4 under acidic condition in presence of Cu3(1,3,5-benzenetricarboxylate)2 metal organic framework (MOF) in the precursor solution, which acts as the source of Cu2+ playing a crucial role for the formation of the hollow spheres. Four different ILs were investigated from combinations of two different cations [1-ethyl-3-methylimidazolium (EMI+) and 1-butyl-3-methylimidazolium (BMI+)] and four different anions [hexaflurophosphate (PF6−), tetrafluoroborate (BF4−), trifluromethanesulfonate (Otf−) and bis(trifluromethylsulfonyl)imide (TFSI−)]. Influences of the physico-chemical properties such as ionic size, nucleophilicity, viscosity of the IL on the electrochemical properties are discussed. A high energy density of 163 W h kg−1, which is comparable to the energy density of a lithium-ion battery, could be achieved with EMIMBF4 as electrolyte. The present findings would help in further research for developing IL-based supercapacitors.

 Please wait while we load your content...
Please wait while we load your content...