The role of soft colloidal templates in the shape evolution of flower-like MgAl-LDH hierarchical microstructures†
Jie Zhanga,
Xiangli Xieb,
Cunjun Lia,
Hai Wangc and
Linjiang Wang*c
aCollege of Materials Science and Engineering, Guilin University of Technology, Guilin 541004, PR China
bCollege of Chemistry and Bioengineering, Guilin University of Technology, Guilin 541004, PR China
cKey Laboratory of New Processing Technology for Nonferrous Metals and Materials, Ministry of Education, Guilin University of Technology, Guilin 541004, PR China. E-mail: wlinjiang@163.com; Fax: +86 7735896035; Tel: +86 13507736656
First published on 16th March 2015
Abstract
A flower-like MgAl layered double hydroxide (MgAl-LDH) hierarchical microstructure was synthesized by a self-assembly route, employing the anionic surfactant sodium dodecyl sulfate (SDS) as a soft template. X-ray diffraction, Fourier transform infrared spectroscopy, thermogravimetric analysis and Brunauer–Emmett–Teller analysis were employed to characterize the samples. Structural characterization revealed that the hierarchical microstructure materials are composed of nanosheets with a thickness of about 50 nm with a hierarchical porous structure with both large and small mesopores on the surface. The average diameter and specific surface area of the hierarchical porous microstructures were 1.544 nm and 41.20 m2 g−1, respectively. Furthermore, it was found that the diameter and density of the three-dimensional (3D) hierarchical microstructures could be varied by changing the growth parameters, such as growth time, growth temperature, initial reactant concentration, and surfactant type. Compared with two other surfactants, the anionic surfactant sodium lauryl sulfate (SLS) and the cationic surfactant cetyl trimethyl ammonium bromide (CTAB), the anionic surfactant SDS plays dual roles as both a capping agent and through its intercalation in controlling the shape of the MgAl-LDH hierarchical microstructure. Based on the analysis of the evolution of the microstructure and crystalline structure of the MgAl-LDHs, a corresponding formation mechanism of the three-dimensional hierarchical microstructures was proposed. In addition, the thermal stability of the as-prepared hierarchical microstructures of the MgAl-SDS-LDHs was further evaluated. The results show that MgAl-SDS-LDH can be preserved up to 500 °C and eventually be transformed into spinel MgAl2O4 with a similar hierarchical microstructure, indicating the high thermal stability of the hierarchical microstructure.
Introduction
Layered double hydroxides (LDHs) are a class of anionic clay minerals that consist of positively charged layers with an interlayer region containing charge compensating anions and solvent molecules.1 The general formula for a LDH can be described as [M1−x2+Mx3+(OH)2] x+[An−]x/n·zH2O, where M2+ and M3+ are di- and tri-valent metal cations occupying octahedral positions within the host layers of hydroxide sheets, An− is an intercalated anion that compensates for the charge on the layers such as Cl−, NO3−, SO42−, CO32− and x is normally between 0.2–0.4.2 It has been demonstrated that the structure and morphology have significant effects on the physical and chemical properties of LDH materials. However, the general morphology of LDHs synthesized by hydrothermal and coprecipitation methods is a typical hexagonal lamellar structure due to the structural characteristics of LDHs.3 Recently, hierarchically structured LDHs, which possess dual or multiple morphologies and structures, are attracting significant attention owing to their high surface areas, large pore volumes and microstructures. These structural features make LDHs a promising candidate for a variety of applications such as catalysis, pollution control, and electrochemistry.4 For example, the activity of LDH-based materials increases notably with an increase in the surface area, because a large surface area is associated with more catalytically active sites and in turn higher activity.4 Similarly, in electrochemistry, a large specific surface area and abundant pore structure can expose a greater number of active points, which is advantageous for full contact between the electrolyte and the active substance, but also conducive to rapid electron transfer in the active material and the surface of the electrode.5,6 Recently, various methods including structural reconstruction,7 in situ growth,8 exfoliation and assembly,9 and microwave irradiation,10 have been developed and attempted to prepare hierarchically structured LDHs with various morphologies for a variety of practical applications. However, so far the processes of the approaches for fabricating hierarchical LDHs are somewhat complex and uncontrollable. Therefore, finding facile and effective ways for the synthesis of LDHs with complex hierarchical structures is highly desirable.Template methods can effectively control the morphology, structure and size of the synthesized material, and thus have become important methods for the preparation of nanomaterials.11 According to their structural characteristics, template methods can be divided into hard-template and soft-template methods. For the hard-template method, relatively hard template agents, such as monodispersed inorganics, high polymer or resin micro (nano) particles, are used as templates. Chemical materials are deposited on the surface of the templates and finally form core–shell structures after the templates are removed.12 Zhang et al.13 described the synthesis of 3D hierarchical LDH/C microspheres by combining the biological template method (for Al2O3/C) and the hydrothermal method (for LDH/C). Xu et al.8 reported an in situ growth process to prepare a hierarchical 3D composite composed of graphene layers with layered double hydroxide (LDH) nanosheet arrays grown on both sides. The hard-template method has good monodispersity and high repeatability, but the preparation process is complex and the reaction conditions are rigorous. Besides, in order to retain the structural features, the crystalline conversion of the inorganic materials will be restricted, which will limit the performance of the inorganics.12 Soft-template agents generally refer to organic molecules or supramolecules with soft structures, such as surfactants or biomolecules.14 In general, soft-template agents can be assembled into novel organic–inorganic materials due to the strong interactions between the soft-template agents and inorganic species. Moreover, the morphology of the soft-template is diverse and easy to be built without a complicated process. The simple operation process and mild conditions mean the soft-template method has significant advantages in the structure and diverse composition of the composite materials.15
In this work, we developed a facile method to prepare 3D flower-like MgAl-SDS-LDH hierarchical microstructures by assembling LDH nanosheets into flower-like microspheres under hydrothermal conditions. The SDS acts as a soft template and the LDH nanosheets can be efficiently packed onto it to form microspheres with a hierarchical porous structure. The as-prepared product exhibited a special 3D architecture with a large mesoporous structure and high specific surface area. The work provides a promising approach for the design and synthesis of 3D flower-like microstructured MgAl-SDS-LDH which has potential applications in catalysis, adsorption and electrochemistry.
Experimental
Materials synthesis
Magnesium nitrate hexahydrate (Mg(NO3)2·6H2O), aluminium nitrate nonahydrate (Al(NO3)3·9H2O), urea (CO(NH2)2), sodium dodecyl sulfonate (SDS), sodium lauryl sulfate (SLS), cetyl trimethyl ammonium bromide (CTAB) and ethanol were all purchased from Shantou Xilong Chemical Reagents Co. Ltd. and were of analytical purity. All of the chemical reagents were used as received without further purification.The 3D flower-like microstructured MgAl-LDH was synthesized via a soft-template method under hydrothermal conditions. Typically, 1.2307 g Mg(NO3)2·6H2O, 0.9003 g Al(NO3)3·9H2O, and 1.4414 g urea were dispersed in 150 mL deionized water with ultrasonic treatment for 30 min to obtain a solution consisting of 0.032 mol L−1 Mg2+, 0.016 mol L−1 Al3+, and 0.16 mol L−1 urea. 0.9805 g anionic surfactant SDS was dissolved in 90 mL deionized water to obtain a solution consisting of 0.04 mol L−1 SDS. Then, the two solutions were mixed together and stirred with a magnetic stirrer for 30 minutes at room temperature. The mixed solution was transferred into a 500 mL autoclave pressure vessel and heated at 160 °C for 6 h. The collected sample was washed two times with deionized water and three times with ethanol, and then dried at 70 °C for 24 h in a vacuum oven. The resulting product was denoted as MgAl-SDS-LDH. In order to discuss the influence of the surfactant type on the morphology of the product, samples with the anionic surfactant SLS and the cationic surfactant CTAB as the soft-templates were prepared using the above procedure, and were termed as MgAl-SLS-LDH and MgAl-CTAB-LDH, respectively. In detail, 1.0368 g anionic surfactant SLS was dissolved in 90 mL deionized water to obtain a solution consisting of 0.04 mol L−1 SLS and 1.2545 g cationic surfactant CTAB was dissolved in 90 mL deionized water to obtain a solution consisting of 0.04 mol L−1 CTAB. In addition, a blank sample was prepared using the same experimental process without a soft-template, and was termed as MgAl-CO3-LDH.
Characterization
The morphology and elemental composition analysis of the samples were carried out using a field emission scanning electron microscope (Japan-S-4800) equipped with an energy-dispersive X-ray spectrometry (EDS) analyzer. The phase composition and purity of the as-prepared samples were analyzed by powder X-ray diffraction (XRD) with Cu Kα (λ = 1.5405 Å) incident radiation using a PANalytical X’pert PRO diffractometer at 40 kV voltage and 40 mA current. Fourier transform infrared (FT-IR) spectra were recorded on a NICOLEF-470 Fourier infrared spectrometer. Thermogravimetric analysis (TGA) was carried out on a NETZSCH STA-449C thermal analyzer with a heating rate of 10 °C min−1 at a temperature ranging from room temperature to 800 °C. The specific surface area was determined using a Nova 1200e Accelerated Surface Area and Porosimetry System (America) according to a Brunauer–Emmett–Teller (BET) plot of the nitrogen adsorption isotherm at the relative pressure range of 0.05–0.3. The Barrett–Joyner–Halenda (BJH) pore volume and NLDFT pore size distribution were obtained from the N2 adsorption–desorption isotherms recorded at 77 K. Before the BET measurements, the samples were degassed at 100 °C for 5 h.Results and discussion
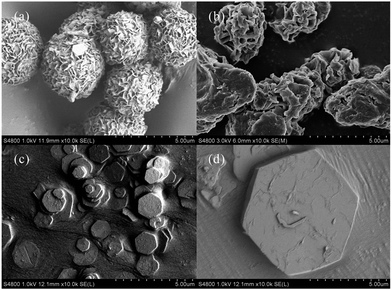
Fig. 1 shows the morphologies and structures of the MgAl-LDHs synthesized at 160 °C for 6 h with the surfactants SDS, SLS, CTAB as soft-templates. As can be seen, the morphology of the MgAl-SDS-LDH sample was flower-like nanospheres formed by bent nanoplates (Fig. 1a). The average diameter of such nanospheres was 4 μm and the size distribution was fairly narrow. Besides, the single nanosphere was self-assembled by masses of interconnected nanoplates with a thickness of about 50 nm. As a result of the self-assembly, there were many open-pore structures at the surface of the nanospheres and the size of the open-pore was about 200 nm with a bent shape and uniform distribution. Fig. 1b shows that the morphology of the MgAl-SLS-LDH sample was spherical aggregate structures which consisted of a number of poorly-defined nanoplates. The shape and size of the spherical aggregates were irregular with a poorly-defined crystallization and poor dispersion compared with the MgAl-SDS-LDH. Besides, the size of the open-pore at the surface of the spherical aggregate structures was uneven and the thickness of the poorly-defined nanoplates was larger than that of the MgAl-SDS-LDH. For the MgAl-CTAB-LDH that was synthesized with the cationic surfactant CTAB as the soft-template, the morphology was thin hexagonal platelets which was similar with that of the MgAl-CO3-LDH (Fig. 1d) and the size distribution of the MgAl-CTAB-LDH platelets was not uniform, with the majority of the crystallites ranging from 1 μm to 2 μm (Fig. 1c). From the SEM images of the LDH particles shown in Fig. 1, it can be seen that MgAl-LDH particles with a 3D flower-like morphology (Fig. 1a and b) can be produced in the anionic surfactant SDS and SLS aqueous solutions. But the deformation degree or curvature radius differs depending on the surfactant anion and chemical composition of the LDH sheets.16The XRD patterns of the MgAl-SDS-LDH (Fig. 2a) and MgAl-SLS-LDH (Fig. 2b), synthesized with the anionic surfactants SDS and SLS as the soft-templates respectively, exhibited characteristic diffractions of hydrotalcite with a larger interlayer spacing compared with that of the MgAl-CTAB-LDH (Fig. 2c). The three main characteristic diffraction peaks corresponding to the (003), (006), and (009) diffraction planes of the MgAl-SDS-LDH appeared at 3.55°, 7.09°, and 10.63°, respectively. The d003 value (d-spacing) of the MgAl-SDS-LDH was 2.48 nm. Given that the thickness of the brucite-like layer of the LDH is about 0.48 nm,17 the interlayer distance of the MgAl-SDS-LDH was 2 nm including the hydrogen domains on both sides of the layers and the chain length of the SDS anion, which is mostly made up of the chain length of SDS anion (1.85 nm).15 The results imply that the SDS anions form a vertical intersectional monolayer arranged in the interlayer space, where the sulfonate groups attach to the hydroxyl layers through strong hydrogen bonds and electrostatic attraction. For the MgAl-SLS-LDH, the interlayer distance was 2.71 nm, demonstrating the SLS anions also arrange as a vertical intersectional monolayer in the interlayer space. The XRD pattern of the MgAl-CTAB-LDH was similar to that of the MgAl-CO3-LDH. The d003 value of the MgAl-CTAB-LDH was 0.75 nm, which was the same as that reported in the literature for MgAl-CO3-LDH.18 The results prove that the cationic surfactant can not enter the interlayer of the LDH, which is consistent with the SEM observation results (Fig. 1c).
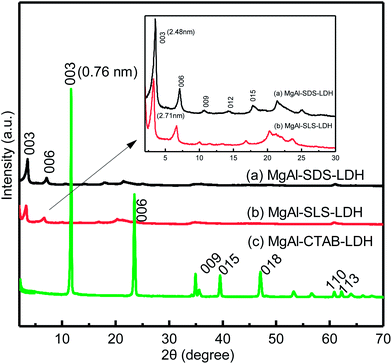 | ||
| Fig. 2 XRD patterns of the MgAl-LDH samples synthesized at 160 °C for 6 h with different surfactants as soft-templates: (a) MgAl-SDS-LDH, (b) MgAl-SLS-LDH, and (c) MgAl-CTAB-LDH. | ||
The EDS spectrum of the MgAl-SDS-LDH flower-like nanospheres (Fig. 3a) shows the peaks corresponding to C, O, Mg, Al and S. Obviously, the S peak in the EDS comes from the anionic surfactant SDS. The results indicate that the MgAl-LDH and the related anionic surfactant SDS co-exist in the composites which is consistent with the XRD results (Fig. 2a). Besides, the atom contents of Mg and Al are 4.19% and 1.85%, respectively. Thus, the Mg/Al atom ratio of the LDH phase was about 2, which was similar to the ratio in the reactant mixture. The EDS spectrum of the MgAl-SLS-LDH spherical aggregate structures (Fig. 3b) also revealed peaks corresponding to C, O, Mg, Al and S, in which the S comes from the anionic surfactant SLS. However, the EDS spectrum of the MgAl-CTAB-LDH (Fig. 3c) shows the existence of C, O, Mg and Al without N, indicating the cationic surfactant CTAB does not exist in the composite. From the above results, it was found that the SDS and SLS anions play similar roles in the formation process of the flower-like structures, due to their similar molecular structures and chemical properties.
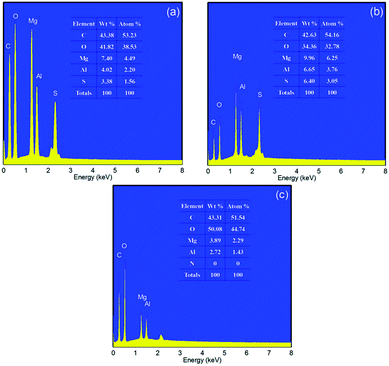 | ||
| Fig. 3 EDS spectra of the MgAl-LDH samples synthesized at 160 °C for 6 h with different surfactants as soft-templates: (a) MgAl-SDS-LDH, (b) MgAl-SLS-LDH, and (c) MgAl-CTAB-LDH. | ||
It was found that the concentration of the surfactant SDS aqueous solution had significant effects on the morphology of the samples. When the concentration of SDS was as low as 0.005 mol L−1, the sample exhibited a rose-like structure formed by hexagonal nanoplates as shown in Fig. 4a. The average size of such nanoplates was 5 μm with a thickness of 200 nm. The size of the open-pore structures formed by the nanoplate aggregation was about 1 μm with a distribution ranging from 500 nm–3 μm. When the concentration was increased to 0.02 mol L−1, the morphology of the sample was still a rose-like structure formed by hexagonal nanoplates (Fig. 4b). The rose-like structure had a tendency to form a spherical structure and the average size of the hexagonal nanoplates was about 2 μm with a thickness of 100 nm. The size of the open-pore structures was about 500 nm with a distribution ranging from 200 nm–2 μm. However, combining with Fig. 4a, c and d, it was found that the size of the nanoplates decreased gradually and the number of nanoplates increased. Due to the high lateral growth rate of the LDH crystals along the a direction, the formed curved LDH nanoplates aggregate together and self-assemble into a three-dimensional ordered spherical structure with a perpendicularly oriented and interlaced growth mode on the surface of the generated microspheres, in order to minimize their surface energy in the course of the reaction. As a result, the morphology of the rose-like structure had a tendency to form a spherical structure. With the concentration further increased to 0.04 mol L−1, the morphology of the sample was hydrangea-like nanospheres formed by bent nanoplates (Fig. 4c). The average diameter of such nanospheres was 4 μm and the size distribution was fairly narrow. The average size of the bent nanoplates was about 800 nm with a thickness of about 50 nm. As a result of the nanoplate self-assembly, there were many open-pore structures at the surface of the nanospheres and the size of the open-pore was about 200 nm with a bent shape and uniform distribution. When the concentration was adjusted to 0.06 mol L−1, as shown in Fig. 4d, the morphology of the sample was still hydrangea-like nanospheres. The average diameter of such nanospheres increased to 6 μm. However, the dispersion of the hydrangea-like nanospheres was reduced and the nanosphere structures aggregated together with some amorphous crystallization at the surface. Therefore, with the increase of the concentration of the SDS aqueous solution, the morphology of the as-synthesized products changed from rose-like structures, to hydrangea-like structures, to aggregated nanospheres. The morphology changes are ascribed to the shape and number of the surfactant SDS micelles in the aqueous solution. It is well known that the lowest concentration of a surfactant which is required to begin to form micelles is the CMC (Critical Micelle Concentration). When the concentration of the surfactant is lower than the CMC, the surfactant molecules dispersed in water exist in the form of single molecules while the concentration of metal ions is relatively larger. As a result, the sample exhibits a rose-like structure with large hexagonal nanoplates. When the concentration of the surfactant exceeds the CMC, the concentration of surfactant molecules is relatively larger and the shape of the surfactant micelles is spherical or axiolitic, and the micelles can serve as templates to induce the nucleation and growth of LDH nanoplates. As a result, the ideal 3D flower-like microstructures with a good dispersion can be obtained when the concentration of the surfactant SDS aqueous solution is 0.04 mol L−1. Under such a concentration condition, the surfactant SDS can form spherical micelles with the hydrophobic groups pointed toward the inside and the hydrophilic groups toward the outside for inducing the crystallization and growth of LDH nanoplates along the spherical micelle interface through electrostatic interactions between the positively charged SDS head group and the Mg2+ and Mg3+ metal cations.
 | ||
| Fig. 4 SEM images of the MgAl-SDS-LDH samples synthesized at 160 °C for 6 h with different concentrations of SDS: (a) 0.005 mol L−1, (b) 0.02 mol L−1, (c) 0.04 mol L−1, and (d) 0.06 mol L−1. | ||
Fig. S1† shows the XRD patterns of the MgAl-SDS-LDHs synthesized with different concentrations of SDS. As shown in Fig. S1a,† when the surfactant concentration was 0.005 mol L−1, the characteristic diffraction peaks of the sample were consistent with that of the MgAl-CO3-LDH, indicating that the surfactant SDS was not involved in the formation of the LDH and the resulting product was MgAl-CO3-LDH. When the surfactant concentration was increased to 0.02 mol L−1 (Fig. S1b†), the characteristic diffraction peak intensity of the MgAl-CO3-LDH significantly decreased while the MgAl-SDS-LDH characteristic diffraction peaks appeared in the low diffraction region. The results indicated that at this concentration, the surfactant molecules started to form spherical micelles in the solution for inducing divalent and trivalent metal ions to form LDH crystals, and the resulting product was a mixture of the MgAl-SDS-LDH and MgAl-CO3-LDH. When the surfactant concentration increased to 0.04 mol L−1 (Fig. S1c†), the characteristic diffraction peaks of the MgAl-CO3-LDH all disappeared and the characteristic diffraction peak intensity of the MgAl-SDS-LDH increased, indicating the growth of LDH crystals has experienced a phase change process, the introduction of the surfactant changed the morphology as well as the composition of the product. When the concentration continued to increase to 0.06 mol L−1 (Fig. S1d†), the characteristic diffraction peak intensity of the MgAl-SDS-LDH decreased, indicating the crystallinity of the sample decreased. The XRD results further confirmed the growth mechanism of the flower-like structured MgAl-SDS-LDH, at lower surfactant concentration, the salt concentration in the solution is relatively large and the crystals develop into the conventional hexagonal nanosheet structured MgAl-CO3-LDH. With the increase of surfactant concentration, a large number of surfactant molecules formed spherical micelles and part LDH crystals began to grow along the surfactant micelles and formed the flower-like structured MgAl-SDS-LDH with DS− in the interlayer. When the concentration continues to increase, excessive surfactant micelles aggregate together, leading to the MgAl-SDS-LDH to exhibit an aggregate structure with a bad dispersion.
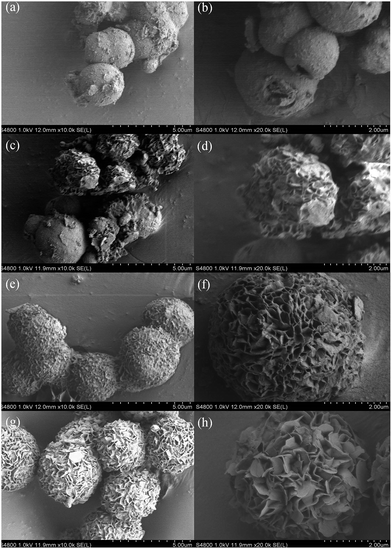
Fig. 5 shows the morphology evolution of the MgAl-SDS-LDH flower-like structures at 160 °C for different hydrothermal reaction times. When the time was as short as 1 h, the sample exhibited two kinds of structures including flower-like structure and microcrystal sphere with a bad dispersion (Fig. 5a). The average diameter of the microcrystal sphere was approximately 1.5 μm. However, a large number of microcrystal spheres had not yet fully crystallized and only part of the spherical particle exhibited a flower-like structure with open-pores distributed throughout its surface. With the crystallization time increased to 3 h, the sample exhibited all spherical flower-like structures (Fig. 5b). The average size of the spherical structures was 2 μm and the open-pore distribution on the surface was uniform. When the crystallization time was 6 h, the morphology of the sample was hydrangea-like nanospheres (Fig. 5c). The average diameter of such nanospheres was about 4 μm and the size distribution was narrow. Besides, the size of the open-pores distributed on the surface of the flower-like nanospheres was more uniform. When the crystallization time was increased to 10 h (Fig. 5d), however, the morphology of the sample became irregular. The results indicated that when the time was too short, the sample had not yet begun to grow petals and only formed microcrystal spheres. With the increase of time, the petals of the flower-like structures began to grow and develop to well-defined nanoplates. However, when the time was too long, the size of the petals increased and the flower-like structures grew outward and linked together to form aggregated structures. As a result, the ideal 3D flower-like microstructures with a good dispersion could be obtained when the hydrothermal reaction time was 6 h.
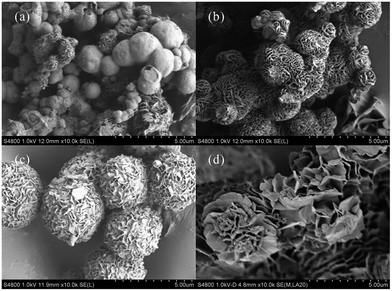 | ||
| Fig. 5 FESEM images of the MgAl-SDS-LDH samples synthesized at 160 °C for different hydrothermal reaction times: (a) 1 h, (b) 3 h, (c) 6 h, and (d) 10 h. | ||
Fig. S2† shows the XRD patterns of the MgAl-SDS-LDHs synthesized at 160 °C for different hydrothermal reaction times. As shown in Fig. S2a,† when the crystallization time was 1 h, there are three sharp diffraction peaks at 3.17°, 6.40°, and 9.63°. When the time was increased to 3 h (Fig. S2b†), the three sharp diffraction peaks disappeared and two relatively broad diffraction peaks appeared at 7.04° and 10.60°, corresponding to the (006) and (009) diffraction peaks of the MgAl-SDS-LDH. When the time was increased to 6 h (Fig. S2c†), the intensity of the two diffraction peaks increased, meanwhile a relatively strong diffraction peak appeared at 3.55°, corresponding to the (003) diffraction peak of the MgAl-SDS-LDH. The results show that the growing speeds are quite different in different directions. The (006), (009) crystal face developed earlier than the (003) crystal face, but the growing speed of the (003) crystal face is much faster than the (006), (009) crystal face. When the time was further increased to 10 h (Fig. S2d†), the peak shape and position of the sample remain the same and the intensity of the diffraction peaks increased indicating the crystallinity of the sample increased.
Fig. 6 illustrates the morphology change of the MgAl-SDS-LDH flower-like structures synthesized at different hydrothermal temperatures. As can be seen, with the increase of the hydrothermal temperature, the size and crystallinity of the samples significantly increased. In detail, when the temperature was 100 °C, the morphology of the sample was microcrystal spheres with no nanoplates at the surface (Fig. 6a and b), indicating the LDH nanoplates haven’t begun to crystallize under such temperature. When the hydrothermal temperature was increased to 120 °C, nanospheres formed with LDH nanoplates began to form (Fig. 6c and d). The average diameter of the nanospheres was about 2.5 μm. However, the intercrossed nanoplates distributed on the surface of the nanospheres were not uniform and the distance between adjacent nanoplates was about 100 nm. When the temperature was increased to 140 °C, the average diameter of the nanospheres was about 4 μm and the size distribution was fairly narrow (Fig. 6e and f). The thickness of the nanoplates was about 20 nm and the distance between adjacent nanoplates was about 200 nm. When the hydrothermal temperature was increased to 160 °C, the sample exhibited a more regular nanosphere structure with a more uniform size (Fig. 6g and h). Furthermore, it was noted that the size of a single nanoplate on the surface of the sample as well as the distance between adjacent nanoplates was about 300 nm. The results indicated that the influence of the hydrothermal temperature on the sample morphology was similar to the hydrothermal reaction time. When the temperature was too low, the sample had not yet begun to grow petals and only formed microcrystal spheres. With increasing temperature, the petals of the flower-like structures began to grow and developed to well-defined nanoplates. Meanwhile, the thickness of the petals increased. As a result, the ideal 3D spherical flower-like microstructures with a good dispersion were obtained when the hydrothermal temperature was 160 °C.
Fig. S3† shows the XRD patterns of the MgAl-SDS-LDHs synthesized at different hydrothermal temperatures for 6 h. As shown in Fig. S3a,† when the temperature was 100 °C, there are three sharp diffraction peaks at 3.20°, 6.42°, and 9.65°. When the temperature was increased to 120 °C (Fig. S3b†), the intensity of the three diffraction peaks was significantly increased. However, when the temperature was further increased to 140 °C (Fig. S3c†), the intensity of the three diffraction peaks began to decrease. Meanwhile, three new diffraction peaks which correspond to the (003), (006), and (009) diffraction planes of the MgAl-SDS-LDH appeared at 3.55°, 7.09°, and 10.63°, respectively. When the temperature was further increased to 160 °C (Fig. S3d†), the intensity of the three new diffraction peaks increased while the first three diffraction peaks all disappeared. The results show that the hydrothermal temperature has a significant effect on the composition and crystallinity of the samples. When the hydrothermal temperature was 160 °C, the sample had a single composition with a good crystallinity.
Based on the above discussion, a possible formation mechanism for the 3D flower-like microstructured MgAl-SDS-LDH is illustrated in Scheme 1. When the concentration of the surfactant exceeds the CMC, anionic surfactants in the aqueous solution aggregate to form spherical micelles with the hydrophobic groups toward the inside and the hydrophilic groups toward the outside. The negatively charged hydrophobic head groups should easily attract the positively charged M2+ metal cations via electrostatic interactions. At the same time, due to the strong electrostatic forces between the hydrotalcite layer and the anionic surfactant, the anionic surfactant is then intercalated in the interlayer through ion exchange, leading to a significant increase of the interlayer gallery. Then the LDH crystals grow along the spherical micelle interfaces.15 Their structure is based on M2+(OH)6 octahedral units sharing edges to build M(OH)2 brucite-like layers, in which trivalent cations replace some of the divalent ions giving positively charged sheets and the charge is balanced by intercalated anions in the hydrated interlayer regions.4 Consequently, due to the high lateral growth rate of the LDH crystals along the a direction, the formed curved LDH nanoplates aggregate together and self-assemble into 3D ordered spherical structures with a perpendicularly oriented and interlaced growth mode on the surface of the generated microspheres, in order to minimize their surface energy in the course of reaction.
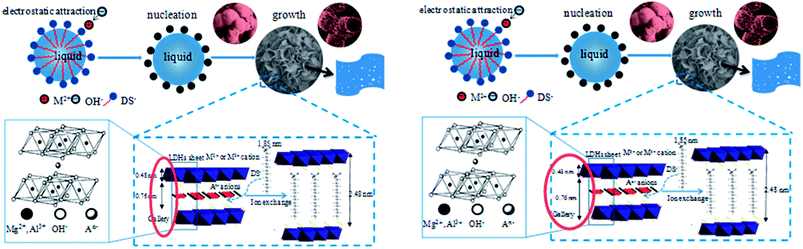 | ||
| Scheme 1 Schematic illustration of the formation mechanism for the 3D flower-like MgAl-SDS-LDH microstructures. | ||
Fig. 7 shows the N2 adsorption–desorption isotherms and pore size distribution curve of the 3D flower-like MgAl-SDS-LDH microstructures obtained using the Brunauer–Emmett–Teller (BET) method. It was observed that the N2 adsorption–desorption isotherm of the MgAl-SDS-LDH microstructures exhibit a typical IV isotherm with a H3-type hysteresis loop (P/P0 > 0.4), indicating the existence of mesopores.19 Besides, the H3-type hysteresis loop is the most common for plate-like particles with slit-shaped pores according to the IUPAC classification and the narrow H3-type loop also generally indicates the existence of uniform pores of a narrow size distribution.20 As estimated by the BET analysis, the specific surface area of the MgAl-SDS-LDH microstructures was 41.20 m2 g−1. It is speculated that the large increase of surface area derives from a well-formed 3D flower-like architecture with a hollow interior coupled with small and uniform particle sizes.20 From the pore size distribution curves, we can see that the MgAl-SDS-LDH microstructures had a wide hierarchical pore distribution (Fig. 7 insert). The pore diameter and pore volume of such flower-like microstructures were 1.544 nm and 0.076 cm3 g−1 while those of the hexagonal lamellar structured LDH were 3.829 nm and 0.021 cm3 g−1, respectively.
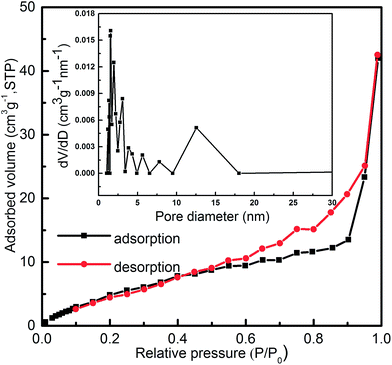 | ||
| Fig. 7 Nitrogen adsorption–desorption isotherms and the pore size distribution curve (inset) of the 3D flower-like MgAl-SDS-LDH microstructures with the SDS concentration of 0.04 mol L−1. | ||
In addition, we investigated the effect of the heat treatment of the MgAl-SDS-LDH on its microstructure and crystal phase. As shown in Fig. 8a, the overall flower-like microstructure can be retained without the occurrence of structure collapse, indicating the hierarchical microspheres have very good thermal stability. Compared with the original sample, the average diameter of such flower-like nanospheres decreased to 2 μm and the thickness of the single nanoplates reduced to 20 nm due to the removal of water molecules intercalated in the interlayer galleries and the loss of the interlayer SDS template. Furthermore, XRD was used to verify the crystal phase. Surprisingly, the MgAl-SDS-LDH was completely converted into spinel MgAl2O4 (PDF#86-0096) and the crystallinity degree became poor (Fig. 8b). This is understandable as the structure of the MgAl-SDS-LDH has changed. From the XRD pattern and its formation mechanism, we reasonably inferred that the intercalated water molecule was released, and the OH group plate layer linked with metal atoms became O linked with metal atoms. Recently, the transition metal oxide spine is becoming attractive due to its wide application in many fields such as lithium-ion batteries, supercapacitors, photocatalysis and so on. Thus, the conversion reactions in our work provide a new opportunity for the synthesis of transition metal oxides in the future.
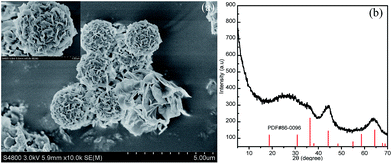 | ||
| Fig. 8 (a) FESEM image of the MgAl-SDS-LDH after calcination at 500 °C for 3 h and its corresponding XRD pattern (b). | ||
Fig. 9 shows the TG and DTG curves of the MgAl-SDS-LDH 3D flower-like micro-nanostructures. The process for weight loss consisted of two remarkable steps in the TG curve from room temperature up to 800 °C, including desorption of water physically adsorbed on the external surface of the crystallites and removal of water intercalated in the interlayer galleries and dehydroxylation of the brucite-like layers, respectively. The first decomposition step ended at 160 °C, with a weight loss of 7.8% corresponding to the removal of physically absorbed and interlayer water molecules.21 The second stage (160–600 °C), with a 48.0% weight change, corresponded to the dehydroxylation of the brucite-like layers and the loss of interlayer sodium dodecyl sulfate.22 Hence, the total weight loss of the MgAl-SDS-LDH between 25 °C and 600 °C was 55.8%. In the DTG profile of the MgAl-SDS-LDH, the two stages were accompanied by two distinct endothermic peaks, centered at 110 °C and 429 °C, respectively.
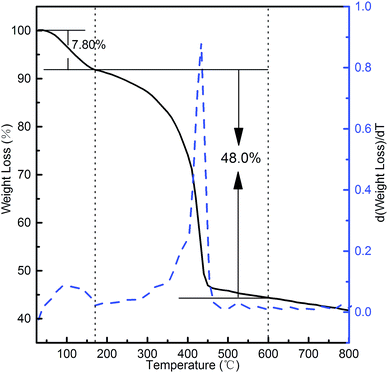 | ||
| Fig. 9 TG and DTG curves of the 3D flower-like MgAl-SDS-LDH microstructures synthesized at 160 °C for 6 h with the SDS concentration of 0.04 mol L−1. | ||
Fig. 10 shows the FT-IR spectra of the pure SDS, MgAl-SDS-LDH and MgAl-SDS-LDH calcined at 500 °C for 3 h (C-MgAl-SDS-LDH). The broad band observed around 3500 cm−1 was associated with the hydroxyl stretching band arising from metal-hydroxyl groups and hydrogen bonds of interlayer water molecules. The absorption at 1630 cm−1 was associated with the bending vibration of water.4 For pure SDS, the three characteristic bands at 2850 cm−1, 2918 cm−1, and 2956 cm−1 (Fig. 10a) were assigned to the stretching vibration of C–H groups and the characteristic band at 1467 cm−1 was associated with the CH2 bending vibration. The two characteristic bands at 1198 cm−1 and 1065 cm−1 in SDS, were attributed to the anti-symmetric and symmetric stretching vibrations of S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, shifted to 1167 cm−1 and 1049 cm−1 in the MgAl-SDS-LDH (Fig. 10b). The red shift of the two characteristic bands may arise from the hydrogen bond and electrostatic interactions between the S
O, shifted to 1167 cm−1 and 1049 cm−1 in the MgAl-SDS-LDH (Fig. 10b). The red shift of the two characteristic bands may arise from the hydrogen bond and electrostatic interactions between the S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups and the LDH layer surface. The other bands at the low frequency region (<800 cm−1) were attributed to metal-oxygen and metal-hydroxyl vibration modes in the lattice of the LDH. For C-MgAl-SDS-LDH (Fig. 10c), the three characteristic strong bands at 2850 cm−1, 2918 cm−1, and 2956 cm−1 and the two characteristic strong bands at 1198 cm−1 and 1065 cm−1 in SDS all disappeared, indicating the surfactant soft-template decomposed completely. The above results confirmed that the formation of the flower-like morphology is related to the presence of anionic surfactant which can aggregate to form spherical micelles in aqueous solution and then serve as a template for inducing the crystal nucleation and growth.
O groups and the LDH layer surface. The other bands at the low frequency region (<800 cm−1) were attributed to metal-oxygen and metal-hydroxyl vibration modes in the lattice of the LDH. For C-MgAl-SDS-LDH (Fig. 10c), the three characteristic strong bands at 2850 cm−1, 2918 cm−1, and 2956 cm−1 and the two characteristic strong bands at 1198 cm−1 and 1065 cm−1 in SDS all disappeared, indicating the surfactant soft-template decomposed completely. The above results confirmed that the formation of the flower-like morphology is related to the presence of anionic surfactant which can aggregate to form spherical micelles in aqueous solution and then serve as a template for inducing the crystal nucleation and growth.
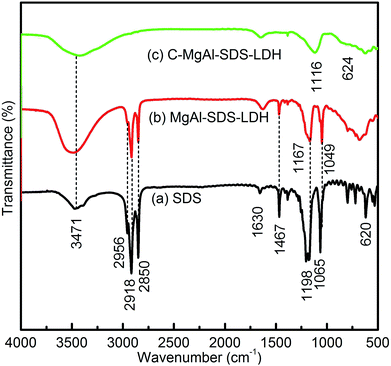 | ||
| Fig. 10 FT-IR spectra of (a) surfactant SDS, (b) MgAl-SDS-LDH and (c) MgAl-SDS-LDH calcined at 500 °C for 3 h (C-MgAl-SDS-LDH). | ||
Conclusions
In summary, we have developed a facile soft template combined with solvothermal method to prepare 3D flower-like MgAl-SDS-LDHs with a hierarchical microstructure by taking advantage of the dual roles of the SDS soft template. The morphology and crystal structure of the as-prepared MgAl-SDS-LDH can be rationally tuned by changing the hydrothermal temperature, reaction time, and surfactant concentration. Compared with two other different types of surfactants, SDS plays a crucial role in the formation of the flower-like MgAl-LDH hierarchical microstructures, acting as a soft template. The obtained flower-like MgAl-SDS-LDH hierarchical microstructures nanostructured microspheres are composed of LDH nanosheets and have a hierarchical porous structure. Based on our investigation, the surfactant type and its appropriate concentration are believed to be responsible for the formation of the MgAl-SDS-LDHs. A plausible formation mechanism for the 3D flower-like MgAl-SDS-LDH was also discussed. It is expected that our described synthesis strategies for anionic clay mineral LDHs with hierarchical microstructures may be extended to the preparation of cationic clay minerals, such as montmorillonite.Acknowledgements
This work was supported by the Natural Science Foundation of China (no. 41272064, 51462007) and Guangxi Natural Science Foundation (no. 2011GXNSFD018008, 2014GXNSFAA118349).Notes and references
- L. Wang, S. Su, D. Chen and C. A. Wilkie, Polym. Degrad. Stab., 2009, 94, 770–781 CrossRef CAS PubMed
.
- Z. Liu, R. Ma, Y. Ebina, N. Iyi, K. Takada and T. Sasaki, Langmuir, 2007, 23, 861–867 CrossRef CAS PubMed
.
- S. Huang, H. Peng, W. W. Tjiu, Z. Yang, H. Zhu, T. Tang and T. Liu, J. Phys. Chem. B, 2010, 114, 16766–16772 CrossRef CAS PubMed
.
- J. Yu, G. Fan, Y. Yang and F. Li, J. Colloid Interface Sci., 2014, 432, 1–9 CrossRef CAS PubMed
.
- J. Shi, Y. Hara, C. L. Sun, M. A. Anderson and X. D. Wang, Nano Lett., 2011, 11, 3413–3419 CrossRef CAS PubMed
.
- F. Shao, J. Sun, L. Gao, S. W. Yang and J. Q. Luo, ACS Appl. Mater. Interfaces, 2011, 3, 2148–2153 CAS
.
- S. Huang, X. Cen, H. D. Peng, S. Z. Guo, W. Z. Wang and T. X. Liu, J. Phys. Chem. B, 2009, 113, 15225–15230 CrossRef CAS PubMed
.
- J. Xu, S. Gai, F. He, N. Niu, P. Gao, Y. Chen and P. Yang, J. Mater. Chem. A, 2014, 2, 1022–1031 CAS
.
- H. L. Kang, G. L. Huang, S. L. Ma, Y. X. Bai, H. Ma, Y. L. Li and X. J. Yang, J. Phys. Chem., 2009, 113, 9157–9163 CrossRef CAS PubMed
.
- T. Yan, H. Y. Zhu, R. Y. Li, Z. J. Li, J. K. Liu, G. L. Wang and Z. Q. Gu, Electrochim. Acta, 2013, 111, 71–79 CrossRef PubMed
.
- L. Yan, R. Li, Z. Li, J. Liu, Y. Fang, G. Wang and Z. Gu, Electrochim. Acta, 2013, 95, 146–154 CrossRef CAS PubMed
.
- E. Geraud, V. Prevot and F. Leroux, J. Phys. Chem. Solids, 2006, 67, 903–908 CrossRef CAS PubMed
.
- T. Zhang, Y. Zhou, Y. Wang, X. Bu, H. Wang and M. Zhang, Appl. Clay Sci., 2015, 103, 67–70 CrossRef CAS PubMed
.
- L. Sun and C. Hu, Mater. Res. Bull., 2011, 46, 1922–1927 CrossRef CAS PubMed
.
- Y. Yang, G. Fan and F. Li, Mater. Lett., 2014, 116, 203–205 CrossRef CAS PubMed
.
- B. Li, J. He and D. G. Evans, Chem. Eng. J., 2008, 144, 124–137 CrossRef CAS PubMed
.
- X. M. Lu, L. M. Meng, H. P. Li, N. Du, R. J. Zhang and W. G. Hou, Mater. Res. Bull., 2013, 48, 1512–1517 CrossRef CAS PubMed
.
- H. Kang, Y. Shu, Z. Li, B. Guan, S. Peng, Y. Huang and R. Liu, Carbohydr. Polym., 2013, 100, 158–165 CrossRef PubMed
.
- X. Wang, Y. Xiao, J. Wang, L. Sun and M. Cao, J. Power Sources, 2014, 274, 142–148 CrossRef PubMed
.
- T. Yan, R. Li and Z. Li, Mater. Res. Bull., 2014, 51, 97–104 CrossRef CAS PubMed
.
- Y. X. Yan, Q. Liu, J. Wang, J. Wei, Z. Gao, T. Mann, Z. S. Li, Y. He, M. L. Zhang and L. H. Liu, J. Colloid Interface Sci., 2012, 371, 15–19 CrossRef CAS PubMed
.
- B. R. Venugopal and M. Rajamathi, J. Colloid Interface Sci., 2011, 355, 396–401 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra01561h |
| This journal is © The Royal Society of Chemistry 2015 |