One pot oxidative cleavage of cyclohexene to adipic acid using silver tungstate nano-rods in a Brønsted acidic ionic liquid†
Abstract
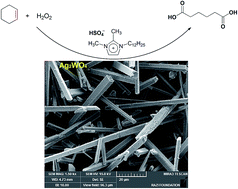
A green and facile method for oxidation of cyclohexene to adipic acid is introduced using 30% H2O2 as oxidant. The catalytic system comprises small amounts of Ag2WO4 nano-rods and a Brønsted acidic ionic liquid (1,2-dimethyl-3-dodecylidazolium hydrogensulfate).
- This article is part of the themed collection: Ionic Liquids: Editors collection for RSC Advances

 Please wait while we load your content...
Please wait while we load your content...