Coupling electric energy and biogas production in anaerobic digesters – impacts on the microbiome†
Abstract
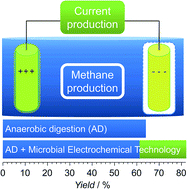
The combination of anaerobic digestion (AD) and microbial electrochemical technologies provides the opportunity to efficiently produce methane and electrical energy from complex biomass. Enhanced methane production and system stability have been reported but the causes (electrolysis or microbial–electrochemical interactions) are less understood. Using the model substrate corn silage it is demonstrated that, for conditions allowing microbiome growth and adaptation, the methane yield of combined reactors remains constant (216 (±29) mL godm−1) while a second product, electrons (q = 14.4 (±0.8) kC, jmax = 1.34 mA cm−2 geometric current density), is also generated. The combined strategy allowed up to a 27% increase in total yield while the reactor community and its dynamics over time were not affected. A typical AD composition of Firmicutes, Bacteroidetes, Proteobacteria, and Synergistetes (bacteria) as well as Methanosarcina, Methanoculleus and Methanobacterium (archaea) was found in the bulk liquid. Specific enrichments of Geobacter (anode) and Methanobacterium (cathode) were of functional relevance.

 Please wait while we load your content...
Please wait while we load your content...