Gas-sensing and electrical properties of perovskite structure p-type barium-substituted bismuth ferrite
Guangzhi Dong,
Huiqing Fan*,
Hailin Tian,
Jiawen Fang and
Qiang Li
State Key Laboratory of Solidification Processing, School of Materials Science and Engineering, Northwestern Polytechnical University, Xi'an 710072, China. E-mail: hqfan3@163.com; Fax: +86-29-88492642; Tel: +86-29-88494463
First published on 20th March 2015
Abstract
Pure and Ba-substituted bismuth ferrite (BiFeO3, BFO) powders were successfully synthesized via a sol–gel method. The effects of the Ba-substitution on the morphology, gas-sensing and electrical properties of BFO were studied. The gas-sensing tests show that the sensor based on Bi0.9Ba0.1FeO2.95 (BBFO10) has high sensitivity, quick response, effective selectivity and excellent long-time stability. The conduction mechanism and gas-sensing mechanism of a BBFO10 sample were investigated by the impedance spectroscopy and it was found that the conduction is dominated by p-type hole conduction. The conductivity of the sensor is dependent on the oxygen partial pressures and the type of gas atmosphere. The enhanced gas-sensing performances of the BBFO10 sensor are attributed to the higher oxygen vacancy concentration which was induced by the substitution of Bi3+ ion by an aliovalent Ba2+ ion at the A-site of the perovskite structure.
1. Introduction
Metallic oxide-based gas sensors have attracted much research interest due to the growing attention to environmental problems, chemical process control and medical applications. Among the available semi-conductive materials, binary metallic oxides such as ZnO, SnO2 and Fe2O3 usually exhibit excellent sensing properties.1–5 Yet it is a pity that their long-term reliability is still questionable, especially when working in a humid environment.6 It was reported that some ternary metal oxides with a perovskite ABO3 structure have more stable and reliable gas-sensing properties than binary metal oxides do, which could be attributed to the ternary oxides consisting of two differently sized cations and their ability to accept a variety of dopants.7 Ferrites with perovskite-structure, such as BiFeO3, LaFeO3, PrFeO3, EuFeO3, GdFeO3, were often found to have different degrees of gas-sensing ability.6,8,9Bismuth ferrite (BiFeO3, BFO) is well known for its multiferroic properties owning both ferroelectric ordering and antiferromagnetic ordering at room temperature, and it was always considered to be a promising candidate for application in memory, spintronics and magnetoelectric sensor devices.10,11 Although BFO is also a p-type semiconductor oxide that exhibits gas-sensing properties to some extent, the researches on that area are still limited.12–14 As we know, properly designed aliovalent dopants can enhance the gas response and gas selectivity of oxide semiconductor sensors by electronically sensitizing and chemically sensitizing them, respectively.
Herein, we prepared pure and bivalent Ba-substituted BiFeO3 samples utilizing sol–gel method. The influences of the Ba-substitution on the morphology, gas-sensing and electrical properties of BFO were studied in detail. The conduction and gas-sensing mechanism of the samples were also discussed.
2. Experimental
2.1. Materials and synthesis
BiFeO3 (BFO) and Bi0.9Ba0.1FeO2.95 (BBFO10) powders were synthesized via sol–gel method using bismuth nitrate pentahydrate (Bi(NO3)3·5H2O), barium nitrate (Ba(NO3)2), iron nitrate nonahydrate(Fe(NO3)3·9H2O, 32 mmol), acetic acid (CH3COOH, 25 mmol), ethylene glycol ((CH2OH)2, 1 mol) and citric acid monohydrate (C6H8O7·H2O, 32 mmol) as starting ingredients. Firstly, stoichiometric bismuth nitrate and barium nitrate were dissolved in acetic acid, the iron nitrate and citric acid monohydrate were separately dissolved in half of the prescribed amount of ethylene glycol. After stirring for half an hour, nitrates solutions were mixed together, and then the citric acid monohydrate solution was added. A stable sol was obtained by aging the final transparent precursor for 24 h, after drying the sol at 100 °C for 24 h, and the obtained gel was calcined at 450 °C for 2 h to remove the organics. Finally, after sintering at 850 °C for 2 h, the samples were obtained.2.2. Materials characterization
Powder X-ray diffraction (XRD) data were collected by using an automated diffractometer (X'Pert PRO MPD, Philips, Eindhoven, The Netherlands) with a nickel filter (Cu-Kα radiation) in 2θ range of 10–130° at room temperature. The surface morphologies of the samples were observed by using a field-emission scanning electron microscopy (SEM; Supra 55, Zeiss, Oberkochen, German). The X-ray photoelectron spectroscopy (XPS) measurements were performed using an Omicron energy analyser (PHI-5400, Perkin Elmer, Waltham, MA, USA). An AC impedance analyzer (4294A, Agilent, San Diego, CA, USA) was connected with gas response instrument to collect the impedance data, which performed at different temperatures and gas atmospheres over the frequency range of 40 Hz to 10 MHz under a bias ac voltage of 1 V.2.3. Gas sensor fabrication and measurements
The typical fabrication process of the gas sensors can be described as follows.15 The as-synthesized powders were mixed and ground with glycerol as adhesive in an agate mortar to form a homogeneous paste, respectively. An alumina tube with Au electrodes and platinum wires was used as a substrate which was coated with the paste. A Ni–Cr alloy crossed alumina tube was applied as a heating resistor for both substrate heating and temperature controlling. Each element was dried under infrared light for several minutes and then calcined at 500 °C for 2 h in air. In order to improve their stability and repeatability, the gas sensors were aged at 300 °C for 10 days in air.16The gas-sensing measurements were performed on a gas response instrument (HW-30A, Hanwei Ltd, Zhengzhou, China). In the measuring electric circuit of a gas sensor, a load resistor is connected in series with a gas sensor. The circuit voltage (Vc) is 5 V, and the output voltage (Vout) is the terminal voltage of the load resistor (RL). The working temperature of the sensor is adjusted by varying the heating voltage (Vh). When a given amount of tested gas was injected into the chamber, the resistance of the sensor changed, and the output voltage changed accordingly. The gas-response sensitivity (S) is defined as the ratio of Rgas/Rair, where Rgas and Rair are the resistance values measured in reducing atmosphere and in air, respectively.
3. Results and discussion
3.1. Crystalline structure, morphology and composition
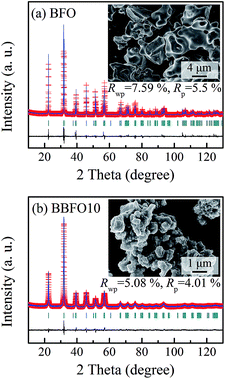
The XRD results of BiFeO3 (BFO) and Bi0.9Ba0.1FeO2.95 (BBFO10), as shown in Fig. 1, exhibit a single-phase perovskite structure and no secondary impurities phase was observed. The diffraction peaks are indexed to be the rhombohedral BiFeO3 with space group R3c.XRD Rietveld refinements were carried out with an R3c space group at room temperature by using FullProf program,17 and the refined lattice parameters are shown in Table 1. The unit cell volume of BBFO10 sample is larger than BFO, which might be attributed to the larger radius of Ba2+ ion than Bi3+ ion. The insets of Fig. 1 are the SEM micrographs of BFO and BBFO10, and it is observed that the grain size decreases obviously as the Bi3+ ions were replaced by Ba2+ ions at A-site.
| Sample | BFO | BBFO10 |
|---|---|---|
| a/Å | 5.5797(1) | 5.5986(3) |
| c/Å | 13.8719(2) | 13.7934(9) |
| V/Å3 | 374.0116 | 374.4245 |
| Rp/% | 5.50 | 4.01 |
| Rwp/% | 7.59 | 5.08 |
The XPS spectra for BFO and BBFO10 samples were measured and shown in Fig. 2. There are XPS photoelectron peaks of Bi, Fe, O and C elements on the pure BFO surface, and the C 1s peak located at 284.6 eV is used as the criterion to rectify the binding energy of XPS spectra. Besides the characteristic photoelectron peaks for Bi 4f7/2, Bi 4f5/2, O 1s, Fe 2p3/2 and Fe 2p1/2, the characteristic peaks of Ba 3d5/2 and Ba 3d3/2 can also be observed in the survey spectra of BBFO10.
To explore the effects of doping on oxygen state, the O 1s XPS spectra are displayed in Fig. 2(b). The O 1s XPS spectra show an asymmetric peak very close to 529 eV, while an additional peak near 531 eV can be obviously observed in both BFO and BBFO10 samples. Both curves can be Gaussian fitted by two symmetrical peaks, which are normally assigned as the low binding energy (LBE) peak and the high binding energy (HBE) peak of O 1s, respectively. The LBE peak is ascribed to the O 1s binding energy of the BFO phase, while the HBE peak is related with the loss of oxygen in the sample.18–20 Comparing the area ratio of the fitted HBE peak with LBE peak, it is noted that the area ratio of HBE peak is higher in BBFO10 than that of pure BFO, indicating the higher concentration of oxygen vacancies in BBFO10 sample. The formation of these oxygen vacancy defects may be attributed to the substitution of aliovalent Ba2+ ion at Bi3+ site in BiFeO3 to maintain charge neutrality.21 The valence state of Fe was also checked by XPS, as shown in the inset of Fig. 2(b). No obvious differences could be observed for the two samples. The peak at 711.1 eV combined with two satellite peaks at 719.2 and 724.5 eV demonstrate that the valence state of Fe in both samples is Fe3+, and there is no evidence for Fe2+ existence within a few atomic percent resolution.22
3.2. Gas sensing properties
Considering the sensitivity of gas sensors is greatly influenced by operating temperature, parallel experiments were carried out in the range of 300–430 °C to optimize the appropriate working temperature of the sensors. Typical response and recovery curve of BBFO10 sensor which was exposed to 100 ppm ethanol at different working temperatures are shown in Fig. 3(a). The output voltage decreases along with the operating temperature and reaches a minimum value at 400 °C, and the output voltage increases with further increase of the operating temperature to 430 °C. It means that the best operating temperature for BBFO10 sensor is around 400 °C which might be attributed to the competition between adsorption and desorption of the chemisorbed gases.23The sensitivities of the sensors based on BFO and BBFO10 (defined as the ratio of Rgas/Rair) at different working temperatures, are displayed in the Fig. 3(b). It is observed that the sensitivity of BBFO10 sensor is obviously higher than the undoped BFO sensor over the full operating temperature range, which means that the partial substitution of Bi-site by Ba ion enhanced the gas sensitivity of BiFeO3 effectively.
The gas-concentration dependence is illustrated by the response recovery characteristics of BBFO10 sensor which was exposed to ethanol vapor with different concentrations of 5, 10, 30, 50, 100, 300 and 500 ppm at 400 °C, as shown in Fig. 4(a). The sensor exhibits good sensitivity in various ethanol concentrations even exposed to as low concentration as 5 ppm ethanol, and the sensitivity increases dramatically as the concentration of ethanol vapor increases. Besides, the sensors exposed in different concentrations show a quick response and short recovery time. The response and recovery curve of BBFO10 sensor under 100 ppm ethanol vapor was chosen and displayed in Fig. 4(b), and the gas response and recovery time are 3 s and 10 s, respectively.
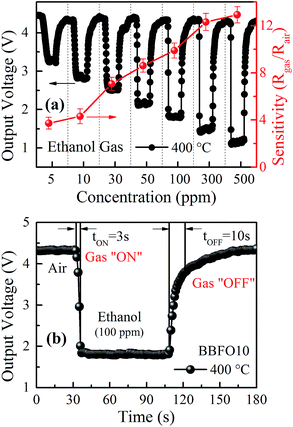 | ||
| Fig. 4 (a) Gas response and recovery curve of BBFO10 sensor exposed to ethanol at concentrations ranging from 5 to 500 ppm at 400 °C and (b) 100 ppm. | ||
Fig. 5(a) displays the stability of the BBFO10 sensor, which could be continuously operated over many cycles without obvious loss of sensitivity, and the results could be repeated after two months, illustrating good reproducibility and long-term stability of the sensor. In order to estimate the sensitivity for different gases, the response of BFO and BBFO10 sensor were examined in different gases with equal concentration of 100 ppm at operating temperature of 400 °C, and the results are shown in Fig. 5(b). The results show that the response of the BBFO10 sensor is much higher than that of the pure BFO sensor for all the gas tested. The sensor shows much stronger responses to ethanol, acetone and gasoline, while little or no response to toluene, methanol, ammonia and formaldehyde. It is also noteworthy that the representative indoor pollutant like toluene have minimum interference to the detection of other gases, which had been a difficult task using conventional n-type oxide semiconductor gas sensors.24
 | ||
| Fig. 5 (a) Stability of BBFO10 sensor to 100 ppm of ethanol at 400 °C, (b) sensitivity for different gases of BBFO10 sensor to different test gases with a concentration of 100 ppm at 400 °C. | ||
3.3. Gas sensing mechanism
For better understanding of the conduction mechanism of Bi0.9Ba0.1FeO2.95 sensor, the variable frequency impedance measurements were performed and shown in the Fig. 6. In order to determine the influence of oxygen partial pressure on the conductivity, the BBFO10 sensor was exposed to O2, air and N2 atmosphere at 400 °C for 20 h, respectively, and the complex impedance spectra were displayed in Fig. 6(a). Resistance values, the reciprocal of conductance, could be obtained from intercepts on the real Z′ axis. The impedance data, regardless of the processing conditions or measured temperatures, show a single semicircle. It means that the electrical properties of samples are dominated by one electrical component (Grain).The impedance spectra for the O2- and air-processed samples are similar, but the conductivities of the O2-annealed samples are a little higher, indicating a p-type conduction mechanism,25 and the hole concentration can be derived from and controlled by the schematic reaction:
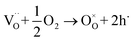 | (1) |
According to the eqn (1), the conductivities of BBFO10 sample are affected dramatically by hole concentration which is dominated by oxygen partial pressure. As shown in the Fig. 6(a), the N2-annealed samples are much more insulating due to the fact that the hole concentration decreases dramatically.
The imaginary parts of impedance (Z′′) and modulus (M′′) as a function of frequency are combined and shown in Fig. 6(b). The data in Z′′/M′′ spectroscopic plots show a single peak in both cases with maxima at similar frequencies, indicating that the sample was electrically homogeneous.25 The temperature dependence of impedance spectroscopy for BBFO10 sensor is shown in Fig. 6(c). It could be observed that the diameter of the semicircle decreases monotonously, which means the resistance decreases as the temperature increases. The corresponding resistances evaluated from the impedance spectrum are shown in Fig. 6(e). This is due to that the conductivity and density of carriers in semiconductors greatly increase with temperature.26
To study the effects of the gases on the resistance of the BBFO10 sensor, impedance spectroscopy is also measured when the sensor is exposed to 100 ppm concentration of different gases at 400 °C, including ethanol, toluene, methanol, gasoline, acetone, ammonia, formaldehyde, and the results are summarized in Fig. 6(d) and (f). The resistance values all increase different degrees when the sensor was exposed to in the reducing gases, and among them, the resistance increases most when the sensor were exposed to ethanol gas which indicating an effective ethanol gas sensitivity.
According to the Wolkenstein's model,27 the adsorbed neutral oxygen molecules (O2)ads at the semiconductor surface are partially ionized into O2−, O− or O2− ions by attracting electron from the valence band of semiconductor at different temperatures. Since the sensors are operated at 400 °C, the O− species is more dominated than any other oxygen adsorbate.
| (O2)gas ↔ (O2)ads | (2) |
| (O2)ads + e− ↔ (O2−)ads | (3) |
| (O)ads + e− ↔ (O−)ads | (4) |
For the p-type semiconductor, this reduction of the electrons number will change the electric surface's charge status of the sensors. It means that an underlying positive accumulation zone formed by holes, inducing a lower resistance layer which covers the whole surface of the gas sensor. Thus the surface resistance decreases by the presence of atmospheric oxygen. This view has been confirmed by the variation in conductivity of sensors under different oxygen partial pressure, as discussed earlier.
This leads to the formation of an electronic core–shell configuration (Fig. 7(a and b)), and the conduction in p-type oxide semiconductors can be explained by the parallel competition between the resistive core (Rbulk) and the conductive hole accumulation layer (RHAL) shell in addition to the serially connected neck component. As suggested by Pokhrel et al.,28 the response to reducing gas in p-type sensors is not affected significantly by the particle size of sample. Because if the neck configuration of particles remains similar, the coarsening of particles will not decrease gas response to a great extent.29
 | ||
| Fig. 7 The schematic diagram of the proposed gas-sensing mechanism for p-type semiconducting sensor: (a) and (c) in air, (b) and (d) in reducing gas, (e) simplified equivalent circuit. | ||
Correspondingly, accompanied with the electrons attracted by the adsorbed neutral oxygen molecules, the formation of a thick space-charge layer will increase the potential barrier, as displayed in the schematic model of the depletion layer. Furthermore, a larger quantity of oxygen vacancy in BBFO10 sample induces higher adsorptions of oxygen without lowering the expansion level of the depletion layer for pure BFO sample, which results in a further decrease of the resistance of the BBFO10 sensor.
When the sensors are exposed to an atmosphere containing reducing gas, the oxygen adsorbates are removed by reduction reaction. This process returns the trapped electrons in the valence band and results in a recombination of electrons and holes which increases the layer resistance. It is described in the specific cases of ethanol and acetone by the reaction as follows.
| CH3CH2OH + 6Oads− → 2CO2gas + 3H2Ogas + 6e− | (5) |
| CH3COCH3 + 8Oads− → 3CO2gas + 3H2Ogas + 8e− | (6) |
For BBFO10, the substitution of Bi3+ by Ba2+ increases the oxygen vacancy concentration. The oxygen vacancies react with the atmospheric oxygen to generate more holes and then make the sample more conductive.30
 | (7) |
 ,
, 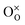 , h˙ represent oxygen vacancy, lattice oxygen and hole, respectively. In this case, more oxygen gas can combine with these surface defects after debonding the molecules, and the conductance change in the Ba-substituted BFO is more significant compared to that of the pure BFO. Thus, the Ba-substituted BFO sensor that has a significantly better sensing performance than pure BFO sensor is mainly due to the larger quantity of oxygen vacancies.
, h˙ represent oxygen vacancy, lattice oxygen and hole, respectively. In this case, more oxygen gas can combine with these surface defects after debonding the molecules, and the conductance change in the Ba-substituted BFO is more significant compared to that of the pure BFO. Thus, the Ba-substituted BFO sensor that has a significantly better sensing performance than pure BFO sensor is mainly due to the larger quantity of oxygen vacancies.
4. Conclusions
In summary, the gas-sensing properties, conduction and gas-sensing mechanism of Ba-substituted BFO sample are investigated and compared with pure BFO. It is found that the conduction of BBFO10 is dominated by p-type hole conduction. The gas-sensing test revealed that the sensor based on the BBFO10 sample exhibited a high sensitivity, quick response time, effective sensitivity for different gases and excellent long-time stability. It is proposed that the enhanced gas-sensing properties of Ba-substituted BFO sample could be attributed to the higher concentration of oxygen vacancy than pure BFO, and the oxygen vacancy is induced by the substitution of Bi3+ ion by aliovalent Ba2+ ion at A-site of perovskite structure.Acknowledgements
This work was supported by the National Natural Science Foundation (51172187), the SPDRF (20116102130002, 20116102120016) and 111 Program (B08040) of MOE, the Xi'an Science and Technology Foundation (CX12174, XBCL-1-08), the Shaanxi Province Science Foundation (2013KW12-02), Aeronautical Science Foundation of China (2013ZF53072), the SKLP Foundation (KP201421), and the Fundamental Research Funds for the Central Universities (3102014JGY01004) of China.Notes and references
- J. Xu, Q. Pan, Y. A. Shun and Z. Tian, Sens. Actuators, B, 2000, 66, 277–279 CrossRef CAS.
- J. Chen, L.-n. Xu, W.-y. Li and X.-l. Gou, Adv. Mater., 2005, 17, 582–586 CrossRef CAS.
- X. Xu, X. Li, W. Wang, B. Wang, P. Sun, Y. Sun and G. Lu, RSC Adv., 2014, 4, 4831–4835 RSC.
- J. Sun, P. Sun, D. Zhang, J. Xu, X. Liang, F. Liu and G. Lu, RSC Adv., 2014, 4, 43429–43435 RSC.
- H. Song, T. Chen, X. Zhang and X. Jia, CrystEngComm, 2015, 17, 1173–1181 RSC.
- M. Siemons, A. Leifert and U. Simon, Adv. Funct. Mater., 2007, 17, 2189–2197 CrossRef CAS.
- H. Obayashi, Y. Sakurai and T. Gejo, J. Solid State Chem., 1976, 17, 299–303 CrossRef CAS.
- X. Niu, W. Du and W. Du, Sens. Actuators, B, 2004, 99, 399–404 CrossRef CAS PubMed.
- V. Lantto, S. Saukko, N. Toan, L. Reyes and C. Granqvist, J. Electroceram., 2004, 13, 721–726 CrossRef CAS.
- J. Wang, J. B. Neaton, H. Zheng, V. Nagarajan, S. B. Ogale, B. Liu, D. Viehland, V. Vaithyanathan, D. G. Schlom, U. V. Waghmare, N. A. Spaldin, K. M. Rabe, M. Wuttig and R. Ramesh, Science, 2003, 299, 1719–1722 CrossRef CAS PubMed.
- G. Catalan and J. F. Scott, Adv. Mater., 2009, 21, 2463–2485 CrossRef CAS.
- X. L. Yu, Y. Wang, Y. M. Hu, C. B. Cao and H. L. W. Chan, J. Am. Ceram. Soc., 2009, 92, 3105–3107 CrossRef CAS PubMed.
- M. Dziubaniuk, R. Bujakiewicz-Korońska, J. Suchanicz, J. Wyrwa and M. Rękas, Sens. Actuators, B, 2013, 188, 957–964 CrossRef CAS PubMed.
- A. Poghossian, H. Abovian, P. Avakian, S. Mkrtchian and V. Haroutunian, Sens. Actuators, B, 1991, 4, 545–549 CrossRef.
- H. Tian, H. Fan, H. Guo and N. Song, Sens. Actuators, B, 2014, 195, 132–139 CrossRef CAS PubMed.
- Y. Cai, H. Fan, M. Xu, Q. Li and C. Long, CrystEngComm, 2013, 15, 7339–7345 RSC.
- J. Rodrigues-Carvajal, FULLPROF, a Rietveld refinement and pattern matching analysis program, Laboratoire Leon Brillouin, CEA-CNRS, France, 2000 Search PubMed.
- L. Fang, J. Liu, S. Ju, F. Zheng, W. Dong and M. Shen, Appl. Phys. Lett., 2010, 97, 242501–242503 CrossRef PubMed.
- C. Rath, P. Mohanty, A. Pandey and N. Mishra, J. Phys. D: Appl. Phys., 2009, 42, 205101 CrossRef.
- M. Naeem, S. Hasanain, M. Kobayashi, Y. Ishida, A. Fujimori, S. Buzby and S. I. Shah, Nanotechnology, 2006, 17, 2675 CrossRef CAS PubMed.
- B. Ramachandran, A. Dixit, R. Naik, G. Lawes and M. R. Rao, J. Appl. Phys., 2012, 111, 023910 CrossRef PubMed.
- W. Eerenstein, F. Morrison, J. Dho, M. Blamire, J. Scott and N. Mathur, Science, 2005, 307, 1203 CrossRef CAS PubMed.
- L. Huang and H. Fan, Sens. Actuators, B, 2012, 171, 1257–1263 CrossRef PubMed.
- H.-J. Kim and J.-H. Lee, Sens. Actuators, B, 2014, 192, 607–627 CrossRef CAS PubMed.
- N. Masó and A. R. West, Chem. Mater., 2012, 24, 2127–2132 CrossRef.
- N. Khemiri, F. Chaffar Akkari, M. Kanzari and B. Rezig, Thin Solid Films, 2008, 516, 7031–7035 CrossRef CAS PubMed.
- F. F. Vol'kenshteĭn, Electronic processes on semiconductor surfaces during chemisorption, Plenum Pub Corp, 1991 Search PubMed.
- S. Pokhrel, C. Simion, V. Quemener, N. Barsan and U. Weimar, Sens. Actuators, B, 2008, 133, 78–83 CrossRef CAS PubMed.
- J.-W. Yoon, J.-K. Choi and J.-H. Lee, Sens. Actuators, B, 2012, 161, 570–577 CrossRef CAS PubMed.
- P. Knauth and H. L. Tuller, J. Am. Ceram. Soc., 2002, 85, 1654–1680 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |