Continuous cyclo-polymerisation of l-lactide by reactive extrusion using atoxic metal-based catalysts: easy access to well-defined polylactide macrocycles†
Abstract
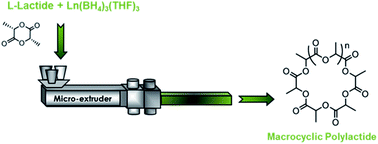
The bulk polymerisation of L-lactide was assessed using the alkaline-earth and lanthanide borohydride complexes Mg(BH4)2 (1), Ca(BH4)2(THF)2 (2) and Ln(BH4)3(THF)3 with Ln = Nd (3), Sm (4) and La (5). All five catalysts display good activities, with up to 77% conversion in 20 min with 5. Lanthanide-based catalysts 3, 4 and 5, which were found to lead to the highest conversions, were tested in reactive extrusion polymerisation conditions in a twin-screw micro-extruder. High activities were also observed in theses experimental conditions, affording up to 73% conversion in only 20 min with lanthanum-based catalyst 5. More interestingly, the polylactides formed display a macrocyclic structure with molecular weights up to 30 000 g mol−1 along with relatively narrow dispersities (1.23–1.79). The macrocycles are formed via intramolecular transesterification reactions taking place during the ring opening polymerisation. Furthermore, the reactive extrusion polymerisations operate at a temperature as low as 130 °C, this monomer usually being polymerized at 185 °C. This is the first example of a cyclic polyester obtained directly via a continuous process.

 Please wait while we load your content...
Please wait while we load your content...