Photocatalytic and photoelectrochemical activity of N-doped BiPO4 photocatalyst
Liaona She,
Guoqiang Tan*,
Huijun Ren,
Jing Huang,
Chi Xu and
Ao Xia
School of Materials Science and Engineering, Shaanxi University of Science & Technology, Xi'an, Shaanxi 710021, China. E-mail: tan3114@163.com; Tel: +86 13759878391
First published on 15th April 2015
Abstract
N-doped BiPO4 photocatalysts were successfully synthesized via a facile microwave hydrothermal method. XRD, Raman, and XPS results confirmed that the nitrogen atoms were incorporated into the lattice of BiPO4 to replace oxygen atoms. The doped nitrogen did not affect the crystalline structure of BiPO4. With the increase of nitrogen content, the nanorod sizes of N-doped BiPO4 were decreased gradually compared with those of pure BiPO4. The photocatalytic activity of N-doped BiPO4 was remarkably improved. Greater than 96% photodegradation of RhB under the exposure of UV light was achieved within 15 min with 21 at%, and its degradation rate was about 2.1 times greater than pure BiPO4. This phenomenon could be attributed to the fact that the nitrogen doped into BiPO4 improved the separation efficiency of photogenerated electrons and holes.
1. Introduction
Nowadays, semiconductor photocatalysis has attracted considerable attention for environment remediation and solar energy conversion.1–3 Among various semiconductor oxide photocatalysts, TiO2 has been considered as one attractive photocatalyst due to its high photocatalytic activity, excellent stability, and low cost.4–6 Nevertheless, the rapid recombination rate of photogenerated electron–hole pairs in the TiO2 system leads to its low quantum efficiency.7 Therefore, the development of more efficient photocatalysts is of great significance and necessity, especially in view of serious energy shortages.8Recently, BiPO4, an oxoacid salt photocatalyst, is discovered by Zhu's group and exhibits more attractive photocatalytic activity than P25 (TiO2) for the degradation of methylene blue (MB) dye under UV light irradiation.9 The photocatalytic activities strongly depend on the crystal structure. BiPO4 appears in three main crystalline phases and among these three crystal phases, monoclinic BiPO4 exhibits the highest photocatalytic activity due to the most distorted PO4 tetrahedron.10 Nevertheless, similar to TiO2, the wide band gap energy of monoclinic BiPO4 limits the utilization of solar light. Therefore, it is highly desirable to develop an effective method to further improve the photocatalytic activity of BiPO4 under UV light irradiation or induce its visible light photocatalytic activity. One way to improve the photocatalytic activity of BiPO4 is to dope the anionic impurities into the BiPO4 matrix. For example, Liu et al. synthesized the fluorine doped BiPO4 photocatalysts and the obtained results indicated that the photocatalytic activity of BiPO4 could be improved by F-doping.11 Moreover, Lu et al. prepared in situ carbon hybridized BiPO4 films through calcination of amorphous complex precursor films on the Ti substrate under various temperatures, and it was found that the film prepared at 400 °C presented the highest photocatalytic activity.12 Nitrogen element is widely used in the modification of photocatalysts due to its comparable atomic size with oxygen, high stability, and small ionization energy.13–15 For example, Shang et al. prepared the nitrogen-doped Bi2WO6 photocatalysts and found that the doped samples exhibited 2–3 times higher photocatalytic activities than the undoped one, doping Bi2WO6 with N could not only broaden the range of light adsorption in the visible region, but also nitrogen could inhibit the photogenerated electron–hole recombination rate.16 Wang et al. used the sol–gel method to synthesize N-doped BiVO4 photocatalyst and reported that the doped nitrogen resulted in a red shift in the absorption edge.8 Nitrogen doped bismuth niobate (N-Bi3NbO7) hierarchical architectures were prepared by Hou et al., which exhibited higher photocatalytic activities in the decomposition of organic pollutant under visible-light irradiation than Bi3NbO7 nanoparticles due to the large specific surface area.17 On the basis of these results, it can be inferred that the introduction of nitrogen, irrespective of the approaches of nitrogen doping, indeed improved the photocatalytic activity of semiconductor photocatalysts. Although there have been a large number of reports on the modification of BiPO4, to the best of our knowledge, it is rare to prepare and investigate N-doped BiPO4 catalysts.
In this work, N-doped BiPO4 photocatalysts have been first prepared by the microwave hydrothermal method at 200 °C. The structure, morphology, optical property, photocatalytic and photoelectrochemical activity are investigated in detail. Besides, a possible mechanism for the enhanced photocatalytic activity by N-doping is also proposed.
2. Experimental
Pure and N-doped BiPO4 photocatalysts were synthesized via a facile microwave hydrothermal process. All of the reagents used in this study were of analytical grade without any further purification. In a typical preparation, 3 mmol Bi(NO3)3·5H2O and equal molar Na3PO4·12H2O were put into a beaker. Then, 50 mL of distilled water was added to the beaker and magnetically stirred for 30 min to form a homogeneous solution at room temperature. Afterwards, different amounts of NaN3 were added into the suspension with the molar ratio of N and Bi from 0 at% to 21 at%. After stirring for another 30 min, each precursor was transferred into a 100 mL Teflon-lined stainless autoclave. The microwave hydrothermal reactions were carried out at 200 °C for 60 min, and then cooled down to room temperature naturally. The as-prepared samples were rinsed sequentially with absolute ethanol and water for several times so as to be neutral. Finally, the resulting products were obtained and dried at 80 °C for 10 h.The crystalline structures of the samples were characterized by X-ray powder diffraction (XRD) (D/max-2200PC, Rigaku) with Cu Kα radiation (λ = 0.15406 nm) in the range of 2θ = 15–70°. Raman scattering spectra measurements were performed with an Ar+-ion laser excitation at 532 nm (Model JY HR800, Horiba). The morphologies and microstructures of the prepared samples were examined by field-emission scanning electron microscopy (FE-SEM) (Model JSM-6700, JEOL) and transmission electron microscopy (TEM) (TecnaiG2F20 S-TWIN, FEI) systems. The specific surface area was measured by a Brunauer–Emmett–Teller specific surface area instrument (BET) (3H-2000BE T-A, Beishide Instrumentation Technologies Ltd, Beijing). The surface analysis was studied by X-ray photoelectron spectroscopy (XPS) (XSAM800, Kratos Ltd, Japan). The binding energy data was calibrated with the C 1s signal at 284.6 eV of the surface adventitious carbon. The photoabsorption performance was characterized by a UV-vis diffuse reflectance spectroscopy in the wavelength range of 200–800 nm (DRS) (Agilent Cary 5000, America) using BaSO4 as the reference.
Photocatalytic activities of pure and N-doped BiPO4 photocatalysts were determined by the decolorization of Rhodamine B (RhB) aqueous solution under ultraviolet light irradiation of a 300 W high-pressure mercury lamp in a quartz photochemical reactor. The initial concentration of RhB solution was 5 mg L−1. For each experiment, 0.050 g photocatalyst was added into 50 mL RhB solution and the solution was stirred in the dark for 30 min before illumination to ensure the establishment of an adsorption–desorption equilibrium between photocatalyst and RhB. At given time intervals, 5 mL of the suspension was collected and centrifuged to remove the photocatalyst particles. The concentration of RhB was then determined by measuring the absorbance at λmax = 554 nm, using a UV-vis spectrophotometer (Model SP-756p, Shanghai Optical Spectrometer Company).
For the fabrication of the film electrode, a total of 0.2 g synthesized powder sample was added into a mixed solution with 2 mL anhydrous ethanol and 0.2 mL acetyl acetone. After a period of time, it became into the stable suspension with ultrasonic dispersion. Then the powder was spread out on 1.5 × 2 cm2 ITO glass substrate with spin-coating method for 3 times. After liquid volatilization, this membrane electrode was calcinated at 150 °C for 3 h. Photoelectrochemical measurements were performed on a electrochemical workstation (CHI660E, China) in a standard three-electrode system with the as-prepared sample as the working electrode, a Pt foil as the counter electrode, and Ag/AgCl (saturated KCl) as the reference electrode. A 300 W mercury lamp was utilized as the light source. A 0.1 M Na2SO4 aqueous solution was used as the electrolyte. The impedance spectra were interpreted by a nonlinear least-squares fitting procedure using commercial software (ZsimpWin).
3. Results and discussion
The effect of N-doping on the phase structure of BiPO4 is examined by XRD (Fig. 1(a)). The diffraction peaks of all samples can match well with the monoclinic phase of BiPO4 with lattice parameters of a = 6.752, b = 6.933 and c = 6.468 Å (JCPDS 15-0767) (standard XRD pattern shown at the bottom of Fig. 1(a)). Compared with pure BiPO4, no impurity peaks arise in N-doped BiPO4, which indicates that N-doping does not affect the crystal structure of BiPO4. Additionally, no extra phases related to N compounds are detected for N-doped BiPO4 even at high content. This may be due to the low N contents in these samples, which are beyond the detection limit of the XRD technique. Fig. 1(b) exhibits a magnified view of (200) diffraction peak in the range of 27–28°, the enlarged peak centered at 27.2° first shifts slightly to low angle for the higher value of the ionic radius of N3− (0.171 nm) than that of O2− (0.140 nm) when the content of nitrogen is below 5 at%. However, the peaks display a high angle shift (lower d value) when the content of nitrogen is above 5 at%. This can be ascribed to the presence of compressive strain in N-doped BiPO4 lattice, which may emanate from the differences in the bonding characteristics between nitrogen and oxygen. This is important for significant modification to the electronic states due to the replacement of oxygen atoms with nitrogen atoms upon N doping, which is required for the desired substantial shift in the optical absorption toward the long-wavelengths region.13 According to the line width analysis of the (200) diffraction peak based on the Scherrer formula, the average crystallite sizes of all samples are 46.99, 48.98, 46.96, 42.54 and 42.47 nm, respectively. The crystallite sizes of N-doped BiPO4 are slightly smaller than those of pure BiPO4. Therefore, it can be proposed that N-doping may lead to a slight lattice distortion in the structure of BiPO4. To further investigate the effect of N-doping on the crystal structure of BiPO4, the lattice parameters, cell volumes, bond lengths, and bond angles of all samples are refined using the Rietveld analysis and listed in Table 1. These data reveal that the lattice parameters and cell volumes first increase and then decrease with the increasing of N-doping. The increasing reason for 5 at% may be attributed to the incorporation of nitrogen into BiPO4 lattice to replace oxygen, resulting in the increase of volume, whereas excessive nitrogen may generate compressive strain in BiPO4 lattice and compress grain volume. Besides that, it can be seen in Table 1 that the bond lengths and bond angles of samples also change. With the content of nitrogen increasing, the bond angles of Bi–O4–P range from 133.298° to 58.623°, which suggests that the doped nitrogen has an important effect on the bond angles of BiPO4.| Samples | Lattice parameters/Å | Cell volumes/Å3 | Bond lengths of Bi–O4/Å | Bond lengths of P–O/Å | Bond angles of Bi–O4–P/° |
|---|---|---|---|---|---|
| 0 at% | a = 6.7452 | 293.76 | 1.873 | P–O1: 1.615 | 133.298 |
| b = 6.9308 | P–O2: 1.211 | ||||
| c = 6.4680 | P–O3: 1.525 | ||||
| β = 103.7091 | P–O4: 1.414 | ||||
| 5 at% | a = 6.7487 | 294.02 | 1.922 | P–O1: 1.609 | 133.592 |
| b = 6.9326 | P–O2: 1.257 | ||||
| c = 6.4681 | P–O3: 1.522 | ||||
| β = 103.6898 | P–O4: 1.396 | ||||
| 9 at% | a = 6.7441 | 293.60 | 1.898 | P–O1: 1.607 | 131.94 |
| b = 6.9285 | P–O2: 1.151 | ||||
| c = 6.4678 | P–O3: 1.531 | ||||
| β = 103.7093 | P–O4: 1.381 | ||||
| 15 at% | a = 6.7437 | 293.41 | 1.933 | P–O1: 1.604 | 61.544 |
| b = 6.9277 | P–O2: 1.213 | ||||
| c = 6.4652 | P–O3: 1.521 | ||||
| β = 103.7280 | P–O4: 1.397 | ||||
| 21 at% | a = 6.7422 | 293.38 | 1.978 | P–O1: 1.593 | 58.623 |
| b = 6.9272 | P–O2: 1.160 | ||||
| c = 6.4646 | P–O3: 1.519 | ||||
| β = 103.6929 | P–O4: 1.242 |
The Raman spectra of pure BiPO4 and N-doped BiPO4 are shown in Fig. 2. In all five spectra, the observed intense band at 170 cm−1 and a shoulder at 231 cm−1 are ascribed to the Bi–O stretching vibration. The bands center at 1038 and 970 cm−1 are due to the asymmetric (ν3) and symmetric (ν1) stretching vibrations of the PO4 group, respectively. The bands at 554 and 596 cm−1 can be assigned to the ν4 bending vibration modes of PO4 groups. The weak bands at 406 and 460 cm−1 correspond to the ν2 bending vibration of the PO4 unit.18 With the increasing of nitrogen content, no apparent change of Raman shifts can be observed except for the intensity of inherent Raman peaks decreases. This indicates a breaking of the symmetry of the BiPO4 molecular structure, it can be inferred that the nitrogen is doped in the BiPO4 lattice which corroborates the XRD results.19,20 From the refinement results of bond angles (Table 1), we can see that the bond angle of 21 at% changes mightily, which further proves that the symmetry of the BiPO4 molecular structure is indeed broken.
XPS analysis is conducted to investigate the surface composition and the chemical state of 0 at% and 21 at%. According to the results of XRD and Raman, it is suggested that N3− ions are dopant in BiPO4 crystal lattice. As shown in the overall XPS spectra of 0 at% and 21 at% (Fig. 3(a)), there are no obvious differences between the two spectral lines. The C 1s peak at 284.6 eV is probably attributed to the signal from carbon in the instrument.21 In order to investigate the doped nitrogen chemical states in the sample, the N 1s high-resolution XPS spectrum is analyzed. As shown in Fig. 3(b), the N 1s peak is found at 398.98 eV only, which may be suitable for entering into the BiPO4 lattice to replace oxygen.8 Fig. 3(c) shows the XPS spectra for the Bi 4f region of 0 at% and 21 at%. By fitting the curves, two strong symmetrical peaks are obtained at 158.98 and 164.28 eV, corresponding to the Bi 4f7/2 and Bi 4f5/2 signals of 0 at%; peaks at 159.18 and 164.48 eV correspond to the Bi 4f7/2 and Bi 4f5/2 signals of 21 at%, respectively, which are characteristic of the Bi3+ species.22 We can conclude that N-doping has no effect on the chemical state of the bismuth ion in BiPO4. It is worth noting that the binding energy of Bi 4f peak of 21 at% increases to a higher value compared with that of 0 at%, suggesting that some of the lattice oxygen atoms are replaced by nitrogen atoms and doping nitrogen can lead to a decrease of electron density on Bi due to the lower electronegativity of nitrogen compared to that of oxygen.8 The binding energy of P 2p is 132.38 eV (Fig. 3(d)) in the oxide form of 0 at%, which can be characteristic of P species in the PO4 tetrahedron.23 However, in the case of 21 at%, the binding energy of the peak of P 2p increases about 0.3 eV, which is attributed to the change of the chemical environment surrounding P. The same results occur in the binding energies of O 1s (Fig. 3(e)). On the basis of XPS results, it is suggested that the presence of N3− ions in BiPO4 crystal lattice can influence the chemical environment surrounding the Bi, P and O elements.
The morphology and microstructure of N-BiPO4 are shown in Fig. 4. It is clearly seen that all samples are nanorods, indicating that nitrogen doping does not change the morphologies of BiPO4. However, with the increasing nitrogen content, the nanorod sizes of N-doped BiPO4 are decreased gradually in comparison with those of pure BiPO4, thus resulting in the increase of specific surface areas (from 1.63 m2 g−1 to 4.83 m2 g−1), as shown in Fig. 4(j). This indicates that nitrogen doping can inhibit the grain growth of BiPO4. The selected area electron diffraction (SAED) patterns (the insets of Fig. 4(c) and (i)) suggest that both 0 at% and 21 at% are single-crystal structures. The HRTEM images obtained from Fig. 4(b) and (h) areas marked with rectangles are shown in Fig. 4(c) and (i). The clear lattice fringe indicates the high crystallinity. It can be seen that the fringe spacing is ca. 0.306 nm, which corresponds to the (120) plane of monoclinic BiPO4 (JCPDS no. 15-0767).
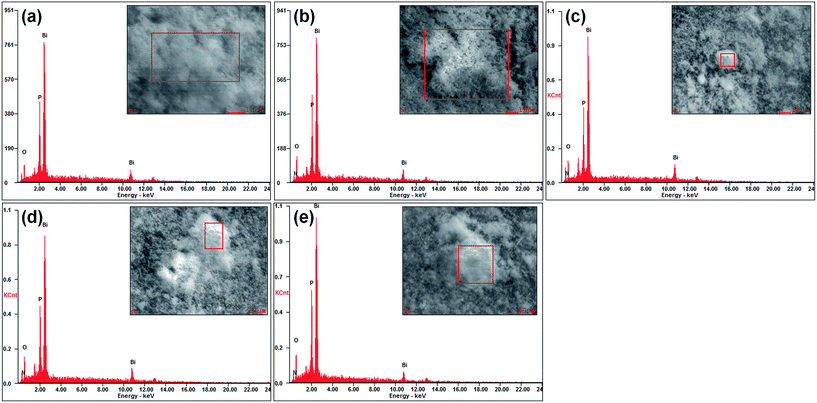
EDS is a semi-quantitative method to detect the chemical composition of samples. The EDS spectra of all samples are displayed in Fig. 5. From the spectra, it can be seen that 0 at% only contains Bi, P, and O elements, while 5 at%, 9 at%, 15 at% and 21 at% all contain Bi, P, O and N elements. However, it is found that there is a great deviation in O and N contents. It may be because EDS is only a semi-quantitative method. The molar ratio of N and Bi is about 1.9%, 4.5%, 7.0% and 12% for 5 at%, 9 at%, 15 at% and 21 at%, respectively, as listed in Table 2. Therefore, with the increase of NaN3 addition, the molar ratio of N and Bi gradually increases, which indicates that more nitrogen enters into the lattice of BiPO4. Combined with XRD, Raman and XPS analyses, it further proves that partial N ions enter into the lattice of BiPO4 crystallite changing the crystal structure of BiPO4.
| Samples | Element O/atom% | Element P/atom% | Element Bi/atom% | Element N/atom% | Total |
|---|---|---|---|---|---|
| 0 at% | 49.53 | 25.95 | 24.52 | 0.00 | 100.00 |
| 5 at% | 54.82 | 23.47 | 21.31 | 0.40 | 100.00 |
| 9 at% | 54.42 | 22.63 | 21.96 | 0.99 | 100.00 |
| 15 at% | 55.19 | 22.65 | 20.71 | 1.45 | 100.00 |
| 21 at% | 52.80 | 23.42 | 21.23 | 2.55 | 100.00 |
The formation process of pure BiPO4 and N-doped BiPO4 is shown in Fig. 6. Bi(NO3)3·5H2O and Na3PO4·12H2O are dissolved in the aqueous solution at room temperature with the hydrolysis process. At 200 °C, Bi3+ and PO43− react with each other to form BiPO4 crystal nucleus, the produced BiPO4 crystal nucleus grow quickly, then the larger size BiPO4 crystals are obtained. With the addition of NaN3, because of the presence of sterical hindered in solution, the growth of BiPO4 crystal is restrained and thus a large number of smaller size nanorods are formed.
Fig. 7(a) shows the UV-vis diffuse reflectance spectroscopy (DRS) of pure BiPO4 and N-doped BiPO4. From the spectra, it can be seen that all samples exhibit intense absorption bands with a steep edge in the UV light region, which indicates that the absorption of UV-light is not due to the transition of impurity levels but to the intrinsic transition of band gap energy level.24 The band gap energy (Eg) values are calculated from the UV-vis DRS using the equation Eg = 1240/λ, where λ is the wavelength of the absorption onset. The band gaps are 4.02 eV, 4.11 eV, 4.12 eV, 4.19 eV and 4.22 eV for 0 at%, 5 at%, 9 at%, 15 at% and 21 at%, respectively, which indicates that the band gap energy increases gradually with the increase of the content of nitrogen.
To further characterize the electrical properties of the surfaces, Mott–Schottky plots are performed. Fig. 7(b) shows the Mott–Schottky (MS) plots measured at an AC frequency of 100 Hz for of 0 at% and 21 at% electrodes in 0.1 M Na2SO4. The positive slopes indicate the typical n-type characteristics for these two semiconductors. The linear range fitted in Fig. 7(b) can be ascribed to the effect of potential on capacitance of depletion layer in the BiPO4 membrane electrode, which can be interpreted by eqn (1),
 | (1) |
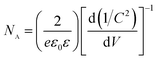 | (2) |
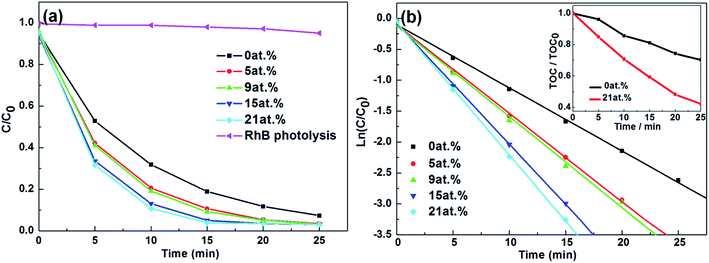
The photocatalytic activities of pure BiPO4 and N-doped BiPO4 photocatalysts are evaluated by measuring the degradation of RhB under UV light irradiation. As a comparison, results of blank photolysis experiment for RhB is also provided. It can be seen that without the presence of a catalyst, the rate of RhB degradation can be neglected under UV light irradiation. As shown in Fig. 8(a), when the nitrogen is introduced into BiPO4, all N-doped BiPO4 exhibit much higher photocatalytic activities than that of pure BiPO4. For 21 at%, the photodegradation rate of RhB reaches nearly 89% after irradiation for 10 min. However, photodegradation rates are just 68%, 79%, 81% and 87% for 0 at%, 5 at%, 9 at% and 15 at% after the same time of irradiation.
According to the Langmuir–Hinshelwood first-order reaction kinetics behavior, the pseudo-first-order rate constants (k) for different photocatalysts can be calculated by eqn (3),
| ln(C/C0) = −kt | (3) |
To evaluate the stability and reusability of N-doped BiPO4, the recycle degradation experiments of RhB are conducted under UV light irradiation, as shown in Fig. 9(a). It is found that 21 at% does not exhibit significant loss of photocatalytic activity after four recycles for the photodegradation of RhB, which indicates that the 21 at% has high stability. Furthermore, the XRD patterns of 21 at% before and after 100 min UV light irradiation are carried out, as shown in Fig. 9(b). The XRD patterns are almost identical, indicating that the 21 at% is stable during the photodegradation process and has the ability to be reused.
 | ||
| Fig. 9 (a) Cycling runs of 21 at% for the degradation of RhB under UV light; (b) XRD patterns of 21 at% before and after 100 min UV light irradiation. | ||
It is well known that the separation efficiency of charge carriers play an important role in the photocatalytic reaction.28 Photocurrent is an effective approach to investigate the separation efficiency of photogenerated carriers, the higher photocurrents mean better electron and hole separation efficiencies, and thus higher photocatalytic activities.29,30 The photoelectrochemical properties of pure BiPO4 and N-doped BiPO4 electrodes are measured in Na2SO4 electrolyte under UV light irradiation. The plots of transient photocurrent responses of pure BiPO4 and N-doped BiPO4 versus time are recorded for several on–off cycles of UV light irradiation, as shown in Fig. 10. As seen, it is obvious that the photocurrent is drastically increased when the irradiation is turned on, whereas the photocurrent value rapidly decreases to zero as soon as the irradiation is turned off. The photocurrent generated from N-doped BiPO4 are all larger than that of pure BiPO4, and 21 at% exhibits the highest photocurrent intensity when the UV illumination is turned on. This obvious enhancement of photocurrent indicates a smaller recombination and a more efficient separation of photogenerated electron–hole pairs for the N-doped BiPO4.
The electrochemical impedance spectra (EIS) of 0 at% and 21 at% films are measured under UV light irradiation. EIS has been widely employed in studying the separation efficiency of photogenerated electron–hole pairs and the property of carriers.31 Fig. 11 shows the Nyquist plots of 0 at% and 21 at% under UV-light irradiation (denoted by solid data points). It is clear that the radius of the Nyquist circular decreases after the photoirradiation, respectively, which indicates that the separation rate of photogenerated electron–hole pairs improves greatly. In addition, the EIS Nyquist plots of 0 at% and 21 at% show that the arc radius of 21 at% electrode is smaller than that of 0 at% with or without UV irradiation, which means that the 21 at% electrode has the smaller electric resistance, and thus an effective separation of photogenerated electron–hole pairs occurs in the 21 at% film electrode. Based on corresponding equivalent circuit (inset of Fig. 11), impedance spectra are simulated by the ZsimpWin software and a series of circuit parameters are obtained, as shown in Table 3. Here, RL is the solution resistance, Q is the electrochemical double-layer capacitance, R1 is the electrolytic resistance, Csc is the space charge capacitance, and R2 is the charge-transfer resistance. The size of the arc radius can be shown by the value of charge-transfer resistance (R2) which indicates the rate of the electrochemical reaction. It could be seen from Table 3 that the R2 value of 0 at% decreases from 5422 Ω to 4431 Ω after the illumination, while the R2 value of 21 at% is reduced from 4303 Ω to 3381 Ω after the irradiation. This result clearly indicates that the 21 at% possesses the stronger ability in separation of photoinduced electron–hole pairs than that of 0 at%, which is advantageous to the photocatalytic degradation to the RhB solution.
 | ||
| Fig. 11 EIS Nyquist plots (solid data points) and Z-fit equivalent circuit (hollow data points) of 0 at% and 21 at%. | ||
| RL (Ω) | Q (Ssen) | n | R1 (Ω) | C (μF) | R2 (Ω) | |
|---|---|---|---|---|---|---|
| 0 at% (dark) | 104.3 | 1.11 × 10−5 | 0.6406 | 35.54 | 13.14 | 5422 |
| 0 at% (UV) | 101.5 | 1.35 × 10−5 | 0.6102 | 39.58 | 13.03 | 4431 |
| 21 at% (dark) | 118 | 1.40 × 10−5 | 0.6389 | 46.17 | 12.23 | 4303 |
| 21 at% (UV) | 94.38 | 2.26 × 10−5 | 0.507 | 71.73 | 12.59 | 3381 |
4. Conclusions
In summary, N-doped BiPO4 photocatalysts with significantly enhanced photocatalytic activities have been successfully synthesized by a microwave hydrothermal method, and their photoelectrochemical activities have also been investigated. Nitrogen atoms were incorporated into BiPO4 lattice to replace oxygen atoms, which were verified by XRD, Raman and XPS analyses. Compared with pure BiPO4, N-doped BiPO4 exhibited much higher photocatalytic activity for the degradation of RhB, which was ascribed to the higher separation efficiency of photogenerated electron–hole pairs. In our work, although the doped nitrogen increased the specific surface areas of BiPO4, they were always in the same order of magnitude. Therefore, the specific surface area was not the decisive factor for the photocatalytic activity. Although N-doped BiPO4 exhibits outstanding UV light driven photocatalytic activity, it does not have visible light driven activity. The study not only demonstrates a facile fabrication method for N-doped BiPO4 with outstanding UV light photocatalytic activity but also provides new insights into the design of new highly active photocatalysts for future practical applications.Acknowledgements
This work is supported by the Project of the National Natural Science Foundation of China (Grant no. 51172135); the Academic Leaders Funding Scheme of Shaanxi University of Science & Technology (2013XSD06); Doctorate Scientific Research Initial Fund Program of Shaanxi University of Science & Technology (BJ4-13); the Graduate Innovation Fund of Shaanxi University of Science & Technology (SUST-A04).References
- L. W. Zhang and Y. F. Zhu, Catal. Sci. Technol., 2012, 2, 694–706 CAS.
- X. C. Wang, K. Maeda, A. Thomas, K. Takanabe, G. Xin, J. M. Carlsson, K. Domen and M. Antonietti, Nat. Mater., 2009, 8, 76–80 CrossRef CAS PubMed.
- A. Kubacka, M. Fernández-García and G. Colón, Chem. Rev., 2012, 112, 1555–1614 CrossRef CAS PubMed.
- A. Fujishima, X. Zhang and D. Tryk, Surf. Sci. Rep., 2008, 63, 515–582 CrossRef CAS PubMed.
- J. Zhang, Y. P. Zhang, Y. K. Lei and C. X. Pan, Catal. Sci. Technol., 2011, 1, 273–278 CAS.
- X. B. Chen, S. H. Shen, L. J. Guo and S. S. Mao, Chem. Rev., 2010, 110, 6503–6570 CrossRef CAS PubMed.
- X. B. Chen and S. S. Mao, Chem. Rev., 2007, 107, 2891–2959 CrossRef CAS PubMed.
- M. Wang, Q. Liu, Y. S. Che, L. F. Zhang and D. Zhang, J. Alloys Compd., 2013, 548, 70–76 CrossRef CAS PubMed.
- C. S. Pan and Y. F. Zhu, Environ. Sci. Technol., 2010, 44, 5570–5574 CrossRef CAS PubMed.
- C. S. Pan, D. Li, X. G. Ma, Y. Chen and Y. F. Zhu, Catal. Sci. Technol., 2011, 1, 1399–1405 CAS.
- Y. F. Liu, Y. H. Lv, Y. Y. Zhu, D. Liu, R. L. Zong and Y. F. Zhu, Appl. Catal., B, 2014, 147, 851–857 CrossRef CAS PubMed.
- B. Lu, X. G. Ma, C. S. Pan and Y. F. Zhu, Appl. Catal., A, 2012, 435, 93–98 CrossRef PubMed.
- T. C. Jagadale, S. P. Takale, R. S. Sonawane, H. M. Joshi, S. I. Patil, B. B. Kale and S. B. Ogale, J. Phys. Chem. C, 2008, 112, 14595–14602 CAS.
- Z. S. Lin, A. Orlov, R. M. Lambert and M. C. Payne, J. Phys. Chem. B, 2005, 109, 20948–20952 CrossRef CAS PubMed.
- F. Peng, L. F. Cai, H. Yu, H. J. Wang and J. Yang, J. Solid State Chem., 2008, 181, 130–136 CrossRef CAS PubMed.
- M. Shang, W. Z. Wang, L. Zhang and H. L. Xu, Mater. Chem. Phys., 2010, 120, 155–159 CrossRef CAS PubMed.
- J. G. Hou, R. Cao, Z. Wang, S. Q. Jiao and H. M. Zhu, J. Hazard. Mater., 2012, 217, 177–186 CrossRef PubMed.
- M. L. Zhao, G. S. Li, L. P. Li, L. S. Yang and J. Zheng, Cryst. Growth Des., 2012, 12, 3983–3991 CAS.
- K. Bhattacharyya, S. Varma, A. K. Tripathi, S. R. Bharadwaj and A. K. Tyagi, J. Phys. Chem. C, 2008, 112, 19102–19112 CAS.
- F. Dong, H. Q. Wang and Z. B. Wu, J. Phys. Chem. C, 2009, 113, 16717–16723 CAS.
- J. Su, X. X. Zou, G. D. Li, X. Wei, C. Yan, Y. N. Wang, J. Zhao, L. J. Zhou and J. S. Chen, J. Phys. Chem. C, 2011, 115, 8064–8071 CAS.
- H. F. Cheng, B. B. Huang, P. Wang, Z. Y. Wang, Z. Z. Lou, J. P. Wang, X. Y. Qin, X. Y. Zhang and Y. Dai, Chem. Commun., 2011, 47, 7054–7056 RSC.
- C. S. Pan, J. Xu, Y. J. Wang, D. Li and Y. F. Zhu, Adv. Funct. Mater., 2012, 22, 1518–1524 CrossRef CAS PubMed.
- G. S. Li, D. Q. Zhang and J. C. Yu, Chem. Mater., 2008, 20, 3983–3992 CrossRef CAS.
- G. Wang, Q. Wang, W. Lu and J. Li, J. Phys. Chem. B, 2006, 110, 22029–22034 CrossRef CAS PubMed.
- H. Maeda, K. Ikeda, K. Hashimoto, K. Ajito, M. Morita and A. Fujishima, J. Phys. Chem. B, 1999, 103, 3213–3217 CrossRef CAS.
- K. V. Kumar, K. Porkodi and F. Rocha, Catal. Commun., 2008, 9, 82–84 CrossRef CAS PubMed.
- J. Jiang, X. Zhang, P. B. Sun and L. Z. Zhang, J. Phys. Chem. C, 2011, 115, 20555–20564 CAS.
- Y. F. Liu, W. Q. Yao, D. Liu, R. L. Zong, M. Zhang, X. G. Ma and Y. F. Zhu, Appl. Catal., B, 2015, 163, 547–553 CrossRef CAS PubMed.
- Q. J. Xiang, J. G. Yu and M. Jaroniec, J. Phys. Chem. C, 2011, 115, 7355–7363 CAS.
- H. Liu, S. A. Cheng, M. Wu, H. J. Wu, J. Q. Zhang, W. Z. Li and C. N. Cao, J. Phys. Chem. A, 2000, 104, 7016–7020 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |