Control of Microcystis aeruginosa growth and associated microcystin cyanotoxin remediation by electron beam irradiation (EBI)
Shuyu Liuab,
Yueping Zhaoa,
Fang Ma*b,
Liyan Mad,
Kevin O'sheac,
Cen Zhaoc,
Xiaohui Hua and
Minghong Wu*a
aSchool of Environment and Chemical Engineering, Shanghai University, 201800, P.R. China. E-mail: mhwu@shu.edu.cn; Fax: +86-021-66137787; Tel: +86-21-66137787
bHarbin Institute of Technology, State Key Laboratory of Urban Water Resource and Environment, Harbin 150090, P.R. China. E-mail: liushuyu@shu.edu.cn; Fax: +86-021-66137787; Tel: +86-1350-194-7933
cDepartment of Chemistry and Biochemistry, Florida International University, Miami, FL 33199, USA
dKey Laboratory of East China Sea and Oceanic Fishery Resources Exploitation, Ministry of Agriculture, Chinese Academy of Fishery Sciences, Shanghai, 200090, China
First published on 3rd March 2015
Abstract
Microcystin-LR (MC-LR), a problematic potent cyanotoxin, is produced by a variety of cyanobacteria. The presence of MC-LR in drinking water is a severe threat to human health as well as an environmental concern. The control of these algal blooms and their associated toxins is critical for ensuring safe drinking water to significant populations. To the best of our knowledge, this is the first detailed study about the application of Electron Beam Irradiation (EBI) for the control of Microcystis aeruginosa algae cultures and simultaneous degradation of MC-LR. The effects of EBI dose on MC production and removal efficiency were investigated by measuring intercellular and extracellular MC concentrations. The dramatic decreases in MC concentration in the cells and solution were observed under our experimental conditions. The correlation between Chl-a and MC concentrations is eliminated. The inhibition of cell growth and degradation of MC-LR by EBI is highly efficient during radiolysis.
1. Introduction
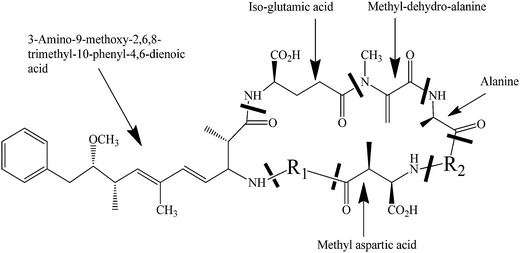
Cyanobacteria, which are known as blue-green algae, commonly exist in the sources of drinking water and can lead to harmful algal blooms (HABs). Cyanobacteria can produce a range of potent toxins such as nodularin, cylindrospermopsin and microcystin,1,2 which threaten the sources of drinking water and human health. Recently, due to the increased presence of harmful algal blooms (HABs) in water bodies, they have become one of the most important environmental problems. Microcystis aeruginosa (M. aeruginosa), the most common cyanobacterial blooms, has been reported to predominate 90% of HABs in natural water bodies and produces toxic microcystins (MC).3 Cynotoxin microcystins are potent hepatotoxins, which affect the inhibition of protein synthesis.4,5 Moreover, microcystins act as tumor promoters6 and may induce oxidative DNA damage in human hepatoma cell line HepG2.7 The microcystin structure is shown in Fig. 1.Due to the significant increases in the occurrences and volume of toxic algal blooms in industrial and potable water, effective treatments are critical to control and eliminate HABs. A number of studies have been reported for the treatment of MC.8–10 The conventional removal methods, such as filtration, flotation or coagulation, are often not viable for the removal of cyanotoxins.11 Although chemical oxidation treatments using chlorine and ozone have been studied for the removal of cyanotoxins, the by-products such as trihalomethanes (THMs) (by chlorination) and bromate (by ozonation) are a concern because of the associated negative health consequences.12,13 Advanced oxidation processes (AOPs) have been widely studied for the removal of a variety of pollutants and toxins from wastewater;11,14,15 however, they involve the generation of a highly reactive hydroxyl radical to react with pollutants, which leads to degradation.
Among the advanced oxidation processes, electron beam irradiation (EBI) is a promising technique with the advantages of a high removal efficiency and lower temperature requirement compared with other traditional treatment methods.16 EBI can produce reactive species, such as eaq−, ˙H, ˙OH and H2O2, by the radiolysis of water, as shown in eqn (1). These produced reactive species are capable of efficiently degrading the pollutants from wastewater.
| H2O → eaq− (0.27) + ˙H (0.06) + ˙OH (0.28) + H2 (0.05) + H2O2 (0.07) + H3O+ (0.27) | (1) |
EBI applications have been reported for the treatment of a variety of pollutants, such as polychlorinated biphenyls, thioanisole, azo dye, and polycholoro diabenzo-p-dioxin (PCDD), in wastewater.17–19 However, a few applications on the treatment of M. aeruginosa have been reported. Herein, we report, for the first time, the treatment of a toxin producing culture M. aeruginosa, focusing on the development of utilizing EBI on controlling MC concentration in the algae cell and free MC in the solution. We investigated the controlling effect of EBI on MC production by varying the different doses irradiation. Our results demonstrate that EBI can be widely applied for the purification of cyanotoxin based polluted water.
2. Materials and methods
2.1 Culture of M. aeruginosa
M. aeruginosa specimen (FACHB 905) culture was obtained from the Freshwater Algae Culture collection of the Institute Hydrobiology (Chinese Academy of Sciences, Wuhan, China). It was cultured in an autoclaved BG-11 medium at pH around 7.5. Cultures were incubated at 28 °C in the Light-Emitted Feeding Chamber with an automated light/dark cycle of 14 h light/10 h dark.2.2 Electron beam irradiation
The electron beam irradiation experiments were conducted at the Institute of Radiation Application, Shanghai University using a linear electron accelerator (GJ-II, XianFeng, Shanghai) with 1.0 MeV operating voltage and 1.0 mA beam intensity. Six groups of 100 mL algal suspension were irradiated in glass Petri dishes (90 mm in diameter). The irradiation dose was controlled by setting specific irradiation time to give 0 (control), 1, 2, 3, 4 and 5 kGy dose.2.3 Chl-a measurement
The concentration of Chl-a was calculated using ODs at 663, 645, 630 and 750 nm with 5 mL of the culture extract in 90% acetone and 10% water solution. The ODs were measured using an UV-Vis spectrophotometer (U-3100, Hitachi). The Chl-a concentration was calculated by the following equation:20| Chl-a (mg L−1) = [11.64(A1 − A4) − 2.16(A2 − A4) + 0.10(A3 − A4)]v/vg | (2) |
2.4 Microcystin analysis method
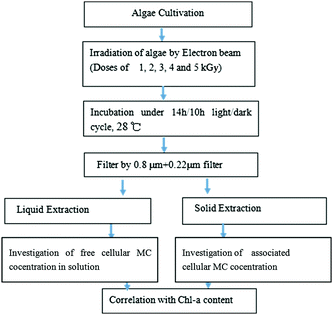
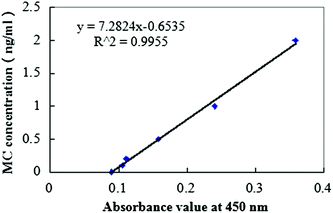
The procedures are summarized in Fig. 2. For each treatment, 25 mL of M. aeruginosa culture was filtered through a 0.8 μm pore size membrane filter. Then, the sediment was frozen and thawed 3 times with a small amount of ultra-pure water. The sample was centrifuged for 10 min at 7000 rpm and 4 °C, and the supernatant was filtered through a 0.22 μm filter membrane, then the filtrate was subjected to an ELISA kit for the determination of the concentration of MC.The ELISA kit (from Chinese Academy of Sciences) was used to determine the concentration of MC. The procedure is described as follows: first, 50 μL of the standard solution and 50 μL of the antibody solution were added into the wells, and then the wells were covered with a parafilm or tape and the contents were mixed for 30 seconds. Subsequently, the strips were incubated for 90 minutes. Then, 100 μL of the enzyme conjugate solution was added and the contents were mixed for 30 seconds. Furthermore, 100 μL of substrate (color) solution was added and mixed for 30 seconds. Finally, 50 μL of stop solution was added to the wells in the same sequence as for the substrate (color) solution. The absorbance at 450 nm was obtained using a microplate ELISA photometer (TU-1901, Puxi Company) within 15 minutes after the addition of the stopping solution. All the measurements were carried out in triplicate and the data are expressed in the form of mean ± standard deviation. The calibration curve of MC concentration is shown in Fig. 3.
3. Results and discussion
3.1 Changes in the concentration of associated cellular microcystin
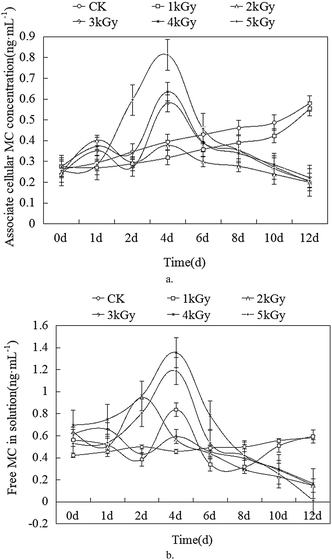
M. aeruginosa culture can produce MC in the cell during the growth process. In order to study the effect of the different doses of EBI on controlling the production of MC, we first measured the intercellular MC concentration using ELISA. As shown in Fig. 4a, MC concentration in the Ctrl and 1 kGy treated M. aeruginosa cells increased steadily during the 12 days growth period. MC concentration in 1 kGy treated sample was lower than that in the ctrl, which indicates that EBI can inhibit MC production and M. aeruginosa cell growth rate. Furthermore, the MC concentrations after EBI were 60%, which is considerably lower than the control experiment. The fluctuation of intercellular MC concentration was observed under 2–5 kGy EBI irradiation. This is likely because the algae cell number and philosophy activity decreasing further gradually interrupt the MC concentration, including the fluctuation of intercellular MC concentration. The further treatment of M. aeruginosa cell by EBI resulted in the significant decrease in intercellular MC concentration during the following days. Our results indicate that an appropriate dose of EBI can effectively inhibit the intercellular MC production in the M. aeruginosa cells.3.2 Changes of free microcystin concentration in the solution
The M. aeruginosa cells under EBI irradiation may lead to release of MC into the solutions. The free MC contents in the solution were also investigated to evaluate the effect of EBI treatment on MC production. Fig. 4b illustrates the free MC concentration in solution as a function of EBI exposure days. MC concentration in 1 kGy group was similar with the Ctrl group after 12 days of EBI irradiation. 2–4 kGy EBI exposure led to a similar decrease in free MC concentration in the solution with the removal percentage of 76% ± 1%. A high dose of EBI can produce more reactive oxygen species (ROS), such as hydroxyl radical (˙OH), in the solution.21 As a result, 5 kGy EBI exposure can significantly remove free MC up to 97%. Our results suggest that the ROS produced in a high dose of EBI irradiation are critical for the removal and degradation of free MC concentration in the M. aeruginosa solution.3.3 Changes in total MC concentration
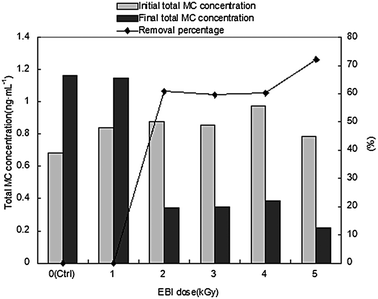
The total MC concentrations were measured to fully evaluate the effect of EBI irradiation on the control of MC production. As shown in Fig. 5, MC concentration increased by 69.8% and 37.2% under Ctrl and 1 kGy EBI irradiation, respectively, after 12 days of growth. MC concentration significantly decreased under 2–5 kGy EBI exposure with the removal percentages of 60.8%, 59.6%, 60.2% and 72.1% in 2, 3, 4 and 5 kGy groups, respectively. This also demonstrates that 5 kGy EBI irradiation can exhibit the best performance for the removal of total MC concentration under our experimental conditions (pH is about 7, room temperature is 24 °C and atmospheric pressure) (Fig. 6).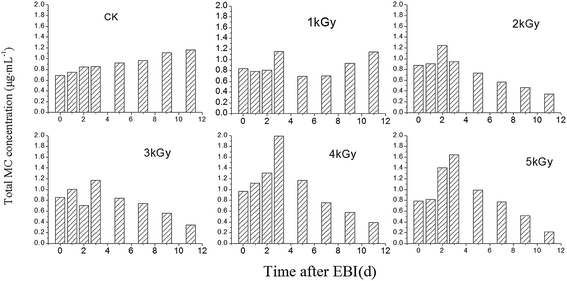 | ||
| Fig. 5 Total MC concentration changing of different treatment (* – a* means the culture time after irradiation). | ||
3.4 Correlation between MC concentration and algae cell growth
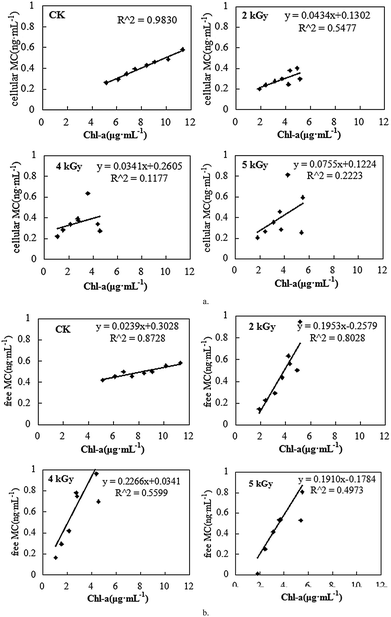
Fig. 7(a) and (b) show MC concentration as a function of Chl-a during the algae growth after irradiation. We calculated an important coefficient for MC production capability and cell growth under different dose of EBI, as shown in Table 1. In the Ctrl group, the good correlation (Ra2 = 0.983) between associated MC concentration and Chl-a concentration indicates that cellular MC production increased with algae cell growth. Rb2 showed that MC in the solution increased with the algae growth, indicating that MC was produced and released during the growing process. When the algae were irradiated by EBI irradiation, both the slopes decreased, indicating that irradiation inhibited algae cell growth and MC production. As a result, MC concentration released into solution decreased simultaneously. The slope (in Fig. 7b) between free MC concentration in solution and Chl-a concentration decreased with the increase in EBI dose, which shows that the free MC in the solution did not keep on increasing with the cell growth.| Parameters | Correlation between associate cellular MC concentration and Chl-a content | Correlation between free MC concentration in solution and Chl-a content | ||
|---|---|---|---|---|
| Slopes | Ra2 | Slopes | Rb2 | |
| Ctrl | Yc = 0.0499x + 0.0053 | 0.983 | Yc = 0.0239x + 0.3028 | 0.8728 |
| 2 kGy | Y2 = 0.0434x + 0.1032 | 0.5477 | Y′2 = 0.1953x − 0.2579 | 0.8028 |
| 4 kGy | Y4 = 0.0341x + 0.2605 | 0.2223 | Y′4 = 0.2266x + 0.0341 | 0.5599 |
| 5 kGy | Y5 = 0.0755x + 0.1224 | 0.1177 | Y′5 = 0.01910x − 0.1784 | 0.4973 |
The cell substance of algae, including protein, carbohydrate and lipid, often have two functions; one is to combine the nutrient for growth, and the other is to resist circumstance intimidation. When electron beam irradiated the solution, it produced a type of extreme condition for M. aeruginosa. As a result, parts of algae cells would die and the cells that survived would produce more carbohydrate or protein to resist environment change, which also contributed to the decrease in MC production. Moreover, the corresponding MC released into the solution decreased as well. Upon EBI treatment, a variety of ROS are produced, which can result in cell damage and ultimately death. Cells that are not killed can recover and counter produce MC, whereas those cells that die release MC into the solution.
4. Conclusions
We systematically investigated the effect of different doses (1–5 kGy) of EBI on the production, release and degradation of cellular MC. The changes in the cellular MC and the free MC content in the solution changing process were tested individually. We found that 1 kGy EBI could control MC production and accumulation. 2–5 kGy EBI could inhibit the MC production in M. aeruginosa cells and also be considered as a good range for the removal of MC from contaminated water bodies. An enhancement in both the cellular MC and solution MC removal was observed with the increase in EBI dose. EBI destroys the correlation between intercellular and exocellular Chl-a and MC concentrations. The results demonstrate that the MC production and release are reduced following EBI and the MC concentration in the solution can be reduced as a function of radiation doses though various algae growth stages. These results can provide an understanding of the removal and degradation of cellular MC and free MC in solution under the EBI, which can improve the viability of EBI technologies for the remediation of contaminated water with microcystin based cyanobacteria and their associated toxins. Ongoing studies are underway to further develop the fundamental understanding and better assess EBI as a potential water treatment for cyanotoxins.Abbreviations
| EBI | Electron beam irradiation |
| MC | Microcystin |
| HAB | Harmful cyanobacterial blooms |
| Microcystis aeruginosa | M. aeruginosa |
| Ctrl | Control |
| Chl-a | Chlorophyll |
| OD | Optical density |
Acknowledgements
This study was supported by the Open Project of State Key Laboratory of Urban Water Resource and Environment (no. HC201323) and the National Natural Science Foundation of China (no.50809037, 41430644, 41273126), the special S&T project on the treatment and control of water pollution (no. 2012ZX07201-003), the Program for Changjiang Scholars and Innovative Research Team in University (no. IRT13078), and the Science and Technology Commission of Shanghai Municipality (13230500600). The authors extend their sincere thanks to all the people in the Key Laboratory of Habin Institute of Technology.References
- G. Zanchett and E. C. Oliveira-Filho, Cyanobacteria and cyanotoxins: from impacts on aquatic ecosystems and human health to anticarcinogenic effects, Toxins, 2013, 5, 1896–1917 CrossRef PubMed.
- A. A. de la Cruz, A. Hiskia, T. Kaloudis, N. Chernoff, D. Hill, M. G. Antoniou, X. He, K. Loftin, K. O'Shea, C. Zhao, M. Pelaez, C. Han, T. J. Lynch and D. D. Dionysiou, A review on cylindrospermopsin: the global occurrence, detection, toxicity and degradation of a potent cyanotoxin, Environ. Sci.: Processes Impacts, 2013, 15, 1979–2003 CAS.
- I. Majsterek, P. Sicinska, M. Tarczynska, M. Zalewski and Z. Walter, Toxicity of microcystin from cyanobacteria growing in a source of drinking water, Comp. Biochem. Physiol., Part C: Toxicol. Pharmacol., 2004, 139, 175–179 CrossRef PubMed.
- L. Voloshko, J. Kopecky, T. Safronova, A. Pljusch, N. Titova, P. Hrouzek and V. Drabkova, Toxins and other bioactive compounds produced by cyanobacteria in Lake Ladoga, Estonian Journal of Ecology, 2008, 57, 100–110 CrossRef.
- J. Mankiewicz, M. Tarczynska, Z. Walter and M. Zalewski, Natural toxins from cyanobacteria, Acta Biol. Cracov., Ser. Bot., 2003, 45, 9–20 Search PubMed.
- R. Nishiwaki-Matsushima, T. Ohta, S. Nishiwaki, M. Suganuma, K. Kohyama, T. Ishikawa, W. W. Carmichael and H. Fujiki, Liver tumor promotion by the cyanobacterial cyclic peptide toxin microcystin-LR, J. Cancer Res. Clin. Oncol., 1992, 118, 420–424 CrossRef CAS.
- B. Zegura, B. Sedmak and M. Filipic, Microcystin-LR induces oxidative DNA damage in human hepatoma cell line HepG2, Toxicon, 2002, 41, 41–48 CrossRef.
- A. M. de Freitas, C. Sirtori, C. A. Lenz and P. G. P. Zamora, Microcystin-LR degradation by solar photo-Fenton, UV-A/photo-Fenton and UV-C/H 2 O 2: a comparative study, Photochem. Photobiol. Sci., 2013, 12, 696–702 CAS.
- L. Li, C. Shao, T.-F. Lin, J. Shen, S. Yu, R. Shang, D. Yin, K. Zhang and N. Gao, Kinetics of Cell Inactivation, Toxin Release, and Degradation during Permanganation of Microcystis aeruginosa, Environ. Sci. Technol., 2014, 48, 2885–2892 CrossRef CAS PubMed.
- J. Andersen, C. Han, K. O'Shea and D. D. Dionysiou, Revealing the degradation intermediates and pathways of visible light-induced NF-TiO2 photocatalysis of microcystin-LR, Appl. Catal., B, 2014, 154–155, 259–266 CrossRef CAS PubMed.
- C. Zhao, M. Pelaez, D. D. Dionysiou, S. C. Pillai, J. A. Byrne and K. E. O'Shea, UV and visible light activated TiO2 photocatalysis of 6-hydroxymethyluracil, a model compound for the potent cyanotoxin cylindrospermopsin, Catal. Today, 2014, 224, 70–76 CrossRef CAS PubMed.
- R. I. Daly, L. Ho and J. D. Brookes, Effect of Chlorination on Microcystis aeruginosa Cell Integrity and Subsequent Microcystin Release and Degradation, Environ. Sci. Technol., 2007, 41, 4447–4453 CrossRef CAS.
- E. Rodriguez, G. D. Onstad, T. P. J. Kull, J. S. Metcalf, J. L. Acero and G. U. von, Oxidative elimination of cyanotoxins: Comparison of ozone, chlorine, chlorine dioxide and permanganate, Water Res., 2007, 41, 3381–3393 CrossRef CAS PubMed.
- V. K. Sharma, T. M. Triantis, M. G. Antoniou, X. He, M. Pelaez, C. Han, W. Song, K. E. O'Shea, A. A. de la Cruz, T. Kaloudis, A. Hiskia and D. D. Dionysiou, Destruction of microcystins by conventional and advanced oxidation processes: A review, Sep. Purif. Technol., 2012, 91, 3–17 CrossRef CAS PubMed.
- C. Zhao, L. E. Arroyo-Mora, A. P. DeCaprio, V. K. Sharma, D. D. Dionysiou and K. E. O'Shea, Reductive and oxidative degradation of iopamidol, iodinated X-ray contrast media, by Fe(III)-oxalate under UV and visible light treatment, Water Res., 2014, 67, 144–153 CrossRef CAS PubMed.
- C. Guignot, N. Betz, B. Legendre, A. Le Moel and N. Yagoubi, Degradation of segmented poly(ether urethane) Tecoflex induced by electron beam irradiation: Characterization and evaluation, Nucl. Instrum. Methods Phys. Res., Sect. B, 2001, 185, 100–107 CrossRef CAS.
- T. Tobien, W. J. Cooper, M. G. Nickelsen, E. Pernas, K. E. O'Shea and K.-D. Asmus, Odor Control in Wastewater Treatment: The Removal of Thioanisole from Water-A Model Case Study by Pulse Radiolysis and Electron Beam Treatment, Environ. Sci. Technol., 2000, 34, 1286–1291 CrossRef CAS.
- G. Mark, H.-P. Schuchmann, M. N. Schuchmann, L. Prager and C. von Sonntag, Electron-Beam Treatment of Aromatic Hydrocarbons that can be Air-Stripped from Contaminated Groundwater. 1. Model Studies in Aqueous Solution, Environ. Sci. Technol., 2003, 37, 372–378 CrossRef CAS.
- K. Hirota, T. Hakoda, M. Taguchi, M. Takigami, H. Kim and T. Kojima, Application of Electron Beam for the Reduction of PCDD/F Emission from Municipal Solid Waste Incinerators, Environ. Sci. Technol., 2003, 37, 3164–3170 CrossRef CAS.
- X.-L. Jin, Q. Xia, X.-Y. Wang, J.-J. Yue and D.-B. Wei, Inactivation of Microcystis aeruginosa with Contact Glow Discharge Electrolysis, Plasma Chem. Plasma Process., 2011, 31, 697–705 CrossRef CAS.
- S. Liu, Y. Zhao, W. Jiang, M. Wu and F. Ma, Inactivation of Microcystis aeruginosa by Electron Beam Irradiation, Water, Air, Soil Pollut., 2014, 225, 1–6 CAS.
| This journal is © The Royal Society of Chemistry 2015 |