Open Access Article
Open Access ArticleNitroaldol (Henry) reaction of 2-oxoaldehydes with nitroalkanes as a strategic step for a useful, one-pot synthesis of 1,2-diketones†
Alessandro Palmieri*,
Serena Gabrielli,
Susanna Sampaolesi and
Roberto Ballini*
“Green Chemistry Group”, School of Science and Technology, Chemistry Division, University of Camerino, via S. Agostino 1, 62032 Camerino, MC, Italy. E-mail: alessandro.palmieri@unicam.it; roberto.ballini@unicam.it; Fax: +39 0737 402297
First published on 15th April 2015
Abstract
The nitroaldol (Henry) reaction of 2-oxoaldehydes with a variety of nitroalkanes, under basic heterogeneous conditions and microwave irradiation, affords 1,2-diketones in a one-pot way. The key step of the process involves the nitrous acid elimination from the nitroalkanol intermediates instead of the standard water elimination.
1,2-Diketones are a powerful class of molecules widely used as key building blocks in organic synthesis1 for the preparation of a large variety of heterocyclic targets,2 and present in several biologically active compounds.3 Given their great importance, over the years a number of procedures have been proposed in the literature for their preparation. The most valuable ones are: via (i) oxidation of alkynes,4 (ii) oxidation of alkenes,5 (iii) oxidation of aryl ketones,6 (iv) rearrangement of α,β-epoxy ketones,7 (v) nucleophilic acylation of esters,8 (vi) reductive cross-coupling reactions of imines with nitriles,9 (vii) carbonation-diketonization of terminal aromatic alkenes with nitroalkanes,10 and (viii) reaction of α-oxo acid chlorides with organostannanes.11 In summary, they could be classified in two distinct approaches: (a) transformation of substrates maintaining the same carbon backbone (e.g. i–iv) and, (b) ex-novo structure construction, by new C–C bonds generation, through a reaction between specific nucleophiles and electrophiles (e.g. v–viii).
Although the first approach requires the preparation of precise substrates, the approach (b) seems to be more flexible but presents important limitations such as the need of very low temperature,8a,b inert atmosphere,8a,b usage of toxic reagents,8a,10,11 and autoclave.11 Furthermore, all the reported procedures involve an articulate work-up, with evident disadvantages from ecological point of view. In this context, the sustainability of a chemical process is one of the main aspect that, nowadays, must be considered, and the implementation of new simple, mild and greener methodologies is of dramatic importance.12
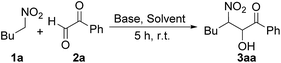
Since its discovery, the nitroaldol (Henry) reaction has become one of the most valuable methods for the generation of C–C bonds13 and as key starting synthetic step for the preparation of important fine chemicals, often with important ecological advantages.14 Thus, following our studies on the nitroaldol reactions, and with the aim to discover a more efficient and general method for the production of the title compounds 5, we focused our attention to the α-oxoaldehydes 2 as strategic partners of nitroalkanes 1 in the Henry reaction. Our idea was the formation of the 3-nitro-2-alkanols 3 in which the elimination of nitrous acid vs. the conventional water elimination is favoured (during experiments, no trace of the dehydrated specie 6, R1 = H, was detected, Scheme 1). The driving force of the reaction can be rationalized with the increased acidity of the proton in 2-position, due to the geminal presence of carbonyl moiety, which leads to the irreversible elimination of HNO2 affording the enolic form 4, which acts as the spontaneous precursor of the 1,2-diketones 5.
In this context, to the best of our knowledge, only a sporadic example of Henry reaction involving nitrous acid elimination was reported in literature,15 which anyway, seems to work in moderate yields just with 2-quinolinecarboxaldehyde and in the presence of a large excess of simple nitroalkanes (3 equivalents).
Initially, with the aim to optimized our approach, we studied the process as two distinct separate steps: (i) the Henry reaction between 1a and 2a, and (ii) the conversion of 3aa into 5aa. Concerning the Henry reaction, we did a deep screening in terms of base type, stoichiometry and solvents (Table 1).
| Entry | Base (g mmol−1) | Solvent | Yielda (%) |
|---|---|---|---|
| a Yield of pure isolated product.b Scale: 1a = 0.5 mmol and 2a = 0.5 mmol.c Scale: 1a = 0.5 mmol and 2a = 0.65 mmol.d Scale: 1a = 0.65 mmol and 2a = 0.5 mmol.e Scale: 1a = 0.75 mmol and 2a = 0.5 mmol. | |||
| ab | TBD on polymer (0.3) | 2-MeTHF | 38 |
| bb | Carbonate on polymer (0.3) | 2-MeTHF | 37 |
| cb | KF/Al2O3 (0.3) | 2-MeTHF | 32 |
| db | Amberlyst A21 (0.3) | 2-MeTHF | 46 |
| eb | Amberlyst A21 (0.4) | 2-MeTHF | 51 |
| fb | Amberlyst A21 (0.5) | 2-MeTHF | 55 |
| gb | Amberlyst A21 (0.6) | 2-MeTHF | 54 |
| hc | Amberlyst A21 (0.5) | 2-MeTHF | 58 |
| id | Amberlyst A21 (0.5) | 2-MeTHF | 76 |
| je | Amberlyst A21 (0.5) | 2-MeTHF | 71 |
| kd | Amberlyst A21 (0.5) | EtOAc | 69 |
| ld | Amberlyst A21 (0.5) | MeCN | 51 |
| md | Amberlyst A21 (0.5) | DCM | 49 |
The best result (entry i, yield = 76%) was obtained using Amberlyst A21 (0.5 g mmol−1), 2-MeTHF (as green solvent)16 at room temperature (5 hours) and in presence of a slight excess of nitroalkane (1.3 equivalents). Once optimized the first step, we explored the reaction conditions for converting the nitro alcohol 3aa into the diketone 5aa, using Amberlyst A21 and 2-MeTHF (Table 2).
| Entry | Amb. A21 (g mmol−1) | T (°C) | Time2 (h) | Yielda (%) |
|---|---|---|---|---|
| a Yield of pure isolated product.b 80 °C (b.p. 2-MeTHF) heated by conventional oil bath.c Heated under microwave irradiation. | ||||
| a | 0.3 | Refluxb | 4 | 63 |
| b | 0.4 | Refluxb | 4 | 71 |
| c | 0.5 | Refluxb | 4 | 72 |
| d | 1.0 | Refluxb | 4 | 51 |
| e | 0.5 | 100c | 1 | 78 |
| f | 0.5 | 110c | 1 | 85 |
| g | 0.4 | 110c | 1 | 83 |
| h | 0.5 | 120c | 1 | 72 |
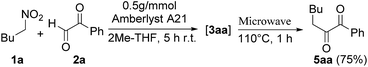
The conversion works efficiently both with 0.4 and 0.5 g mmol−1 of Amberlyst A21, and the best results were obtained under microwave irradiation at 110 °C after 1 hour (83% and 85% respectively).
Then, we combined the two steps to achieve the one-pot protocol and, applying the best reaction conditions, we isolated the diketone 5aa in 75% of overall yields (Scheme 2).
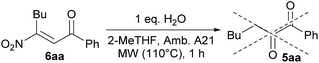
Successively, with the aim to clarify the reaction mechanism, and in particular concerning a possible equilibrium between 3 and 6, we investigated the reaction of the β-nitroenones 6a in presence of water (Scheme 3). Under the optimized reaction conditions, the formation of the diketone 5aa was not observed, demonstrating that the specie 6 is not involved in the mechanism and that the elimination of nitrous acid is the actual synthetic pathway.
Finally, we extended our study to a variety of nitroalkanes and α-oxoaldehydes obtaining in all cases from moderate to good overall yields (Table 3, 42–76%), thus demonstrating the generality of our protocol. In addition, thanks to the mildness of the reaction conditions, several functionalities such as chlorine, fluorine, nitro, ketal, cyano and ester, can be preserved. Furthermore, thanks to the use of Amberlyst A21, as solid supported base, the work-up can be minimized to an easy filtration saving resources and time and thus reducing the waste generation. It is important to point out that the overall transformation (1 + 2 to 5) makes the nitroalkane 1 as a synthetic equivalent of the carbanionic A, while the aldehyde 2 acts as the synthetic equivalent of the acyl cation synthon B (Fig. 1).
| R1 | R2 | R3 | Time1 (h) | Yielda (%) | |||
|---|---|---|---|---|---|---|---|
| a Yield of pure isolated product. | |||||||
| 1a | Bu | H | 2a | Ph | 5 | 5aa | 75 |
| 1b | n-Pr | H | 2a | Ph | 5 | 5ba | 72 |
| 1c | CH3(OCH2CH2O)CCH2 | H | 2a | Ph | 4 | 5ca | 70 |
| 1d | Ph(CH2)2 | H | 2a | Ph | 5 | 5da | 76 |
| 1d | Ph(CH2)2 | H | 2b | 2-F-C6H4 | 5 | 5db | 68 |
| 1e | CH3(CH2)8 | H | 2c | 4-MeO-C6H4 | 6 | 5ec | 62 |
| 1f | Cl(CH2)3 | H | 2c | 4-MeO-C6H4 | 6 | 5fc | 52 |
| 1g | NC(CH2)4 | H | 2d | 2-Napthyl | 6 | 5gd | 74 |
| 1h | CH3(CH2)4 | H | 2d | 2-Napthyl | 6 | 5hd | 71 |
| 1i | MeOOC(CH2)4 | H | 2a | Ph | 5 | 5ia | 70 |
| 1j | Me | Me | 2e | 4-NO2-C6H4 | 12 | 5je | 42 |
| 1k | –(CH2)4– | 2a | Ph | 15 | 5ka | 51 | |
Conclusions
In conclusion we have found a new, general, efficient strategy to provide an easy, sustainable synthesis of 1,2-dicarbonyl derivatives, in fact the title compounds can be prepared in a one-pot way, in good overall yields, and avoiding any elaborate and wasteful work-up. In addition, our report expands the extraordinary synthetic potential of the nitroaldol (Henry) reaction.Acknowledgements
The authors thank the University of Camerino and MIUR-Italy (FIRB National Project “Metodologie di nuova generazione nella formazione di legami carbonio-carbonio e carbonio-eteroatomo in condizioni eco-sostenibili”) for financial support.Notes and references
- (a) B. M. Trost and G. M. Schroeder, J. Am. Chem. Soc., 2000, 122, 3785 CrossRef CAS; (b) A. J. Herrera, M. Rondón and E. Suárez, J. Org. Chem., 2008, 73, 3384 CrossRef CAS PubMed; (c) D. Won Cho, H.-Y. Lee, S. Wha Oh, J. Hei Choi, H. Jung Park, P. S. Mariano and U. Chan Yoon, J. Org. Chem., 2008, 73, 4539 CrossRef PubMed; (d) F. A. Khan, J. Dash and C. Sudheer, Chem.–Eur. J., 2004, 10, 2507 CrossRef CAS PubMed; (e) C. W. Lindsley, Z. Zhao, W. H. Leister, R. G. Robinson, S. F. Barnett, D. Defeo-Jones, R. E. Jones, G. D. Hartman, J. R. Huff, H. E. Huber and M. E. Duggan, Bioorg. Med. Chem. Lett., 2005, 15, 761 CrossRef CAS PubMed.
- For some representative examples, see: (a) E. C. Taylor, J. E. Macor and L. G. Frenc, J. Org. Chem., 1991, 56, 1807 CrossRef CAS; (b) M. H. Nantz, D. A. Lee, D. M. Bender and A. H. Roohi, J. Org. Chem., 1992, 57, 6653 CrossRef CAS; (c) A. Khalaj and M. Ghafari, Tetrahedron Lett., 1986, 27, 5019 CrossRef CAS; (d) A. S. Kiselyov, Tetrahedron Lett., 1995, 36, 493 CrossRef CAS; (e) S. E. Wolkenberger, D. D. Wisnoski, W. H. Leister, Y. Wang, Z. Zao and C. W. Lindsley, Org. Lett., 2004, 6, 1453 CrossRef PubMed.
- (a) K. C. Nicolau, D. L. F. Gray and J. Tae, J. Am. Chem. Soc., 2004, 126, 613 CrossRef PubMed; (b) M. R. Angelestro, S. Mehdi, J. P. Burkhart, N. P. Peet and P. Bey, J. Med. Chem., 1990, 33, 11 CrossRef.
- (a) Y. Ishii and Y. Sakata, J. Org. Chem., 1990, 55, 5545 CrossRef CAS; (b) S. Lai and D. G. Lee, Tetrahedron, 2002, 58, 9879 CrossRef CAS; (c) C. Mousset, O. Provot, A. Hamze, J. Bignon, J.-D. Brion and M. Alami, Tetrahedron, 2008, 64, 4287 CrossRef CAS PubMed; (d) S. Chen, Z. Liu, E. Shi, L. Chen, W. Wei, H. Li, Y. Cheng and X. Wan, Org. Lett., 2011, 13, 2274 CrossRef CAS PubMed; (e) W. Ren, J. Liu, L. Chen and X. Wan, Adv. Synth. Catal., 2010, 352, 1424 CrossRef CAS PubMed; (f) M. Tingoli, M. Mazzella, B. Panunzi and A. Tuzi, Eur. J. Org. Chem., 2011, 399 CrossRef CAS PubMed; (g) Y. Xu and X. Wan, Tetrahedron Lett., 2013, 54, 642 CrossRef CAS PubMed; (h) M. E. Jung and G. Deng, Org. Lett., 2014, 16, 2142 CrossRef CAS PubMed.
- X. Liu and W. Chen, Organometallics, 2012, 31, 6614 CrossRef CAS.
- D. Ghazanfari, F. Najafizadeh and F. Khosravi, Monatsh. Chem., 2004, 135, 1409 CrossRef CAS PubMed.
- C.-L. Chang, M. P. Kumar and R.-S. Liu, J. Org. Chem., 2004, 69, 2793 CrossRef CAS PubMed.
- (a) D. Seyferth, R. M. Weinstein, R. C. Hui, W.-L. Wang and C. M. Archer, J. Org. Chem., 1991, 56, 5768 CrossRef CAS; (b) A. R. Katritzky, Z. Wang, H. Lang and D. Feng, J. Org. Chem., 1997, 62, 4125 CrossRef CAS; (c) P. Liu, Y.-M. Zhang and H.-Li. Zhang, Synth. React. Inorg. Met.-Org. Chem., 2010, 40, 266 CAS.
- H. Chen, G. Fan, S. Li, K. Mao and Y. Liu, Tetrahedron Lett., 2014, 55, 1593 CrossRef CAS PubMed.
- A. Wang, H. Jiang and X. Li, J. Org. Chem., 2011, 76, 6958 CrossRef CAS PubMed.
- T. Kashiwabara and M. Tanaka, J. Org. Chem., 2009, 74, 3958 CrossRef CAS PubMed.
- (a) P. T. Anastas and J. C. Warner, in Green Chemistry: Theory and Practice, Oxford University Press, New York, 1998 Search PubMed; (b) P. T. Anastas and M. M. Kirchhoff, Acc. Chem. Res., 2002, 35, 686 CrossRef CAS PubMed; (c) I. T. Horváth and P. T. Anastas, Chem. Rev., 2007, 107, 2169 CrossRef PubMed; (d) P. Anastas and N. Eghbali, Chem. Soc. Rev., 2010, 39, 301 RSC.
- (a) R. Ballini and A. Palmieri, Curr. Org. Chem., 2006, 10, 2145 CrossRef CAS; (b) G. Rosini and R. Ballini, Synthesis, 1988, 833 CrossRef CAS; (c) R. Ballini, M. Noè, A. Perosa and M. Selva, J. Org. Chem., 2008, 73, 8520 CrossRef CAS PubMed; (d) R. Devi, R. Borah and R. C. Deka, Appl. Catal., A, 2012, 433–434, 122 CrossRef CAS PubMed; (e) D. Kühbeck, B. B. Dhar, E.-M. Schön, C. Cativiela, V. Gotor-Fernández and D. D. Díaz, Beilstein J. Org. Chem., 2013, 9, 1111 CrossRef PubMed; (f) R. Ballini and G. Bosica, J. Org. Chem., 1997, 62, 425 CrossRef CAS; (g) R. Ballini, G. Bosica, D. Livi, A. Palmieri, R. Maggi and G. Sartori, Tetrahedron Lett., 2003, 44, 2271 CrossRef CAS; (h) F. A. Luzzio, Tetrahedron, 2001, 57, 915 CrossRef CAS; (i) D. Kühbeck, B. B. Dhar, E.-M. Schön, C. Cativiela, V. Gotor-Fernández and D. Díaz Díaz, Beilstein J. Org. Chem., 2013, 9, 1111 CrossRef PubMed; (j) P. S. Shinde, S. S. Shinde, S. A. Dake, V. S. Sonekar, S. U. Deshmukh, V. V. Thorat, N. M. Andurkar and R. P. Pawar, Arabian J. Chem., 2014, 7, 1013 CrossRef CAS PubMed; (k) L.-W. Tang, X. Dong, Z.-M. Zhou, Y.-Q. Liu, L. Dai and M. Zhang, RSC Adv., 2015, 5, 4758 RSC.
- (a) R. Ballini, L. Barboni, D. Fiorini, G. Giarlo and A. Palmieri, Green Chem., 2005, 7, 828 RSC; (b) R. Ballini, L. Barboni, D. Fiorini and A. Palmieri, Synlett, 2004, 2618 CrossRef CAS PubMed; (c) R. Ballini, S. Gabrielli and A. Palmieri, Synlett, 2007, 2430 CrossRef CAS PubMed; (d) A. Palmieri, S. Gabrielli and R. Ballini, Chem. Commun., 2010, 6165 RSC; (e) R. Ballini, L. Barboni, D. Fiorini, G. Giarlo and A. Palmieri, Chem. Commun., 2005, 2633 RSC; (f) R. Ballini, G. Bosica, D. Fiorini and A. Palmieri, Synthesis, 2004, 1938 CrossRef CAS PubMed; (g) T. Hara, S. Kanai, K. Mori, T. Mizugaki, K. Ebitani, K. Jitsukawa and K. Kaneda, J. Org. Chem., 2006, 71, 7455 CrossRef CAS PubMed; (h) K. Motokura, M. Tada and Y. Iwasawa, Angew. Chem., Int. Ed., 2008, 47, 9230 CrossRef CAS PubMed.
- A. Nomland and I. D. Hills, Tetrahedron Lett., 2008, 49, 5511 CrossRef CAS PubMed.
- V. Pace, P. Hoyos, L. Castoldi, P. Domínguez de María and A. R. Alcántara, ChemSusChem, 2012, 5, 1369 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra03772g |
| This journal is © The Royal Society of Chemistry 2015 |