Borate ester endcapped fluorescent hyperbranched conjugated polymer for trace peroxide explosive vapor detection†
Abstract
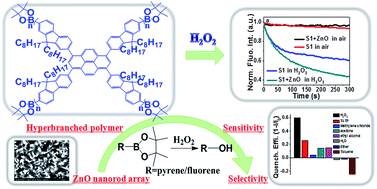
The vapor of peroxide explosives (PEs) is difficult to detect using fluorescent probes because PEs are not typical quenching agents, not having nitro groups or aromatic units that can easily interact with electron-rich probes. Three borate ester endcapped pyrenyl–fluorene copolymers were reported for the detection of PEs, including a hyperbranched polymer (S1) and two linear polymers with borate esters on fluorenyl (S2) or pyrenyl (S3) units. It was found that the hyperbranched polymer S1 has greater steric hindrance, more external borate ester groups, higher HOMO level and higher fluorescence quantum yield, which give it higher sensitivity to H2O2 vapor than S2 and S3. To further amplify the sensing performance toward H2O2 vapor, a polymer/ZnO nanorod array composite was used, exploiting the catalytic ability and high area to volume ratio of the ZnO nanorod array. The fluorescence of the S1 film is quenched by ∼60% and ∼30% under saturated vapor of H2O2 and TATP, respectively, for 300 s at room temperature, and the detection limit for H2O2 is estimated to be 1.6 ppb. These results reveal that the S1/ZnO nanorod array composite is very promising for the preparation of a highly sensitive fluorescence device for detecting the vapor of peroxide explosives.

 Please wait while we load your content...
Please wait while we load your content...