Facile modification of ZIF-8 mixed matrix membrane for CO2/CH4 separation: synthesis and preparation†
Nik Abdul Hadi Md Nordina,
Surya Murali Rachaa,
Takeshi Matsuurab,
Nurasyikin Misdanac,
Nur Aimie Abdullah Sania,
Ahmad Fauzi Ismail*a and
Azeman Mustafaa
aAdvanced Membrane Technology Research Centre (AMTEC), Universiti Teknologi Malaysia, 81310 Skudai, Johor, Malaysia. E-mail: afauzi@utm.my; fauzi.ismail@gmail.com; Fax: +60 75581463; Tel: +60 75535592
bIndustrial Membrane Research Laboratory, Department of Chemical and Biological Engineering, University of Ottawa, Ottawa, Ontario, Canada
cDepartment of Mechanical Engineering Technology, Faculty of Engineering Technology, Universiti Tun Hussein Onn Malaysia (UTHM), 86400 Parit Raja, Johor, Malaysia
First published on 15th April 2015
Abstract
Metal–organic frameworks (MOFs) possess tunable characteristics that allow various modifications to be practiced and suitability for specific applications. In this study, zeolitic imidazole framework 8 (ZIF-8) was synthesized and subjected to ammonia modification under different temperatures and ammonia solution loading. The presence of the N–H group observed after the modification indicates the successful modification of ZIF-8. The modified ZIF-8 showed notable changes with increased phase crystallinity, micropore volume and BET surface area. The unmodified and modified ZIF-8s were then dispersed into a polysulfone (PSf) matrix, and the MMMs were prepared via dry/wet phase inversion. No apparent differences in the membrane’s morphology and thermal stability were noticed between the neat PSf membrane and the MMMs. The MMMs were further subjected to pure CO2 and CH4 gas permeation experiments. CO2 permeance decreased while CO2/CH4 selectivity increased as a result of ZIF-8 modification, due to the decrease in mesopore contribution and the increase in micropore contribution to the gas permeation path. The affinity of the N–H group in the modified ZIF-8 to CO2 also contributed to the increase of CO2 permeance. For example, when the ZIF-8 modified in 25 mL ammonia solution at 60 °C (Z25c) was dispersed in the PSf matrix, the CO2/CH4 selectivity increased to 72% and CO2 permeability to 43% compared to the neat PSf membrane.
1. Introduction
The depletion of natural gas resources as well as their worsening quality are great concern nowadays. It was reported that natural gas usually consists of up to 80% impurities, depending on geographical factors.1 Among the impurities in natural gas, CO2 is considered as the most crucial species that needs to be intensively removed. Aside from environmental issues such as being the main culprit of climate change, the presence of CO2 in the process streams would decrease the heating value, pipeline corrosion, and consequently lead to an increase in operational and maintenance costs. Challenges arise to ensure that the presence of CO2 in pipelines is below 0.2% based on U.S. pipeline specifications before the product is delivered to customers. Hence, an efficient CO2 removal system is necessary. The well-developed processes for CO2 removal such as amine absorption, pressure swing adsorption and cryogenic distillation have been implemented over the years and have demonstrated to be reliable in large scale operation and adaptable for many industries.2,3 However, limitations of the conventional processes such as high solvent losses and degradation, unit corrosion, high maintenance, high energy requirement, and refrigerant toxicity4,5 have driven the search for more economical and efficient gas separation systems.Over the years, attention has been focused on polymeric membranes for natural gas separation. Polymeric membranes are advantageous in terms of versatility in a wide-range of CO2 concentrations, low operating cost, modest energy requirement, and space efficiency compared to the conventional processes.6 In addition, membrane separation as a pressure-driven process would be practical for high pressure systems in natural gas processing and requires no additional compression units to transport the retentate stream.5 However, polymeric membranes are bound by the Robeson trade-off limits between permeability and selectivity, which has hindered its potential application in gas separation.7 Although new classes of polymer such as Thermally Rearrange (TR) polymers and polymers of intrinsic microporosity (PIM) have shown to be promising candidates for gas separation,8 their development is still under investigation and unlikely to be commercially available for large scale membrane production in the next few years.
Limitations suffered by polymeric membranes have led to research for inorganic membranes as alternatives. Inorganic membranes such as zeolites and carbon membranes offer several advantages over conventional polymeric membranes in terms of mechanical strength, thermal stability and resistance towards a wide range of chemicals. Particularly, inorganic membranes provide better gas permeability and gas pair selectivity, exceeding Robeson’s upperbound suffered by polymeric membranes. Despite their prevalence, fabricating continuous defect free inorganic membranes is a challenge due to their brittle structure. As well, requirement of extensive fabrication energy is one of the obstacles that hinder their diverse applications. Hence, recent membrane development has been focused on mixed matrix membranes (MMMs), a combination of a polymeric membrane as the continuous phase with an inorganic particle as the disperse phase. Incorporating filler into the polymer matrix can either improve permeability while maintaining gas pair selectivity9 or improve gas pair selectivity without the expense of permeability10 or improve both permeability and selectivity.11 Besides, the two phases compensate for each phase’s limitations and improved processibility, mechanical strength, thermal and chemical stability also motivate further research on MMMs.12 Previous studies have shown promising results by utilizing zeolites,10,13 carbon nanotubes (CNTs),14 carbon molecular sieves (CMSs),15 activated carbon16 and metal- organic frameworks (MOFs)17 as the disperse phase.
Development of defect-free MMMs remains a challenging task as polymer–filler compatibility is often insufficient to form homogeneous interfaces.18 The polymer–filler incompatibility would provoke filler to repulse the continuous phase and form a “sieve-in-cage” morphology, which is responsible for unselective voids. The unselective voids are more preferable for penetrant to permeate across the membrane since it provides negligible mass transport resistance without discriminating penetrants. Hence, selection of the dispersed phase is a crucial factor to develop defect-free MMMs. Compared to other classes of fillers, MOFs have shown to have good interactions with the polymer matrix through its organic ligands. MOFs are crystalline compounds that consist of metal ions and secondary building unit (SBU) or organic ligands. Besides, large surface area, high micropore volume, various pore sizes, crystallinity and a high metal content has let MOFs emerge as spectacular porous materials for diverse applications.19 Zeolite Imidazole Framework-8 (ZIF-8), a product between Zn2+ with 2-methylimidazole (2-MeIM), is one of the most investigated MOFs. ZIF-8 properties are widely studied to show very good chemical stability against polar and nonpolar solvents,20 reorientation of its structure at high pressure21 and high mechanical strength.22
Nonetheless, MOF membranes suffer from low intrinsic CO2/CH4 selectivity and therefore they have been mainly studied as adsorption media.23–25 In the case of ZIF-8, it offers intrinsic CO2/CH4 selectivity <5, which is significantly lower than other inorganic membranes such as zeolite T26 and carbon membranes.27 Hence, modifications are necessary to further improve its affinity towards CO2 before implementing ZIF-8 as a membrane filler for CO2/CH4 separation. MOFs are known for their tunable properties and ease of modification. Modifications of MOFs are practiced to alter their pore structure, pore aperture, surface functional groups, and chemical stability, depending on specific applications.28–31 Notably, post-synthetic modifications have been demonstrated as a straightforward modification approach with significant improvement of MOF properties. For example, metal ion doping into the MOF to provide open metal sites has significantly improved the gas adsorption capacity of the MOF. Metal ions such as potassium, sodium and lithium are used to provide additional interactions with the quadrupole moments of gases while enhancing molecular sieving through the restricted pore size of a modified MOF.24,32 On the other hand, modifying the MOF through ligands also proved to be a convincing approach. Introducing functional groups such as pyridine and amine to the MOF structure would increase its affinity towards quadrupole moment gases such as CO2 and H2.23,33 For example, Zhang et al.34 demonstrated that ammonia impregnated ZIF-8 provides additional basic sites within the pores which as a result increased CO2 uptake up to 50% as compared to virgin ZIF-8 without changes in the crystal integrity. In short, enhancing the tunability of MOFs would significantly improve their properties and their potential as fillers for MMMs and would give distinctive factors for gas separations.
Although ammonia-based modifications of ZIF-8 have been demonstrated to be an attractive approach to increase its affinity towards CO2, the implementation of the modified particle as the dispersed phase in a MMM is yet to be investigated. This issue is crucial to be investigated since modifications may result in closed pores, which would significantly deteriorate the MMM performance. Herein, we are to report the preparation and characterization of an improved CO2 selective MMM using modified ZIF-8 for CO2/CH4 separation. The ammonia modified ZIF-8 particles were prepared under different modification protocols and their properties were stringently studied before being dispersed into the polymer matrix. Polysulfone (PSf) was selected as the polymeric phase since it is an abundant and cheap material while providing well balanced between CO2 permeability and CO2/CH4 selectivity. Asymmetric MMMs were fabricated using a dry/wet phase inversion method by incorporation of modified ZIF-8s under different modification protocols. The effect of the ZIF-8 embodiment on the overall membrane properties and the CO2/CH4 separation is evaluated.
2. Experimental
2.1 Materials
Zinc nitrate hexahydrate (Zn(NO3)2·6H2O) was purchased from Alfa Aesar. Chemicals such as 2-methylimidazole (2-MeIM), n-hexane, polydimethylsiloxane (PDMS) and triethylamine (TEA) were obtained from Sigma Aldrich. Polysulfone (PSf Udel® P-1700) with a density of 1.24 g cm−3 was procured from Solvay Plastic. Ammonium hydroxide solution (25%), N,N-methylpyrrolidone (NMP), tetrahydrofuran (THF) and methanol were purchased from Merck. All chemicals were used without further purification.2.2 ZIF-8 synthesis
ZIF-8 was prepared by following the same procedure as described in our previous work35 with the ratio of Zn(NO3)2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2-MeIM
2-MeIM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O 1
H2O 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500. Briefly, metal salt solution was prepared by dissolving 2 g of Zn(NO3)2·6H2O (6.72 mmol) in 12.11 g of deionized water (20% of total deionized water). For the ligand solution, 2-MeIM (3.312 g, 40.34 mmol) was dissolved into 48.45 g of deionized water and subsequently 3.0 mL of TEA was added. The metal salt solution was gently added to the ligand solution, resulting in a cloudy solution mixture. The solution was stirred vigorously for 30 minutes, followed by centrifugation of the reaction product. The obtained product was washed several times with deionized water to remove excess reactants and subsequently dried in an oven at 60 °C for 12 hours. The collected powder was ground into fine particles before further drying in an oven at 100 °C for a minimum of 12 hours to further evacuate the guest molecules in the ZIF-8 pores.
500. Briefly, metal salt solution was prepared by dissolving 2 g of Zn(NO3)2·6H2O (6.72 mmol) in 12.11 g of deionized water (20% of total deionized water). For the ligand solution, 2-MeIM (3.312 g, 40.34 mmol) was dissolved into 48.45 g of deionized water and subsequently 3.0 mL of TEA was added. The metal salt solution was gently added to the ligand solution, resulting in a cloudy solution mixture. The solution was stirred vigorously for 30 minutes, followed by centrifugation of the reaction product. The obtained product was washed several times with deionized water to remove excess reactants and subsequently dried in an oven at 60 °C for 12 hours. The collected powder was ground into fine particles before further drying in an oven at 100 °C for a minimum of 12 hours to further evacuate the guest molecules in the ZIF-8 pores.
2.3 ZIF-8 modification parameters
The synthesized ZIF-8s were indigenously modified as follows. Firstly, 1.0 g of the prepared ZIF-8 was uniformly dispersed into a predetermined volume of ammonium hydroxide solution (Table 1) with an additional 10 mL of deionized water. The suspension was then sonicated for 60 minutes to break the particle bulks before being vigorously stirred for 24 h at the specific temperatures. The sample was collected via centrifugation and washed with deionized water (3 times) before being dried in an oven (60 °C) overnight. The ZIF-8 modification parameters are listed in Table 1.| ZIF-8 samples | Ammonia solution (mL) | Temperature | MMM |
|---|---|---|---|
| Z-0 | — | — | M0 |
| Z25a | 25 | Ice bath (4 °C) | M25a |
| Z25b | 25 | Room temperature | M25b |
| Z25c | 25 | 60 °C | M25c |
| Z50a | 50 | Ice bath (4 °C) | M50a |
| Z50b | 50 | Room temperature | M50b |
| Z50c | 50 | 60 °C | M50c |
2.4 Asymmetric flat sheet membrane preparation
An asymmetric flat sheet MMM was prepared from the solution that consisted of PSf (25 wt%), NMP (60 wt%), THF (15 wt%) and ZIF-8 (0.5 wt% of the total solids unless otherwise stated). ZIF-8 was dispersed into NMP/THF solvent and approximately 10% of the polymer was added under stirring for the purpose of priming. The remaining polymer was gradually added and the mixture was kept stirring until the solution became homogeneous. The casting dope was then hand-cast using a casting bar to a thickness of 120–190 μm. After standing on the glass plate for approximately 3 min, the cast film together with the glass plate were immersed in a coagulation bath (water at 27 °C) where the membrane was solidified. Then, the membrane was transferred to fresh water and kept there for 1 day to remove the residual solvent completely. Finally, the membrane was solvent-exchanged by immersing it progressively in methanol and n-hexane, each for 2 h, before being air-dried for 24 hours in the ambient atmosphere. The pristine PSf membrane was prepared by the same process without adding ZIF-8. The surface of the membrane was then brought into contact with 3 wt% PDMS/n-hexane solution for 10 min to seal possible pinholes before it was “cured” at 60 °C for 12 hours. Sample abbreviations are listed in Table 1.2.5 Characterization
Attenuated total reflectance infrared spectroscopy (ATR-IR) analysis was conducted using a Perkin Elmer UATR (Single Reflection Diamond) for Spectrum Two to observe the functional groups of the modified ZIF-8s.A differential scanning calorimeter (DSC) was used to determine the glass transition temperature (Tg) of the prepared membranes after incorporating the filler into polymer matrix using a Mettler Toledo DSC 822e. The membrane sample was cut into small pieces, weighed and placed into a pre-weighed aluminium crucible. Then, the sample was heated from 50 to 400 °C at a heating rate of 10 °C min−1 in the first cycle to remove the thermal history. The sample was cooled from 400 to 30 °C at a rate of 10 °C min−1. The same heating protocol was repeated in the next heating cycle. Tg of the sample was determined as the midpoint temperature of the transition region in the second heating cycle.
Thermogravimetric analysis (TGA) was used to characterize the thermal stability of the prepared samples. TGA records the weight changes of the sample when heated continuously. The sample was heated from 50 to 900 °C at a heating rate of 10 °C min−1 under a nitrogen atmosphere with a nitrogen flow rate of 20 mL min−1.
XRD analysis was used to confirm that the phase of the ZIF-8 was similar to that reported in the literature and to monitor the changes in ZIF-8 crystallinity after modification. X-Ray Diffraction (XRD) analysis using a Siemens D5000 Diffractometer is non-destructive analysis to identify the structure of the sample by measuring 2θ angles. The XRD will emit X-rays towards the sample and the X-rays are diffracted at different angles and intensities by CuKα radiation with a wavelength (λ) = 1.54 Å at room temperature.
The specific surface area and pore volume of the virgin ZIF-8 and modified ZIF-8 crystals were measured using a Micromeritics gas adsorption analyzer ASAP2010 instrument equipped with commercial software for calculation and analysis. The BET surface area was calculated from the adsorption isotherms using the standard Brunauer–Emmett–Teller (BET) equation. The total pore volume was evaluated by converting the adsorption amount at p/p0 = 0.95 to a volume of liquid adsorbate. The mesopore volume was obtained using a BJH plot while the micropore volume was obtained using the t-plot method of Lippens and de Boer with the adsorption data.
Scanning electron microscopy (SEM) was used to observe the membrane structure and morphology. Membrane samples were fractured cryogenically in liquid nitrogen. The samples were coated with gold before they were imaged and photographed by employing a scanning electron microscope (TM3000, Hitachi) with a potential of 10 kV under magnifications ranging from 1000 to 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000.
000.
A transmission electron microscope (TEM) (JEOL, JSM-6701FJEOL 1230) was applied to observe the macrostructures of the ZIF-8. Samples were prepared by dispersing ZIF-8 powder in methanol. A drop of methanol was used for the dispersion of ZIF-8 on carbon-coated copper grids operating at 300 kV.
Gas permeation tests were performed using a constant pressure–variable volume system described elsewhere.36 The membranes were placed into the permeation cell with an effective permeation area of 12.5 cm2 and exposed to pure CH4 or CO2 gas. The feed pressure was 4 bar gauge and the temperature was 27 °C. The pressure-normalized flux (permeance) of gas i was calculated as follows:
 | (1) |
| 1 GPU = 1 × 10−6 cm3 (STP) cm−2 s−1 cmHg−1. |
The selectivity was obtained using eqn (2):
 | (2) |
3. Results and discussion
3.1 Characterization of virgin and modified ZIF-8
The crystallinity of the prepared ZIF-8 was identified via XRD (Fig. 1a). The presence of strong peaks implies a high crystallinity of the prepared ZIF-8 showing good agreement with previous experimental work37 and the simulated pattern;20 where the peaks at 2θ = 7.30, 10.35, 12.70, 14.80, 16.40 and 18.00° correspond to planes (110), (200), (211), (220), (310), and (222), respectively. Fig. 1b represents the TEM image of the ZIF-8. The prepared ZIF-8 possesses rhombic dodecahedron morphology; showing good agreement with the literature.37 The particle size of the prepared samples was estimated from a series of TEM images to be around 133 nm. | ||
| Fig. 1 (a) XRD pattern of the virgin ZIF-8 as compared with the literature37 and the simulated pattern,20 and (b) morphology of the prepared ZIF-8. | ||
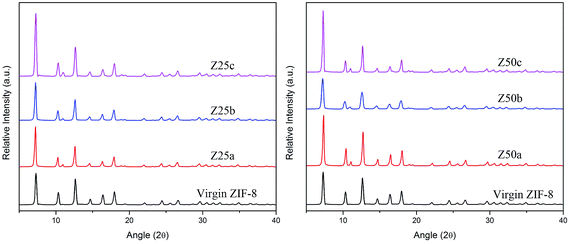
The XRD patterns of ZIF-8s after modification are presented in Fig. 2. The pattern remains unchanged after modification, indicating a strong resistance towards alkaline solution regardless of modification protocols.20,34 In the previous report on amine modification it was demonstrated that the XRD peak intensity decreased after modification presumably due to impregnation of the amine within the ZIF-8 pores causing destructive interference with the XRD peaks.38 However, TGA analysis (see Fig. S1, ESI†) reveals that no weight loss occurred below 150 °C for the virgin and modified ZIF-8, which indicates ammonium hydroxide solution was not impregnated within the pores. Moreover, the XRD peaks were intensified after modification in this study, which further implies that ammonium hydroxide not only was absent within the ZIF-8 pores, but also caused the removal of guest molecules. It was also observed that there was no apparent difference between the peak width of the virgin and modified ZIF-8. This can postulate that the modification does not provide any significant changes in particle size of ZIF-8. A new reflection was also observed at 2θ = 10.96° in all the modified samples presumably due to cage reordering of the ZIF-8 structure.39
N2 adsorption analysis of the prepared ZIF-8s is presented in Table 2. The BET surface area of the prepared pure ZIF-8 is comparable with the literature values.40,41 The increase in BET surface area of the modified ZIF-8 samples, except for Z50b and Z50c, is understandable since the removal of guest molecules increases pore availability as observed by the intensification of XRD peaks after modification. It was also observed that the total pore volume of ZIF-8 increased significantly, again except for Z50b and Z50c, after modification, indicating pore reopening42 and/or formation of new pores due to cage reordering.39
| Sample | BET surface area (m2 g−1) | Mesopore volume (cm3 g−1) | Micropore volume (cm3 g−1) | Total pore volume (cm3 g−1) |
|---|---|---|---|---|
| Z0 | 1031.7 | 0.2427 | 0.2995 | 0.5422 |
| Z25a | 1210.7 | 0.9877 | 0.2576 | 1.2453 |
| Z25b | 1223.4 | 0.3141 | 0.3842 | 0.6983 |
| Z25c | 1250.5 | 0.1951 | 0.3852 | 0.5803 |
| Z50a | 1132.2 | 0.5559 | 0.2964 | 0.8523 |
| Z50b | 1019.5 | 0.2368 | 0.3113 | 0.5481 |
| Z50c | 966.2 | 0.4983 | 0.4805 | 0.9788 |
The types of pore formed/reopened during the modification were highly dependent on the modification temperature. At ice bath temperature, the availability of mesopores increased dramatically while the micropore accessibility was slightly decreased. As modification temperature increased, the decrease in mesopores was significant probably due to pore constriction or the changing of mesopores to micropores (see Fig. S2–S4, ESI†). It was also observed that the amount of ammonia solution used for modification plays an important role in the surface properties of ZIF-8. Introducing 25 mL ammonia solution into ZIF-8 prompted a higher micropore volume whilst constriction of the mesopores was less than with 50 mL ammonia solution. This behavior was presumably caused by the dilution of the ammonium hydroxide solution with 10 mL of deionized water; where a more diluted solution eases the dissolution of guest molecules to evacuate the pores and eases the occurrence of modifications. Hence, the total pore volume increased when a smaller amount of ammonia solution was added, with the exception of Z25c and Z50c due to pore constriction induced at a higher modification temperature.
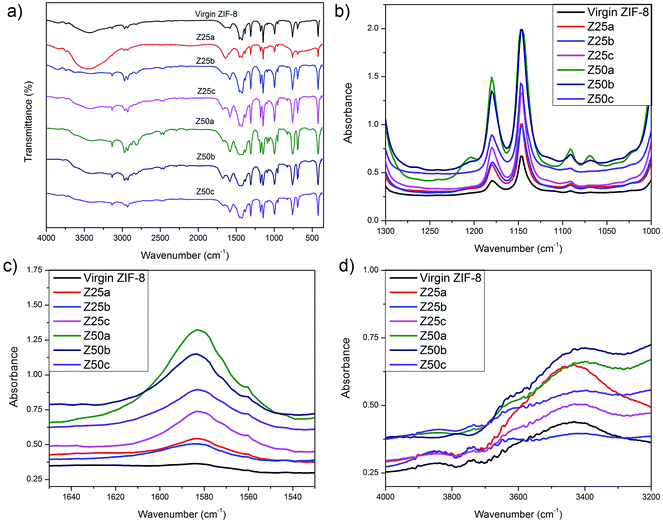
The intensification of the XRD peaks and improved surface properties observed in this work after various modifications contradict with the previous report, where amine would reside within the pores to cause destructive interference on the XRD beam by the blocked pores.34 Hence, further characterizations are necessary to confirm the modification by ammonium hydroxide. Fig. 3a shows the infrared spectrum of the virgin and modified ZIF-8s. Most of the spectra are related to the vibrations of the methylimidazole units and thus can be described based on the origin of the bonds. It was observed that the spectra of the samples are in good agreement with other studies.20,34 The absorption bands between 3135 and 2929 cm−1 can be attributed to the aromatic and the aliphatic C–H stretching of methylimidazole, respectively. The characteristic peak at 1584 cm−1 was due to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching mode, whereas bands between 1350–1500 cm−1 can be assigned to the entire ring stretching.43 The peak at 450 cm−1 shows the distinct stretching vibration of Zn–N.
N stretching mode, whereas bands between 1350–1500 cm−1 can be assigned to the entire ring stretching.43 The peak at 450 cm−1 shows the distinct stretching vibration of Zn–N.
There were no apparent differences after ZIF-8 was subjected to different modification protocols due to the presence of similar functional groups also in the virgin ZIF-8. Hence, the IR absorbance values of some specific functional groups were more thoroughly studied. The modification of the ZIF-8 has led to the intensification of the IR absorbance peaks coming from the C–N stretch (Fig. 3b), N–H bend (Fig. 3c), and N–H stretch (Fig. 3d), thus suggesting that ammonia modifications under various procedures were successful. It was also observed that the peak intensification was more prominent in the Z50-series compared to the Z25-series which was presumably related to the higher ammonium hydroxide content that provided more interactions with the ZIF-8 particles.
The characterization data presented that the ZIF-8 was successfully modified without ammonia solution being impregnated inside its pores. Liu et al.44 simulated that in the ideal crystal structure of amine-modified ZIF-8, the amino group took its place near the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond on the methylimidazole linker (Fig. 4) with a stable structure. The changes of the methylimdazole ligands, which were responsible for a pore opening of 3.4 Å in ZIF-8,45 would significantly influence the textural properties as was observed during the N2 adsorption analysis (see Table 2) and in the XRD patterns (see Fig. 2). It can be postulated that, during modification, the N–H functional group deprotonates the C
C bond on the methylimidazole linker (Fig. 4) with a stable structure. The changes of the methylimdazole ligands, which were responsible for a pore opening of 3.4 Å in ZIF-8,45 would significantly influence the textural properties as was observed during the N2 adsorption analysis (see Table 2) and in the XRD patterns (see Fig. 2). It can be postulated that, during modification, the N–H functional group deprotonates the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C in the imidazole linkers and leads to cage reordering while maintaining its overall structure. However, the simulation demonstrated that the surface area and pore volume should decrease as modification occurs which contradicts with this work. This contradiction is presumably caused by (1) further removal of the guest molecule and pore reopening after modification leading to the increasing surface area and pore volume, overwhelming the pore constriction by the new N–H group, and (2) randomized N–H group attachment (combination of H2N–C
C in the imidazole linkers and leads to cage reordering while maintaining its overall structure. However, the simulation demonstrated that the surface area and pore volume should decrease as modification occurs which contradicts with this work. This contradiction is presumably caused by (1) further removal of the guest molecule and pore reopening after modification leading to the increasing surface area and pore volume, overwhelming the pore constriction by the new N–H group, and (2) randomized N–H group attachment (combination of H2N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–NH2, H–C
C–NH2, H–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–NH2 and/or none in one crystal unit) experienced in this work, whereas the simulation was based on the idealized structure of amine-modified ZIF-8. Nevertheless, this work shows that the amine-group can be introduced through a straightforward approach.
C–NH2 and/or none in one crystal unit) experienced in this work, whereas the simulation was based on the idealized structure of amine-modified ZIF-8. Nevertheless, this work shows that the amine-group can be introduced through a straightforward approach.
 | ||
Fig. 4 Idealized crystal structure of amine-modified ZIF-8 (adapted from Liu et al.44). The NH2–ZIF-8 represents the –NH2 attached on a single side of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, while (NH2)2–ZIF-8 represents the –NH2 group attached on both sides of C C, while (NH2)2–ZIF-8 represents the –NH2 group attached on both sides of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C in the imidazole linkers. C in the imidazole linkers. | ||
3.2 MMM characterization
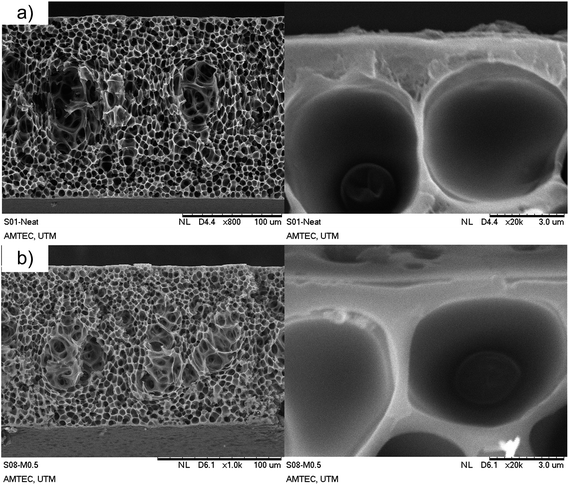
The morphologies of the prepared membranes are presented in Fig. 5. The cross-sectional image of the neat PSf membrane shows an asymmetric structure, with an apparent active layer accompanied by a sponge-like substructure (Fig. 5a). The dry phase inversion was introduced to form a thin-selective layer through evaporation of a volatile solvent (THF). The sponge-like substructure was also observed as a result of the solvent exchange between NMP and water during the wet inversion.A similar cross-sectional morphology was observed for all the prepared MMMs (see Fig. 5b, S5 and S6, ESI†) since small amounts of fillers were incorporated and a similar preparation protocol was used for fabrication. It should be noted that nanoparticles tend to form a bigger cluster due to interactions between their surfaces, especially when a large number of the particles are incorporated. However, no apparent ZIF-8 particles were observed within the membrane cross sectional morphology regardless of the modification procedure. In addition, there were no changes in the membrane functional groups as observed via IR analysis (see Fig. S7, ESI†) after filler (virgin and modified ZIF-8) incorporation. The results suggest that (1) sonication introduced to the dope preparation was able to break the particle cluster; (2) a small amount of ZIF-8 was enough to make significant changes in the membrane morphology and functional group; and (3) uniform distribution of the ZIF-8 particles throughout polymer matrix was possible.
The thermal stability of the prepared membranes was investigated (see Fig. S8, ESI†) and no significant difference between the thermal stability of the pristine PSf and MMMs was observed, which suggested that low ZIF-8 loading did not affect overall thermal stability of the prepared membranes. The Tg values of the prepared membranes are summarized in Table 3. The Tg of the neat PSf membrane is comparable to the value previously reported,46 indicating a high rigidity of the materials. Incorporation of virgin and modified ZIF-8s into the PSf matrix provided notable changes in the membrane’s Tg. The membrane’s Tg decreased after the virgin ZIF-8 was embedded, indicating increased segmental mobility as the particles reside among the polymer chains. Incorporating modified ZIF-8s into the PSf matrix showed a further decrease in the membrane’s Tg. Hence, it can be postulated that better dispersion of the modified ZIF-8s was enabled compared to the virgin ZIF-8.
| Sample | Glass transition temperature (Tg) |
|---|---|
| Neat PSf | 185 |
| M0 | 175 |
| M25a | 165 |
| M25b | 156 |
| M25c | 160 |
| M50a | 151 |
| M50b | 146 |
| M50c | 167 |
3.3 Gas permeation
| Sample | Permeancea (GPU) | Ideal CO2/CH4 selectivityb | |||
|---|---|---|---|---|---|
| CO2 | ±c | CH4 | ±c | ||
| a GPU = 1 × 10−6 cm3 cm−2 s−1 cmHg−1.b Ideal selectivity is based on average of CO2 and CH4 permeance.c ± represents the standard deviation. | |||||
| Neat | 21.27 | 6.35 | 1.08 | 1.33 | 19.83 |
| M0 | 29.19 | 3.68 | 1.26 | 0.05 | 23.16 |
| M25a | 27.05 | 5.99 | 1.18 | 0.18 | 22.66 |
| M25b | 21.21 | 1.26 | 0.73 | 0.01 | 29.04 |
| M25c | 21.16 | 4.84 | 0.62 | 0.228 | 34.09 |
| M50a | 28.19 | 7.87 | 1.26 | 0.35 | 22.61 |
| M50b | 21.74 | 4.94 | 1.02 | 0.39 | 22.72 |
| M50c | 22.41 | 6.77 | 0.77 | 0.17 | 28.66 |
The data are reproduced in Fig. 6 for better interpretation of the effect of the temperature on ZIF-8 modification. The figure shows that, regardless of the modification temperature, all the modified ZIF-8 containing MMMs show CO2 permeance lower than the virgin ZIF-8 containing MMM (M0), despite the enhancement of BET surface area and total pore volume after modification, as observed by the adsorption experiments. This decrease in the membrane permeance is not due to polymer chain rigidification, since the Tg of the MMM decreased when modified ZIF-8 was incorporated. Plausible explanations are (1) CO2 entrapment within the filler due to stronger quadrupole-π electron interactions on the N–H group of the modified ZIF-8 (ref. 34 and 44) which hindered its diffusion; and (2) a decrease in mesopore contribution to the permeation path.
Regarding the M25 series, CO2 permeance decreases progressively from virgin (M0) to ammonium modification at 4 °C (M25a) to 27 °C (M25b) and to 60 °C (M25c). It is recalled that the mesopore volume also decreased progressively from 0.9877 cm3 g−1 of M25a to 0.1951 cm3 g−1 of M25c (see Table 2), while micropore volume increased from 0.2576 cm3 g−1 of M25a to 0.3852 cm3 g−1 of M25c. Therefore, the decrease of CO2 permeance from M25a to M25c is likely due to the decreased contribution of mesopores and increased contribution of micropores to the gas transport channel.
The decrease in CH4 permeance from M25a to M25c seems more severe than the decrease in CO2 permeance, since CO2/CH4 selectivity keeps increasing from M25a to M25c. This is also due to the increase in the micropore contribution from M25a to M25c. Considering the kinetic diameters of CO2 (3.3 Å) and CH4 (3.8 Å), and the size of the micropores that are likely to be originating from the 6-ring window aperture of 3.4 Å,20 it is obvious that CH4 transport through the micropores is prohibited while that for CO2 is allowed. Hence, an increase in the micropore contribution leads to an increase in selectivity. The same trend is observed for the M50 series. However, unlike the M25 series, the selectivity does not change from M50a to M50b but increases significantly from M50b to M50c. This trend parallels the trend in the micropore volume M-50a (0.2964 cm3 g−1) ≈ M50b (0.3113 cm3 g−1) < M50c (0.4805 cm3 g−1). Therefore, suppression of CH4 transport by micropores becomes most effective for M50c.
Hence, the latter two effects, the enhanced interaction between CO2 and CH4 and the hindering effect on modified ZIF-8, have contributed to the significant increase up to 72% in ideal CO2/CH4 selectivity compared to the neat PSf membrane.
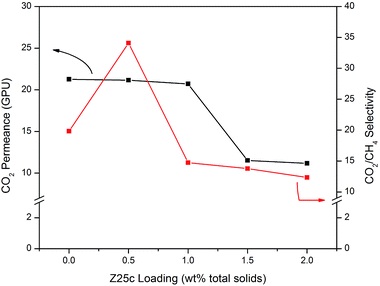
Quite disappointingly, both CO2 permeance and selectivity decreased as Z25c loading was increased from 0.5 wt% to 2.0 wt% as shown in Fig. 7. This is due to the increase in tortuosity of the permeation path in the presence of a large number of filler particles, which increases the mass transport resistance. In addition, severe Z25c agglomeration that might take place at higher loading limits the penetrant’s access to the ZIF-8 pores50 and dwindles its selective features. Consequently, a simultaneous decrease in both CO2 permeance and ideal CO2/CH4 selectivity was observed at higher Z25c loading. Hence, only 0.5 wt% (total solids) of Z25c is necessary to improve the membrane selectivity.
3.4 Comparison with literature
MMMs in which ZIF-8 was incorporated in different polymers have been reported during the past years. Most of the polymer/ZIF-8 MMMs were prepared as dense membranes in earlier studies focusing on the changes in gas solubility and diffusivity with ZIF-8 incorporation and CO2 permeability reported in Barrer. Whereas, asymmetric membranes were prepared in this study since it is more relevant to practical applications and CO2 permeance is reported in GPU. To enable a performance comparison with other ZIF-8 incorporated MMMs, the selective layer thickness of the neat PSf and M25c was evaluated from the FESEM images, as shown in Fig. 8. The selective layer thicknesses for PSf and M25c were estimated to be around 0.238 and 0.341 μm, respectively, and the CO2 permeabilities were calculated to be 5.06 and 7.26 Barrer, respectively.The intrinsic CO2 permeability and CO2/CH4 selectivity of PSf are reported to be 4.4 Barrer and 30.0, respectively.51 Compared to the reported values, the selectivity obtained in this study (19.8) is significantly lower and compensated by higher CO2 permeability (5.06 Barrer). The performances of various polymer/ZIF-8 MMMs reported in the literature are tabulated in Table 5 and compared with M25c of this study. Most notable is the work by Ordoñez et al.50 where 50 wt% (total solids) of ZIF-8 was incorporated in Matrimid®. They achieved CO2/CH4 selectivity of 124.89, which is 187% better than the neat Matrimid® membrane. This level of high ZIF-8 loading is typical of dense MMMs without suffering from defective polymer–filler interface and the dispersed ZIF-8 can be fully utilized to facilitate the separation performance. For this reason, the dense MMMs could surpass the Robeson upper bound limit.
| Polymer | Membrane configuration | Loading (wt% total solids) | % changes in CO2 permeability | % changes in CO2/CH4 selectivity | Reference |
|---|---|---|---|---|---|
| a Volumetric percent. | |||||
| Matrimid | Dense | 50 | −54% | 187% | 50 |
| PPEES | Dense | 10 | 39% | 29% | 52 |
| Matrimid | Dense | 30 | 219% | 15% | 17 |
| Matrimid | Dense | 5 | 25% | 11% | 55 |
| PIM-1 | Dense | 28a | −3% | 31% | 53 |
| PMPS | Dense | 8.3 | 171% | −12% | 54 |
| PSf | Asymmetric | 0.5 | 43% | 72% | This work |
In contrast, the fabrication of an asymmetric membrane does not allow high ZIF-8 loading. In this study, the loading of the modified ZIF-8 was as low as 0.5 wt%, which significantly limited the potential capacity of the ZIF-8 filler.50,52–54 Thus, the MMM fabricated in this study does not stand out among the available data of ZIF-8 MMMs. Nevertheless, the performance of the M25c membrane is considered a remarkable improvement from the neat PSf membrane. The ammonia modification has improved surface area and micropore volume of ZIF-8 with the presence of the N–H functional group. The resulted MMM is more CO2 permeable (7.26 Barrer) and CO2 selective (34.09), than the neat PSf membrane despite its limited filler loading (0.5 wt%). These improvements are in good agreement with simulation44 and experimental34,42 works on CO2 adsorption in amine-modified ZIF-8. Hence, the straightforward modification through ammonium solution on ZIF-8 proposed in this work will motivate research towards the future development of MOF-based MMMs for CO2 removal.
4. Conclusion
ZIF-8s which underwent ammonia modification under various conditions exhibited significant changes in their properties, i.e. phase crystallinity, pore properties and BET surface area increased, while preserving the overall structure. The MMM with modified ZIF-8 has shown a substantial increase in ideal CO2/CH4 selectivity, especially for ZIF-8 modified under 25 mL ammonium hydroxide solution at 60 °C. The formation of new micropores while the disappearance of mesopores have hindered CH4 permeation across the membrane. This, combined with the introduction of the N–H functional group within the ZIF-8 after modification resulted in significant improvements of 43% in CO2 permeability and 72% in ideal CO2/CH4 selectivity with only minimal loading (0.5 wt%). Increasing the filler loading did not necessarily improve the MMM performances in CO2/CH4 separation due to particle agglomeration that restricts penetrant access to the pores. Hence, the ammonia modification proposed in this study has proven to be a straightforward approach to simultaneously increase CO2 permeability and ideal CO2/CH4 selectivity, and would be beneficial for future works focused on gas separation MMM, especially for CO2 separation.Acknowledgements
The authors gratefully acknowledge the Ministry of Education (MOE) for the scholarship and Long-Term Research Grant Scheme (LRGS) Program under Universiti Teknologi Malaysia with the grant number Q.J130000.2452.04H71.References
- T. Cnop, D. Dortmundt and M. Schott, UOP Continued Development of Gas Separation Membranes Tech Paper, 2007 Search PubMed
.
- M. Mofarahi, Y. Khojasteh, H. Khaledi and A. Farahnak, Energy, 2008, 33, 1311–1319 CrossRef CAS PubMed
.
- M. Wang, A. Lawal, P. Stephenson, J. Sidders and C. Ramshaw, Chem. Eng. Res. Des., 2011, 89, 1609–1624 CrossRef CAS PubMed
.
- T. E. Rufford, S. Smart, G. C. Y. Watson, B. F. Graham, J. Boxall, J. C. Diniz da Costa and E. F. May, J. Pet. Sci. Eng., 2012, 94–95, 123–154 CrossRef CAS PubMed
.
- R. W. Baker, in Membrane Technology and Applications, John Wiley & Sons, Ltd, 2012, pp. 325–378 Search PubMed
.
- R. W. Baker and B. T. Low, Macromolecules, 2014, 47, 6999–7013 CrossRef CAS
.
- L. M. Robeson, J. Membr. Sci., 2008, 320, 390–400 CrossRef CAS PubMed
.
- P. M. Budd and N. B. McKeown, Polym. Chem., 2010, 1, 63–68 RSC
.
- S. Kim, L. Chen, J. K. Johnson and E. Marand, J. Membr. Sci., 2007, 294, 147–158 CrossRef CAS PubMed
.
- R. T. Adams, J. S. Lee, T.-H. Bae, J. K. Ward, J. R. Johnson, C. W. Jones, S. Nair and W. J. Koros, J. Membr. Sci., 2011, 367, 197–203 CrossRef CAS PubMed
.
- K. Diíaz, L. Garrido, M. Loópez-Gonzaález, L. F. del Castillo and E. Riande, Macromolecules, 2010, 43, 316–325 CrossRef
.
- Y. Yampolskii, Macromolecules, 2012, 45, 3298–3311 CrossRef CAS
.
- C. Casado-Coterillo, J. Soto, M. T. Jimaré, S. Valencia, A. Corma, C. Téllez and J. Coronas, Chem. Eng. Sci., 2012, 73, 116–122 CrossRef CAS PubMed
.
- A. F. Ismail, N. H. Rahim, A. Mustafa, T. Matsuura, B. C. Ng, S. Abdullah and S. A. Hashemifard, Sep. Purif. Technol., 2011, 80, 20–31 CrossRef CAS PubMed
.
- D. Q. Vu, W. J. Koros and S. J. Miller, J. Membr. Sci., 2003, 221, 233–239 CrossRef CAS
.
- M. Anson, J. Marchese, E. Garis, N. Ochoa and C. Pagliero, J. Membr. Sci., 2004, 243, 19–28 CrossRef CAS PubMed
.
- S. Basu, A. Cano-Odena and I. F. J. Vankelecom, Sep. Purif. Technol., 2011, 81, 31–40 CrossRef CAS PubMed
.
- M. A. Aroon, A. F. Ismail, T. Matsuura and M. M. Montazer-Rahmati, Sep. Purif. Technol., 2010, 75, 229–242 CrossRef CAS PubMed
.
- J. Yao and H. Wang, Chem. Soc. Rev., 2014, 43, 4470–4493 RSC
.
- K. S. Park, Z. Ni, A. P. Cote, J. Y. Choi, R. Huang, F. J. Uribe-Romo, H. K. Chae, M. O’Keeffe and O. M. Yaghi, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 10186–10191 CrossRef CAS PubMed
.
- S. A. Moggach, T. D. Bennett and A. K. Cheetham, Angew. Chem., 2009, 48, 7087–7089 CrossRef CAS PubMed
.
- J. C. Tan, T. D. Bennett and A. K. Cheetham, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 9938–9943 CrossRef CAS PubMed
.
- Y.-S. Bae, O. K. Farha, J. T. Hupp and R. Q. Snurr, J. Mater. Chem., 2009, 19, 2131 RSC
.
- Y.-S. Bae, B. G. Hauser, O. K. Farha, J. T. Hupp and R. Q. Snurr, Microporous Mesoporous Mater., 2011, 141, 231–235 CrossRef CAS PubMed
.
- S. R. Caskey, A. G. Wong-Foy and A. J. Matzger, J. Am. Chem. Soc., 2008, 130, 10870–10871 CrossRef CAS PubMed
.
- Z. Y. Yeo, S.-P. Chai, P. W. Zhu and A. R. Mohamed, Microporous Mesoporous Mater., 2014, 196, 79–88 CrossRef CAS PubMed
.
- W. N. W. Salleh and A. F. Ismail, Sep. Purif. Technol., 2011, 80, 541–548 CrossRef CAS PubMed
.
- J.-R. Li, Y. Ma, M. C. McCarthy, J. Sculley, J. Yu, H.-K. Jeong, P. B. Balbuena and H.-C. Zhou, Coord. Chem. Rev., 2011, 255, 1791–1823 CrossRef CAS PubMed
.
- F.-J. Ma, S.-X. Liu, D.-D. Liang, G.-J. Ren, F. Wei, Y.-G. Chen and Z.-M. Su, J. Solid State Chem., 2011, 184, 3034–3039 CrossRef CAS PubMed
.
- Q.-L. Zhu and Q. Xu, Chem. Soc. Rev., 2014, 43, 5468–5512 RSC
.
- Z. Wang and S. M. Cohen, Chem. Soc. Rev., 2009, 38, 1315–1329 RSC
.
- K. L. Mulfort and J. T. Hupp, Inorg. Chem., 2008, 47, 7936–7938 CrossRef CAS PubMed
.
- S. Choi, T. Watanabe, T.-H. Bae, D. S. Sholl and C. W. Jones, J. Phys. Chem. Lett., 2012, 3, 1136–1141 CrossRef CAS
.
- Z. Zhang, S. Xian, H. Xi, H. Wang and Z. Li, Chem. Eng. Sci., 2011, 66, 4878–4888 CrossRef CAS PubMed
.
- N. A. H. M. Nordin, A. F. Ismail, A. Mustafa, P. S. Goh, D. Rana and T. Matsuura, RSC Adv., 2014, 4, 33292–33300 RSC
.
- A. F. Ismail and P. Y. Lai, Sep. Purif. Technol., 2003, 33, 127–143 CrossRef CAS
.
- A. F. Gross, E. Sherman and J. J. Vajo, Dalton Trans., 2012, 41, 5458 RSC
.
- B. Arstad, H. Fjellvåg, K. Kongshaug, O. Swang and R. Blom, Adsorption, 2008, 14, 755–762 CrossRef CAS
.
- F. Schröder, D. Esken, M. Cokoja, M. W. E. van den Berg, O. I. Lebedev, G. Van Tendeloo, B. Walaszek, G. Buntkowsky, H.-H. Limbach, B. Chaudret and R. A. Fischer, J. Am. Chem. Soc., 2008, 130, 6119–6130 CrossRef PubMed
.
- Y. Pan, Y. Liu, G. Zeng, L. Zhao and Z. Lai, Chem. Commun., 2011, 47, 2071–2073 RSC
.
- J. Cravillon, S. Münzer, S.-J. Lohmeier, A. Feldhoff, K. Huber and M. Wiebcke, Chem. Mater., 2009, 21, 1410–1412 CrossRef CAS
.
- Z. Zhang, S. Xian, Q. Xia, H. Wang, Z. Li and J. Li, AIChE J., 2013, 59, 2195–2206 CrossRef CAS PubMed
.
- Y. Hu, H. Kazemian, S. Rohani, Y. Huang and Y. Song, Chem. Commun., 2011, 47, 12694–12696 RSC
.
- D. Liu, Y. Wu, Q. Xia, Z. Li and H. Xi, Adsorption, 2013, 19, 25–37 CrossRef CAS PubMed
.
- L. Hertäg, H. Bux, J. Caro, C. Chmelik, T. Remsungnen, M. Knauth and S. Fritzsche, J. Membr. Sci., 2011, 377, 36–41 CrossRef PubMed
.
- G. C. Kapantaidakis, S. P. Kaldis, X. S. Dabou and G. P. Sakellaropoulos, J. Membr. Sci., 1996, 110, 239–247 CrossRef CAS
.
- B. Zornoza, A. Martinez-Joaristi, P. Serra-Crespo, C. Tellez, J. Coronas, J. Gascon and F. Kapteijn, Chem. Commun., 2011, 47, 9522–9524 RSC
.
- A. F. Ismail and W. Lorna, Sep. Purif. Technol., 2003, 30, 37–46 CrossRef CAS
.
- R. Banerjee, H. Furukawa, D. Britt, C. Knobler, M. O’Keeffe and O. M. Yaghi, J. Am. Chem. Soc., 2009, 131, 3875–3877 CrossRef CAS PubMed
.
- M. J. C. Ordoñez, K. J. Balkus, J. P. Ferraris and I. H. Musselman, J. Membr. Sci., 2010, 361, 28–37 CrossRef PubMed
.
- M. Mulder, Basic Principles of Membrane Technology, Kluwer Academic Publisher, Netherlands, 2nd edn, 1996 Search PubMed
.
- K. Díaz, M. López-González, L. F. del Castillo and E. Riande, J. Membr. Sci., 2011, 383, 206–213 CrossRef PubMed
.
- A. F. Bushell, M. P. Attfield, C. R. Mason, P. M. Budd, Y. Yampolskii, L. Starannikova, A. Rebrov, F. Bazzarelli, P. Bernardo, J. Carolus Jansen, M. Lanč, K. Friess, V. Shantarovich, V. Gustov and V. Isaeva, J. Membr. Sci., 2013, 427, 48–62 CrossRef CAS PubMed
.
- L. Diestel, X. L. Liu, Y. S. Li, W. S. Yang and J. Caro, Microporous Mesoporous Mater., 2014, 189, 210–215 CrossRef CAS PubMed
.
- Q. Song, S. K. Nataraj, M. V. Roussenova, J. C. Tan, D. J. Hughes, W. Li, P. Bourgoin, M. A. Alam, A. K. Cheetham, S. A. Al-Muhtaseb and E. Sivaniah, Energy Environ. Sci., 2012, 5, 8359–8369 CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra02230d |
| This journal is © The Royal Society of Chemistry 2015 |