Effect of intrinsic and extrinsic factors on the stability of the α-gel phase of a glyceryl monostearate–water system
Abstract
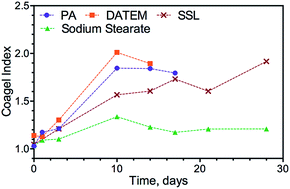
Glyceryl monostearate–water systems (MG-gels) undergo a polymorphic transition from the α-gel phase to the coagel phase. This phase transition results in destabilization and loss of water in monoglyceride-structured systems, commonly found in personal care and food products. In this study, we examined the effect of intrinsic factors (type and concentration of co-emulsifiers) and extrinsic factors (cooling rate and applied shear) on the stability of the α-gel phase. The methods used to study the polymorphic transition were differential scanning calorimetry (DSC) and X-ray diffraction (XRD). The results suggested that the transition from the α-gel phase to the coagel phase caused a change in the gel's physical appearance. More specifically, opaque regions developed in the semi-translucent α-gel upon aging. The stability of the α-gel phase can be increased by using an α-tending co-emulsifier, such as sodium stearyol lactylate (SSL), and by increasing the concentration of the co-emulsifier. Slow cooling rates without shear could also increase the stability of the α-gel phase. In this work, we developed a subα Coagel Index that can be used together with the Coagel Index to characterize the degree of polymorphic transformation of MG-gels.

 Please wait while we load your content...
Please wait while we load your content...