Morphology dependent surface enhanced fluorescence study on silver nanorod arrays fabricated by glancing angle deposition†
Dhruv Pratap Singhab,
Samir Kumara and
J. P. Singh*a
aDepartment of Physics, Indian Institute of Technology Delhi, Hauz Khas, New Delhi 110016, India. E-mail: jpsingh@physics.iitd.ac.in
bMax Planck Institute for Intelligent Systems, Heisenbergstrasse 3, 70569 Stuttgart, Germany
First published on 24th March 2015
Abstract
The nanorods morphology dependence of surface-enhanced fluorescence (SEF) has been investigated for Rhodamine 6G adsorbed onto silver nanorod arrays. SEF activity of the nanorod arrays was observed to increase significantly for uniform and identical shape nanorods grown at a reduced substrate temperature (140 K). On increasing the nanorod length, a significant rise in SEF enhancement factor up to 32 was observed with respect to the conventional silver film. The observation shows the potential of silver nanorod arrays as high-sensitivity substrates for SEF based studies. The variation of SEF efficiency can be qualitatively explained using lightning-rod and surface plasmon resonance effects.
1. Introduction
In the past, glancing angle deposition (GLAD) has emerged as the most promising and versatile technique to grow nanostructures of various shapes and sizes of a wide range of materials.1–5 By manipulating the growth parameters in GLAD, researchers have fabricated different kinds of nanostructures such as slanted nanorods, vertical posts, chevrons, and helices.1–5 These nanostructures find important applications in several fields like gas sensing, optically active films, photo-catalysis, photonic crystals, and in modifying the surface wetting properties.4–12 Recently, the GLAD films of silver metal have attracted a significant interest due to their promising applications in surface plasmon based studies. For example, the silver nanorod arrays fabricated by GLAD method have been identified to produce the high sensitivity Surface Enhanced Raman Scattering (SERS) substrates.5,6,13,14 A remarkable SERS enhancement factor of greater than 108 with applications in sensing the biomolecules and pathogens at very low concentrations have been observed on the silver nanorod arrays.6,13,14 In order to understand the SERS mechanism as well as to explore the possibilities to improve the SERS enhancement factor on nanorod arrays, several theoretical as well as experimental studies have also been performed.6,13–17 Surface enhanced fluorescence (SEF) is another surface plasmon based phenomenon which has recently emerged as a powerful technique to improve the fluorescence sensitivity and collect the fluorescence signals with higher contrast level.18,19 SEF has attracted considerable attention because of its potential applications including medical diagnostics and sensing of the fluorescent biological and chemical molecules.20–22 However, the SEF activity of the silver nanorod arrays is not well explored. Recently in an interesting research by Abdulhalim et al., they performed a comparative study of SEF on the GLAD thin films of different materials like silver, gold, copper, and silicon.23 It was found that the silver columnar films produce the highest SEF enhancement factor. Although, the study identifies the potential of silver GLAD thin films as the SEF active substrates, further research and experiments are still required to improve the enhancement factor for sensitive studies as well as understanding the effect of film morphology on the SEF phenomenon.In the present study, we have investigated the nanorod morphology dependence of SEF for Rhodamine 6G adsorbed onto silver nanorod arrays fabricated by GLAD technique. The nanorod arrays were found to exhibit very good fluorescence enhancement capabilities. Interestingly, the morphology of nanorods grown at reduced substrate temperature was found to produce more fluorescence enhancement compared to the room temperature growth. SEF activity was also shown to depend on the length of the nanorods. Along with the fabrication of high sensitivity substrates for SEF based studies the present study provides the understanding of the morphology dependent SEF response of the standing silver nanorod arrays.
2. Experimental details
Silver nanorod arrays were grown over Si(100) substrates by thermal evaporation of silver powder (99.9%) using GLAD method.1–5 For the growth of nanocolumnar film, the substrates were inclined in polar direction such that the substrate normal made a very high angle (α = 85°) with incident vapor flux. During the deposition, vacuum in the GLAD chamber was better than 2 × 10−6 Torr. To investigate the effect of substrate temperature, the growth of silver nanorods was done on Si substrates under the room temperature RT (∼320 K) and low temperature LT (140 K) conditions. The low substrate temperature was adjusted with a customized substrate heater and controlled supply of the liquid nitrogen to the sample holder. The temperature was measured with an accuracy of ±2 K using a PT100 temperature sensor placed close to the substrates. For the RT growth, no external temperature controlling was utilized. The length of Ag nanorods was varied by controlling the deposition time. For reference, a sample of conventional Ag thin film with plain surface was also grown with the normal incidence (α = 0°) of vapor flux on the Si substrate. Analysis of film morphology was done using scanning electron microscope (SEM, ZEISS EVO 50). For SEF measurements, Rhodamine 6G (Rh6G) was deposited on the Ag nanorod and Ag conventional thin film samples by immersing them in the 1.2 × 10−6 M aqueous dye solution (Rh6G in deionized water) for about 1 hour.24–26 After the immersion, the substrates were rinsed thoroughly with deionized water and dried with gentle nitrogen blow. Fluorescence measurements were performed at multiple locations on the same sample at room temperature using a spectrophotometer (PC1, ISS, Inc.) with Xe arc lamp. The excitation wavelength was 511 nm.3. Results and discussion
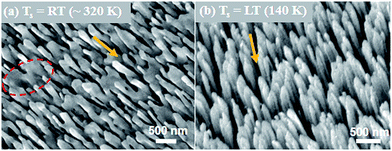
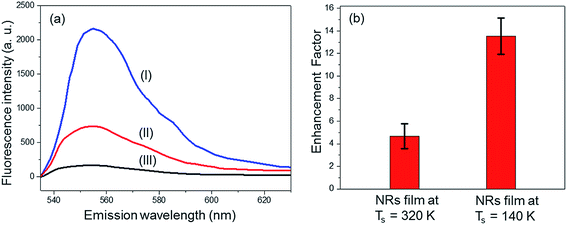
In the case of GLAD, the increase in atomic shadowing effect for high vapor incidence angle (α = 85°) results in the growth of aligned nanorod arrays inclined in the direction of incident vapor flux.1–5 The morphology of nanorod arrays is decided by the competing effect of atomic shadowing and adatom surface diffusion.1,3,27 Since, the adatom surface diffusion strongly depends on the substrate temperature (Ts) therefore the morphology of nanorods film is affected by the substrate temperature during growth.27,28 In the present study, the Ag nanorods films grown under two different substrate temperature conditions that are the room temperature RT (∼320 K) and low temperature LT (140 K) have been evaluated as the SEF substrates. To make a comparative SEF study, the deposition material (1.5 g) and deposition rate (9.7 Å s−1) were kept constant for both the samples. So that only the substrate temperature driven change in nanorods film morphology can be observed. SEM micrographs of the Ag nanorods films grown at RT and LT are shown in Fig. 1(a) and Fig. 1(b) respectively. The growth of inclined Ag nanorods in both the samples can be observed from the SEM images. However, it can be noticed that, at room temperature the nanorods do not possess uniformly aligned and identical shape. The nanorods also get diffuse to form the distorted structures. A dotted red circle in the SEM image marks the apparent distortion in Ag nanorods growth. This observed deviation in morphology of Ag nanorods film under the growth at room temperature can be considered as the effect of high adatom surface diffusion.3,27,28 On reducing the substrate temperature to 140 K, the growth of uniform and identical shaped nanorod arrays all aligned in the direction of incident vapor flux was observed. Interestingly, a large variation in the length of nanorods from about 340 nm at RT to 635 nm at LT was also observed. The observed improvements in the morphology, as well as the length of Ag nanorods at reduced substrate temperature can be considered as the effect of limited adatom surface diffusion.28,29 The mechanism involved in substrate temperature driven morphology of the silver nanorods film is discussed in detail in our earlier work.27 For the SEF measurements, the fluorescence spectra of Rh6G on both the Ag nanorod and reference substrates were collected. The conventional Ag thin film (α = 0°) grown at RT was considered as the reference substrate for SEF measurements. The observed fluorescence spectra are shown in Fig. 2(a). It can be noticed that, on the conventional Ag thin film a weak fluorescence signal with very broad and low intensity emission peak of Rh6G at wavelength of 555 nm was observed. The fluorescence intensity was increased on the Ag nanorods samples. However, it can be noticed that the fluorescence intensity was quite high on the LT Ag nanorod sample compared to the RT Ag nanorod sample. The actual SEF response of the Ag nanorod samples can be understood quantitatively by measuring the enhancement factor EF as follows,24
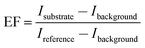 | (1) |
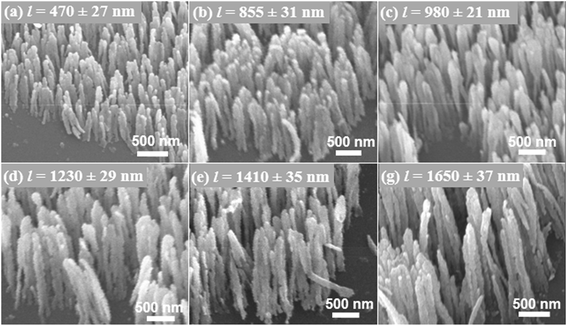
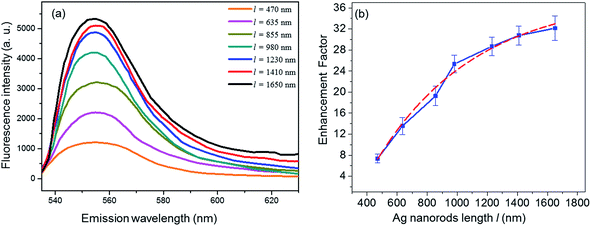
Since the size of Ag nanorods appears to play a crucial role in deciding the enhancement factor. Therefore, once the high enhancement capability of LT Ag nanorods film for the SEF study was realized, more SEF measurements on the LT Ag nanorods film with varying the nanorods length were performed. For this study, the length of Ag nanorods was varied by controlling the deposition time during growth at the low substrate temperature (140 K).27,28 SEM micrographs of the Ag samples of six different nanorod lengths are shown in Fig. 3. Growth of aligned and well-shaped nanorods of different length can be observed from the SEM images. Using the SEM images, the lengths of Ag nanorods were determined to be, l = 470 ± 27, 635 ± 24, 855 ± 31, 980 ± 21, 1230 ± 29, 1410 ± 35, and 1650 ± 37 nm. The average density of nanorods was measured to be 20 × 108 nanorods cm−2, These Ag samples consisting of the nanorod arrays of different lengths have been evaluated as the SEF substrates. The observed fluorescence spectra of Rh6G on these Ag nanorod arrays are shown in Fig. 4(a). Interestingly, it can be observed that the fluorescence intensity increases with an increase in the length of Ag nanorods. Fig. 4(b) plots the calculated enhancement factor as a function of the Ag nanorods length. It can be observed that the enhancement factor increases to a remarkable value that is from about 7.4 to 32.2 on increasing the nanorods length from 470 nm to 1650 nm respectively.
Here, it is interesting to notice that the enhancement factor does not observe a constant rate of increment with increase in the length. An exponential fitting of the experimentally observed trend of the enhancement factor with the length of Ag nanorod is shown by solid red line in the Fig. 4(b). The trends of measured, as well as fitted data plots suggest that, initially the enhancement factor increases very fast but, gradually with an increase in the length, the increment rate appears to decrease by making a little change in the enhancement factor for very large length nanorods. Similar trend in the SERS enhancement factor with the length of Ag nanorod arrays have also been observed by Chaney et al.13
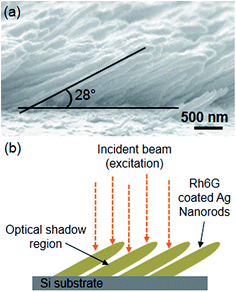
The growth of inclined nanorod arrays increases the porosity, providing more surface area for the dye to be adsorbed upon. The increment in porosity can be considered as an obvious parameter responsible for the observed enhancement in the fluorescence intensity. A cross-sectional SEM image showing the inclined growth of Ag nanorod arrays is given in Fig. 5(a). For this type of growth, it is shown by the schematic in Fig. 5(b) that, if the excitation beam of visible range incidents normal to the substrate plane then it may illuminate only the top surface or apex of the inclined nanorod arrays, leaving a large surface area of the nanorods in shadow. The area in optical shadow may contribute only by scattering or multiple reflections of incident or emitted light. Therefore, in that case only the illuminated top surface of nanorods can make a significant contribution to the fluorescence phenomenon. To make it sure that porosity is not a key factor deciding the fluorescence enhancement by just offering a large surface area to adsorb more Rh6G molecules we have also performed the fluorescence measurements by depositing a constant volume drop on the Ag nanorods samples of different length. The measurement details and results are given in ESI.† We find almost similar trend in increment of the fluorescence signal with the nanorods length for the constant volume and dip coating methods. However, the observed enhancement for constant volume drop method was relatively low. This is because when drop dry on surface it deposits the fluorophores in a non-uniform or most of the time in a ring shape pattern due to the “coffee-stain” effect.30,31 The non-uniform adsorption of fluorophores may not be able to utilize the plasmonic field enhancement of all underlying nanorods and hence, results in relatively less fluorescence enhancement compared to the dip coating method used in this study. From these observations it can be concluded that increase in the porosity cannot be considered as a significant parameter for the observed enhancement in fluorescence intensity on the inclined Ag nanorod arrays. A similar effect has also been observed experimentally by Abdulhalim et al. while measuring the SEF on columnar thin films of different materials.23 They have shown that the porosity of columnar thin film does not play a crucial role in deciding the enhancement factor.
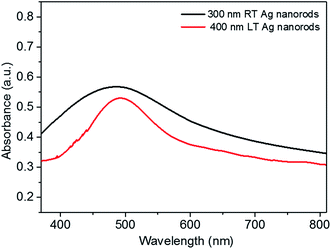
It is known that the external electromagnetic field associated with the excitation beam can induce coherent and confined surface plasmon oscillations within the noble metal nanostructures.18–20,32 These plasmon oscillations produce a local electromagnetic field, which results in the amplification of excitation and emission intensity of the fluorophores in proximity of the nanostructures.18–20,32 In order for metal nanostructures to strongly enhance fluorescence signal, several conditions must be met. One of the most important is the spectral overlap of the resonant plasmon position in the metal nanostructures with excitation/emission spectrum of the fluorophore.19,20,33 Another important aspect of the plasmon resonance is its linewidth, as narrow resonances lead to higher enhancements and higher sensitivity to the local changes in dielectric constant of the environment.19,20,33 Both, the plasmon position and linewidth depends on the shape and size of the nanostructures.33,34 In case of a metal nanorod, following the lightning-rod effect the local electromagnetic field will be weakest at the center of nanorod and highest at the ends.18,28,33,35 For the dense film of inclined Ag nanorod arrays, this elevated electromagnetic field will be experienced near the top end of the nanorods, acting as hotspots. Uniformity in shape and size of nanorods allow the hotspots to produce the narrow plasmon linewidth and high local electromagnetic field. In case of RT, the Ag nanorod arrays possesses deformations and non-uniformity in shape and size whereas the LT nanorod arrays are better aligned and well-shaped. The absorbance spectra of both the RT and LT Ag nanorod arrays deposited on glass substrates are shown in Fig. 6. The peaks in absorption spectra reflect the position of plasmon resonances. The absorption spectra clearly indicate that comparing to the RT growth LT Ag nanorod arrays produce narrower plasmon resonances. This narrow plasmon line width of LT Ag nanorod arrays can be considered to be responsible for the observed high fluorescence enhancement compared to the RT Ag nanorod arrays. In addition to the identical shape and size of nanorods, the plasmonic near-field coupling at the ends of the closely spaced uniformly aligned Ag nanorods in LT growth may also increases the net field experienced by the nearby fluorophores and hence resulting in a strong enhancement in fluorescence.28,32,34,36 It has been observed during the theoretical as well as experimental studies on nanoparticles and nanorods that the plasmon oscillations accentuate with increase in the size and the aspect ratio or the length respectively.13,14,33,35 In that case, following the lightening-rod effect the strength of the local electromagnetic field at the ends will rise with an increase in the length of the nanorods, resulting in the observed rise in fluorescence intensity or the enhancement factor with increase in the length of Ag nanorods. It is noticed that sometimes the plasmonic oscillations in nanostructures may end up with the radiative decay by giving the photo emission.33,37,38 The radiative decay increases the plasmon linewidth and hence limits the local-field enhancement.33,37,38 The probability of radiative decay increases with the volume of the nanostructures.37,38 Therefore, in case of the nanorods an increase in volume with length may enhance the probability of radiative decay. In addition to the radiative decay, following the column broadening effect there will also be a higher probability for two adjacent nanorods to contact each other in the large length nanorods film, which changes the SEF hot spot distribution; i.e., the two adjacent connected nanostructures could result in low localized electric fields, and thus may cause a low SEF enhancement factor for Ag nanorod arrays of large length.13,39 In the present case the observed reduction in the rate of increment in SEF enhancement factor for the large length nanorods can be attributed to the increased contribution of the radiative decay of plasmons and bonding of the adjacent nanorods.
4. Conclusions
In summary, the silver nanorod arrays fabricated using GLAD technique were identified as high sensitivity SEF active substrates. The effect of morphology of nanorods on fluorescence enhancement was investigated. It was observed that the morphology of nanorod arrays grown at low substrate temperature (140 K) produces better SEF active substrates compared to the room temperature growth. SEF activity was also shown to depend on the length of the nanorods. A significant rise in the enhancement factor up to 32 was observed on nanorod arrays with increasing the length during the LT (140 K) growth. The observed morphology dependent variation in SEF efficiency of Ag nanorod arrays is considered as the combined effects of surface plasmon resonance, lightening-rod, near-field coupling, and radiative plasmon decay.Acknowledgements
This research work was supported by the Nano Scale Research Facility (NRF), IIT Delhi, India.References
- K. Robbie, J. C. Sit and M. J. Brett, J. Vac. Sci. Technol., B: Microelectron. Nanometer Struct.--Process., Meas., Phenom., 1998, 16, 1115–1122 CrossRef CAS.
- T. Karabacak, G. C. Wang and T. M. Lu, J. Vac. Sci. Technol., A, 2004, 22, 1778–1784 CAS.
- C. M. Zhou and D. Gall, J. Vac. Sci. Technol., A, 2007, 25, 312–318 CAS.
- A. G. Mark, J. G. Gibbs, T.-C. Lee and P. Fischer, Nat. Mater., 2013, 12, 802–807 CrossRef CAS PubMed.
- M. Suzuki, J. Nanophotonics, 2013, 7, 073598 CrossRef PubMed.
- S. Shanmukh, L. Jones, J. Driskell, Y. Zhao, R. Dluhy and R. A. Tripp, Nano Lett., 2006, 6, 2630–2636 CrossRef CAS PubMed.
- A. Wolcott, W. A. Smith, T. R. Kuykendall, Y. Zhao and J. Z. Zhang, Adv. Funct. Mater., 2009, 19, 1849–1856 CrossRef CAS.
- M. A. Summers, K. Tabunshchyk, A. Kovalenko and M. J. Brett, Photonic Nanostruct., 2009, 7, 76–84 CrossRef PubMed.
- C. Li, Z. Wang, P.-I. Wang, Y. Peles, N. Koratkar and G. P. Peterson, Small, 2008, 4, 1084–1088 CrossRef CAS PubMed.
- S. Hwang, H. Kwon, S. Chhajed, J. W. Byon, J. M. Baik, J. Im, S. H. Oh, H. W. Jang, S. J. Yoond and J. K. Kim, Analyst, 2013, 138, 443–450 RSC.
- D. P. Singh and J. P. Singh, Appl. Phys. Lett., 2013, 102, 243112 Search PubMed.
- D. P. Singh and J. P. Singh, Appl. Phys. A, 2014, 114, 1189–1193 CrossRef CAS.
- S. B. Chaney, S. Shanmukh, R. A. Dluhy and Y.-P. Zhao, Appl. Phys. Lett., 2005, 87, 031908 CrossRef PubMed.
- Y.-J. Liu, H. Y. Chu and Y.-P. Zhao, J. Phys. Chem. C, 2010, 114, 8176–8183 CAS.
- Y.-J. Liu, Z.-Y. Zhang, Q. Zhao and Y.-P. Zhao, Appl. Phys. Lett., 2008, 93, 173106 CrossRef PubMed.
- Y.-J. Liu, Z.-Y. Zhang, Q. Zhao, R. A. Dluhy and Y.-P. Zhao, J. Phys. Chem. C, 2009, 113, 9664–9669 CAS.
- Y.-J. Liu and Y.-P. Zhao, Phys. Rev. B: Condens. Matter Mater. Phys., 2008, 78, 075436 CrossRef.
- M. Moskovits, Rev. Mod. Phys., 1985, 57, 783–826 CrossRef CAS.
- J. R. Lakowicz, Anal. Biochem., 2005, 337, 171–194 CrossRef CAS PubMed.
- K. Aslan, I. Gryczynski, J. Malicka, E. Matveeva, J. R. Lakowicz and C. D. Geddes, Curr. Opin. Biotechnol., 2005, 16, 55–62 CrossRef CAS PubMed.
- M. Hernandez, G. Recio, R. J. Martin-Palma, J. V. Garcia-Ramos, C. Domingo and P. Sevilla, Nanoscale Res. Lett., 2012, 7, 364 CrossRef PubMed.
- F. Xie, M. S. Baker and E. M. Goldys, Chem. Mater., 2008, 20, 1788–1797 CrossRef CAS.
- I. Abdulhalim, A. Karabchevsky, C. Patzig, B. Rauschenbach, B. Fuhrmann, E. Eltzov, R. Marks, J. Xu, F. Zhang and A. Lakhtakia, Appl. Phys. Lett., 2009, 94, 063106 CrossRef PubMed.
- J. Dong, H. Zheng, X. Yan, Y. Sun and Z. Zhang, Appl. Phys. Lett., 2012, 100, 051112 CrossRef PubMed.
- Y. Wang and C. Hu, Thin Solid Films, 2005, 476, 84–91 CrossRef CAS PubMed.
- Y. Sun, X. Q. Yan, Y. Du, Z. Zhang, Y. Huo, F. Gao and H. Zheng, J. Nanosci. Nanotechnol., 2014, 14, 4481 CrossRef CAS PubMed.
- D. P. Singh, P. Goel and J. P. Singh, J. Appl. Phys., 2012, 112, 104324 CrossRef PubMed.
- L. Abelmann and C. Lodder, Thin Solid Films, 1997, 305, 1–21 CrossRef CAS.
- J. P. Singh, T. E. Lanier, H. Zhu, W. M. Dennis, R. A. Tripp and Y. Zhao, J. Phys. Chem. C, 2012, 116, 20550–20557 CAS.
- S. Das, P. R. Waghmare, M. Fan, N. S. K. Gunda, S. S. Roya and S. K. Mitra, RSC Adv., 2012, 2, 8390 RSC.
- R. D. Deegan, O. Bakajin, T. F. Dupont, G. Huber, S. R. Nagel and T. A. Witten, Nature, 1997, 389, 827 CrossRef CAS PubMed.
- E. Fort and S. Grésillon, J. Phys. D: Appl. Phys., 2008, 41, 013001 CrossRef.
- E. M. Goldys and K. Drozdowicz-Tomsi, in Nanowires – Fundamental Research, ed. A. Hashim, InTech, 2011 Search PubMed.
- L. Zhao, K. L. Kelly and G. C. Schatz, J. Phys. Chem. B, 2003, 107, 7343–7350 CrossRef CAS.
- J.-W. Liaw, H.-Y. Tsai and C.-H. Huang, Plasmonics, 2012, 7, 543–553 CrossRef CAS.
- A. M. Funston, C. Novo, T. J. Davis and P. Mulvaney, Nano Lett., 2009, 9, 1651–1658 CrossRef CAS PubMed.
- C. Sönnichsen, T. Franzl, T. Wilk, G. Von Plessen and J. Feldmann, Phys. Rev. Lett., 2002, 88, 077402 CrossRef.
- C. Novo, D. Gomez, J. Perez-Juste, Z. Zhang, H. Petrova, M. Reismann, P. Mulvaney and G. V. Hartland, Phys. Chem. Chem. Phys., 2006, 8, 3540–3546 RSC.
- Y.-J. Liu, H. Y. Chu and Y.-P. Zhao, J. Phys. Chem. C, 2010, 114, 8176–8183 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra03225c |
| This journal is © The Royal Society of Chemistry 2015 |