Copper loaded cross-linked poly(ionic liquid): robust heterogeneous catalyst in ppm amount†
Ali Pourjavadi*a,
Seyed Hassan Hosseinia,
Firouz Matloubi Moghaddamb and
Seyed Ebrahim Ayatib
aPolymer Research Laboratory, Department of Chemistry, Sharif University of Technology, Tehran, Iran. E-mail: purjavad@sharif.edu; Fax: +98 2166165311; Tel: +98 2166165311
bLaboratory of Organic Synthesis and Natural Products, Department of Chemistry, Sharif University of Technology, Tehran, Iran
First published on 6th March 2015
Abstract
A novel heterogeneous copper catalyst was synthesized in which poly(1-vinyl imidazole-co-ionic liquid) was used as a solid heterogeneous support. The catalyst was readily synthesized in a large scale amount. The catalyst has a high loading level of copper ions and can be used in low weight percentages. The resulting catalyst was highly active in the preparation of triazoles by the Huisgen 1,3-dipolar cycloaddition method. In some cases the catalytic turnover number and frequency reached 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 002 and 3889 h−1, respectively. The catalyst was recycled many times without significant loss of activity.
002 and 3889 h−1, respectively. The catalyst was recycled many times without significant loss of activity.
Introduction
After introducing the concept of “click chemistry” by Sharpless and co-workers in 2001, the copper catalyzed reaction of azides with alkynes to produce 1,2,3-triazoles has become the most reported reaction known as a click reaction.1–4 It was found that several members of the 1,2,3-triazole family have biological and pharmaceutical properties.5–7 Also, because of the mild reaction conditions and high efficiency in the synthesis of 1,2,3-triazoles, these compounds are widely used in materials science.8,9 The uncatalyzed reactions of alkynes and azides, known as Huisgen cycloaddition,10 are strongly thermodynamically favored due to the high potential-energy content of the substrates, but have a relatively high kinetic-energy barrier which led to the very slow reaction and poor yield at room temperature.11 Copper(I) catalyzes the reaction of terminal alkynes and azides and dramatically accelerates the reaction rates and produces 1,2,3-triazoles in high yields under mild reaction conditions.12,13 However, the recovery of homogenous copper complexes after completion of the reaction is too difficult. Since, removal of trace amount of metal contamination from the products is essential, especially in pharmaceutical industry, copper catalysts should be completely separated from the products. Even with the extensive methods removal of trace amount of copper catalysts from the products remains a challenge. To solve the catalyst separation problem a rational approach is heterogenizing the homogenous catalysts. Several solid materials have been used for immobilization of copper complexes such as nanosilica,14–16 mesoporous silica,17,18 magnetic nanoparticle,18–22 zeolite,23,24 alumina.25 However, immobilization of copper complexes onto these solid materials suffers from some disadvantages such as multiple tedious steps for preparation of support surface, low loading of copper ion onto the surface of support material, low activity of catalyst after immobilization, high catalyst leaching and low thermal stability of catalyst. In the last decades, several functionalized polymers have been designed for immobilization of copper catalysts.26–34 However, copper ions in this type of catalysts can be easily oxidized to inactive Cu(II) or disproportionate to metallic copper. Also, in most cases polymer loaded copper catalysts showed higher activity only in hazardous organic solvents and reaction should be proceed in an inert atmosphere.On the other hand, decreasing the amount of used copper catalysts in catalytic reactions is very important for industrial applications. Increasing the amount of used catalyst caused more pollution of catalyst and more solvent needs for reaction, separation process and recovery of catalyst. Therefore, developing an effective heterogeneous copper catalyst with high stability, low leaching, high loading and cleaner catalytic system is still demanded.
Herein, we describe the synthesis of copper loaded cross-linked ionic-imidazole polymer as a simple, inexpensive, and high loaded and efficient heterogeneous catalyst for use in the synthesis of 1,2,3-triazoles. Copper metalloenzymes are generated by complexation of imidazole of histidine amino acid in peptide structure and copper ion and these complexations are important in enzymatic reactions.35,36 Therefore, investigation in catalytic activity of imidazole–copper polymer is one of the most interesting topics in polymer chemistry.
Experimental
Reagents and analysis
CuSO4, NaBF4, NaNO3, NaBr, NaOAc, N,N′-methylenebisacrylamide (MBA), 1-vinyl imidazole (Vim) and 3-methacrylamido propyl trimethyl ammonium chloride (MAPTAC) were obtained from Aldrich. 1-Vinyl imidazole was distilled before use and MAPTAC was extracted with diethyl ether. 2,2′-Azobisisobutyronitrile (AIBN, Kanto, 97%) was recrystallized from ethanol.FT-IR spectra of samples were taken using an ABB Bomem MB-100 FTIR spectrophotometer. Thermogravimetric analysis (TGA) was acquired under a nitrogen atmosphere with a TGA Q 50 thermogravimetric analyzer. The morphology of the catalyst was observed using a Philips XL30 scanning electron microscope (SEM).
Synthesis of catalyst
1-Vinyl imidazole (2 g), MAPTAC (4.5 g), MBA (0.5 g) and methanol (50 mL) were loaded into a 100 mL round bottom flask. The mixture was stirred and deoxygenated by argon for 20 min and then AIBN (0.1 g) was added to the mixture and the flask was equipped with a condenser and placed into an oil bath at 75 °C. After 15 h, the solid products were filtered and washed three times with methanol and dried in vacuum at 50 °C and noted as cross-linked poly(imidazole-co-MAPTAC) (P[im/IL][Cl]).The resulting powdered materials were subjected to anion exchange reaction. 0.3 g of powdered P[im/IL][Cl] was added to 50 mL water and an excess amount of salt (10 fold than Cl anions in P[im/IL][Cl]) was added to the solution. The mixture was vigorously stirred (1000 rpm) for 3 days at room temperature. The solid products were then filtered, washed five times with water (5 × 100 mL) and twice with methanol (2 × 20 mL) and dried in vacuum at 50 °C.
0.5 g P[im/IL][Cl] was dispersed in 20 mL aqueous solution of CuSO4 (Cu(II) mmol in solution was 0.5 fold of imidazole groups) by ultra-sonication for 25 min. Then the resulting mixture was stirred at 70 °C for 24 h. Afterward, the solid blue products were filtered and washed with water (5 × 50 mL) and dried at 50 °C to give P[imCu/IL][Cl]. The procedure for synthesis of other catalysts is the same as above method.
Determination of exchange of chloride ion with other ions
After exchange of chloride ions in P[im/IL][Cl] with other anions, the resulting catalysts were separated and the amount of released chloride anion was determined by titration with AgNO3 based on standard Mohr's method. Bromide exchange was determined by ion chromatography. The results showed that the percentages of ion exchange process are 91, 83, 88 and 90% for P[im/IL][Br], P[im/IL][NO3], P[im/IL][OAc] and P[im/IL][BF4], respectively.Determination of the copper content in catalysts
The catalyst (100 mg) was extracted with concentrated HCl (5 × 2 mL) in a screw-capped vessel, followed by treatment with concentrated nitric acid (2 mL) to digest the metal complex. The mixture was then transferred into a volumetric flask (100 mL), diluted 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 for the second time and was analyzed by the ICP analysis.
50 for the second time and was analyzed by the ICP analysis.
General procedure for the synthesis of 1,2,3-triazoles
Alkyne (0.5 mmol), alkyl halide (0.5 mmol), NaN3 (0.6 mmol), sodium ascorbate (10 mg, 10 mol%) and P[imCu/IL][Cl] (5 mg, 20 mol%) were added to a 5 mL round bottom flask and 2 mL water was added and the mixture was stirred at 50 °C for appropriate time. After completion of the reaction (monitored by TLC) ethyl acetate was added to the flask and the catalyst was separated by filtration. The products were extracted with ethyl acetate–water mixture. The combined organic layers were dried over MgSO4 and concentrated under reduced pressure to give the corresponding 1,2,3-triazole. Obtained triazole was purified by recrystallization from EtOAc–hexane.Results and discussion
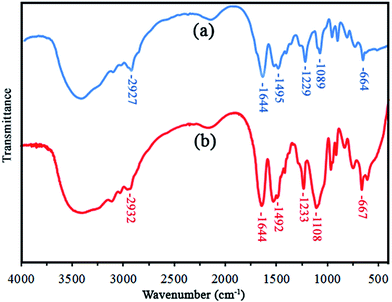
The catalyst was synthesized by free radical polymerization of Vim and MAPTAC in the presence of MBA as cross-linker agent (Fig. 1). Copolymerization was initiated by AIBN and the cross-linked insoluble copolymers were precipitated from the solution.37–41 Afterward, P[im/IL][Cl] was subjected to ion exchange process to produce P[im/IL][Br], P[im/IL][NO3], P[im/IL][OAc] and P[im/IL][BF4]. The resulting powdered P[im/IL] with various anions were dispersed in CuSO4 solution to produce copper loaded P[im/IL] (P[imCu/IL]). Imidazole groups in polymer backbone can strongly absorb copper ions. There are several reports that indicate imidazole–copper complexes improve the catalytic activity of copper ions.32 The presence of ionic monomer MAPTAC in catalyst backbone can improve the substrate diffusion due to their ionic interaction with reactants.The FT-IR spectrum of P[im/IL][Cl] shows the stretching vibration bands at 1644, 1495, 1233 cm−1 which are attributed to carbonyl groups of MAPTAC and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, C
N, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C–N of imidazole rings, respectively. The peak at 2927 cm−1 is attributed to C–H of alkyl chains (Fig. 2). The FT-IR spectra of ion exchanged products are presented at ESI (Fig. S1†).
C and C–N of imidazole rings, respectively. The peak at 2927 cm−1 is attributed to C–H of alkyl chains (Fig. 2). The FT-IR spectra of ion exchanged products are presented at ESI (Fig. S1†).
Similar pattern was observed in the FTIR spectrum of copper loaded catalyst (P[imCu/IL][Cl]) and a new peak also appeared at 1108 cm−1 which is attributed to SO4 anions of CuSO4 (Fig. 2). The result confirms that CuSO4 was successfully absorbed on the cross-linked copolymer.
Fig. 3 shows the thermal gravimetric analysis (TGA) and differential thermal analysis (DTA) of P[imCu/IL][Cl]. The weight loss at 150 °C was attributed to the loss of adsorbed water molecules in cross-linked polymer. The decomposition of catalyst started at 280 °C and it was completely decomposed at 450 °C. This result demonstrates that catalyst is stable at range 25–280 °C which is absolutely suitable for various catalytic reactions. DTA curve shows three distinct peaks at 90, 320 and 450 °C which are attributed to adsorbed water molecules, MAPTAC and imidazole parts in polymer structure, respectively.
The SEM image of catalyst shows a rough surface for P[imCu/IL][Cl] (Fig. 4). This surface morphology improves the catalyst activity due to the higher penetration of substrates onto the catalyst. EDX analysis also shows the presence of copper ion in catalyst structure (Fig. 4).
Atomic absorption and ICP-AES showed that the loading amount of copper ion in catalyst structure is about 1.3 mmol g−1. The high loading level of immobilized copper ion resulted to using lower weight percent of catalyst in reaction. This feature of catalyst is especially useful when the catalyst is applied in large scale production.
Table 1 shows the control experiment and optimization of catalytic reaction by choosing reaction between phenyl acetylene benzyl bromide and sodium azide as model reaction. As seen in Table 1 without catalyst only a small amount of triazole product was obtained even by increasing reaction temperature to 70 °C. Moreover, P[im/IL][Cl] did not show any catalytic effect and less than 12% product was obtained at 100 °C in 8 h. On the other hand, homogenous copper(II) sulfate increased the reaction yield up to 28% at 55 °C. Increasing the reaction temperature up to 110 °C gave 87% yield in 24 h. But in this case mixtures of two regioisomers were obtained. In the next step catalytic performance of P[imCu/IL][Cl] was investigated and it was found that using 0.7 mol% of P[imCu/IL][Cl] increased the reaction yield to 99% in 2 h at 55 °C. To investigate the effect of anion, various catalysts with different anions were examined and the results are presented in Table 1 entries 6–10. As a result, we observed that the order of catalytic activities of the five catalysts were; Cl > OAc > Br > BF4 > NO3. This order of catalytic activity can be attributed to atomic radius of anions and chelation of copper ion by anions. Larger anions reduce substrates penetration onto the polymer network due to larger steric effect. Therefore, chloride counterion with smaller size gave better yield. On the other hand more basic anions like Cl and OAc can chelate copper ions better than less basic ones. Therefore, the transition state of reaction is more stable when P[imCu/IL][Cl] is used than P[imCu/IL][NO3].
| Entry | Catalyst | Cat. loading (mol%) | Solvent | T (°C) | Time (h) | Yieldb (%) | TOF (h−1) |
|---|---|---|---|---|---|---|---|
| a Reaction condition: phenyl acetylene (1 mmol), benzyl bromide (1 mmol), sodium azide (1.2 mmol), sodium ascorbate (10 mol%), solvent (3 mL).b Isolated yield.c The ratio of H2O/t-BuOH is 3/1.d Mainly recovery of the starting materials.e Mixture of regioisomers.f 10 mmol scale of reaction.g Cross-linked poly(vinyl imidazole) was used. Loading amount of copper on P[imCu] is 0.61 mmol g−1.h Catalyst: mixture of vinyl imidazole (2 eq.) and copper sulfate (1 eq.). | |||||||
| 1 | — | — | H2O–t-BuOHc | r.t | 24 | <1d | — |
| 2 | — | — | H2O–t-BuOH | 70 | 8 | <3d | — |
| 3 | P[im/IL][Cl] | 10 mg | H2O–t-BuOH | 100 | 8 | <12d | — |
| 4 | CuSO4 | 5 | H2O–t-BuOH | 55 | 10 | 28 | 0.6 |
| 5 | CuSO4 | 5 | H2O–t-BuOH | 110 | 10 | 87e | 2 |
| 6 | P[imCu/IL][Cl] | 0.7 | H2O–t-BuOH | 55 | 2 | 99 | 71 |
| 7 | P[imCu/IL][Br] | 0.7 | H2O–t-BuOH | 55 | 2 | 83 | 59 |
| 8 | P[imCu/IL][NO3] | 0.7 | H2O–t-BuOH | 55 | 2 | 72 | 51 |
| 9 | P[imCu/IL][OAc] | 0.7 | H2O–t-BuOH | 55 | 2 | 88 | 63 |
| 10 | P[imCu/IL][BF4] | 0.7 | H2O–t-BuOH | 55 | 2 | 73 | 52 |
| 11 | P[imCu/IL][Cl] | 0.7 | H2O–t-BuOH | r.t | 2 | 80 | 57 |
| 12 | P[imCu/IL][Cl] | 0.7 | H2O–t-BuOH | r.t | 9 | 99 | 16 |
| 13 | P[imCu/IL][Cl] | 0.4 | H2O–t-BuOH | 55 | 2 | 99 | 124 |
| 14 | P[imCu/IL][Cl] | 0.1 | H2O–t-BuOH | 55 | 2 | 99 | 495 |
| 15 | P[imCu/IL][Cl] | 0.0013 | H2O–t-BuOH | 55 | 18 | 91f | 3889 |
| 16 | P[imCu/IL][Cl] | 0.1 | H2O | 55 | 2 | 91 | 455 |
| 17 | P[imCu/IL][Cl] | 0.1 | t-BuOH | 55 | 4 | 81 | 203 |
| 18 | P[imCu/IL][Cl] | 0.1 | CH3CN | 55 | 4 | 83 | 208 |
| 19 | P[imCu/IL][Cl] | 0.1 | CH3OH | 55 | 4 | 69 | 173 |
| 20 | P[imCu/IL][Cl] | 0.1 | Toluene | 55 | 4 | 37 | 93 |
| 21 | P[imCu]g | 0.1 | H2O–t-BuOH | 55 | 2 | 79 | 385 |
| 22 | Vim/Cuh | 0.1 | H2O–t-BuOH | 55 | 2 | 99 | 495 |
Since first step of the reaction is nucleophilic substitution of sodium azide to benzyl bromide, basic anions such as Cl and OAc can chelate Na+ in NaN3 and increase the reaction rate for first step and increase the yield of product.
Entry 11 shows that reducing the reaction temperature to room temperature reduced the yield of triazole product at the same reaction time. However, we found that in same condition longer time is required for completion of reaction (entry 12). The optimization of catalyst loading in reaction shows that catalyst is highly active and even 0.1 mol% is enough for reaction and 99% yield is obtained at 55 °C. We decreased the catalyst amount to 0.0013 mol% (130 mol ppm) and surprisingly it was observed that reaction is completed with 91% yield and the turnover frequency of catalyst reached to 3889 h−1. This result shows the high efficiency of P[imCu/IL][Cl] in Huisgen reaction.
Same reaction was performed in various solvents but 3/1 ratio of H2O/t-BuOH shows the best result. It can be seen that the result of catalytic reaction in pure water (entry 16) is as well as using mixture of solvents. This result provides a greener protocol for Huisgen reaction in water.
Entry 21 shows the effect of the presence of ionic monomer in the catalyst structure. It also can be seen that copper is adsorbed to poly(vinyl imidazole) less than ionic catalysts. Therefore, ionic monomer not only improves the catalytic performance but also increase copper adsorption and reduced the amount of used catalyst in the reaction.
To investigate the effect of imidazole groups as ligand we used the mixture of vinyl imidazole and copper sulfate in ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 as a homogenous catalyst. The result showed that imidazole groups increased the activity of copper by complexation (entry 22). However, this catalyst is effective but it is a homogenous catalyst and its separation from the solution is an issue.
1 as a homogenous catalyst. The result showed that imidazole groups increased the activity of copper by complexation (entry 22). However, this catalyst is effective but it is a homogenous catalyst and its separation from the solution is an issue.
In continue, the scope of three-component reaction of alkyl halide, sodium azide and alkyne catalyzed by P[imCu/IL][Cl] was investigated on a number of assorted substrates in optimized condition (Table 2). Various substrates with electron withdrawing and electron donating groups reacted under optimized condition and gave corresponding triazoles in satisfactory yields. Compared to aryl substrates, reaction of alkyl substrates were slightly slower, although the yields of products were still excellent. These results show that P[imCu/IL][Cl] is a powerful catalyst for the synthesis of a broad range of triazoles.
| Entry | Alkyne | Alkyl/Aryl halide | Product | Time (h) | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction condition: alkyne (0.5 mmol), alkyl halide (0.5 mmol), NaN3 (1 mmol), sodium ascorbate (10 mol%), P[imCu/IL][Cl] (0.1 mol%), solvent (H2O/t-BuOH, 3/1), 55 °C.b Isolated yield. | |||||
| 1 |  |
 |
 |
2 | 99 |
| 2 |  |
 |
 |
4 | 98 |
| 3 |  |
 |
 |
2 | 99 |
| 4 | 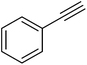 |
 |
 |
1.5 | 96 |
| 5 |  |
 |
 |
3.5 | 97 |
| 6 |  |
 |
 |
5 | 98 |
| 7 |  |
 |
 |
4 | 96 |
| 8 |  |
 |
 |
8 | 94 |
| 9 |  |
 |
 |
3.5 | 98 |
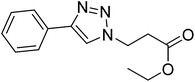
| 10 |  |
 |
 |
3 | 93 |
| 11 |  |
 |
 |
3 | 94 |
| 12 |  |
 |
 |
2 | 97 |
| 13 | 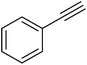 |
 |
 |
4 | 93 |
| 14 |  |
 |
 |
5 | 98 |
| 15 |  |
 |
 |
6 | 90 |
| 16 |  |
 |
 |
5 | 89 |
| 17 | 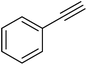 |
 |
 |
2 | 90 |
| 18 |  |
 |
 |
4 | 96 |
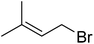
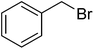
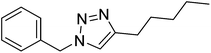
| 19 | 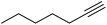 |
 |
 |
3 | 95 |
| 20 |  |
 |
 |
4 | 95 |
| 21 |  |
 |
 |
6 | 96 |
| 22 |  |
 |
 |
3 | 94 |
| 23 | 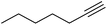 |
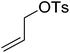 |
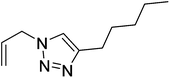 |
3 | 95 |
| 24 |  |
 |
 |
6 | 90 |
| 25 |  |
 |
 |
2 | 92 |
| 26 |  |
 |
 |
4 | 90 |
| 27 | 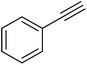 |
 |
 |
3 | 95 |
| 28 | 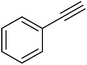 |
 |
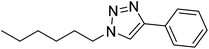 |
3 | 95 |
| 29 |  |
 |
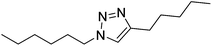 |
4 | 92 |
| 30 |  |
 |
 |
3 | 93 |
| 31 |  |
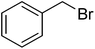 |
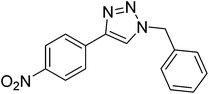 |
2 | 95 |
| 32 |  |
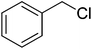 |
 |
3 | 95 |
| 33 |  |
 |
 |
3 | 91 |
| 34 |  |
 |
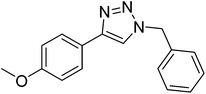 |
3 | 86 |
| 35 |  |
 |
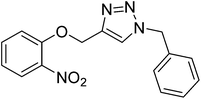 |
4 | 90 |
| 36 |  |
 |
 |
4 | 85 |
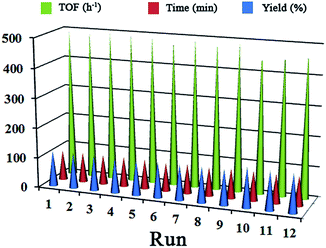
Along with the high activity of P[imCu/IL][Cl] in a low weight percent, another useful advantage of the catalyst is its reusability. At the end of a reaction, the catalyst was easily separated from solution by simple filtration. The reusability of P[imCu/IL][Cl] was investigated in three component reaction between phenyl acetylene, benzyl bromide and sodium azide as model reaction (Table 2, entry 1). In order to prevent catalyst mass losing, at the end of each cycle the catalyst was separated by centrifuge, washed and dried for next runs. As shown in Fig. 5 the catalyst was reused 12 times and no significant loss of activity was observed.
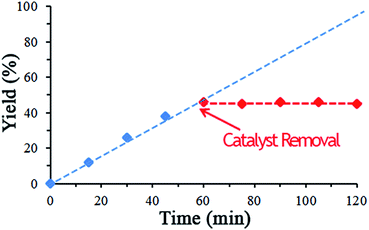
In order to investigate catalyst leaching, reaction between phenyl acetylene, benzyl bromide, NaN3 was chosen as model reaction. The reaction was performed in optimized condition and after half of the reaction time (60 min) the catalyst was separated from the solution by hot filtration using hot ethyl acetate. The rest of reaction mixture (in the absence of catalyst) was allowed to stir for another 60 min. As seen in Fig. 6 no triazole was produced after catalyst removal. Moreover, atomic absorption analysis of triazole product shows no copper ion in it. These results show that catalyst is truly heterogeneous and catalyst leaching is negligible under reaction condition.
Conclusion
In conclusion, we have prepared a novel heterogeneous copper loaded catalyst based on cross-linked poly(ionic liquid)s. The resulted catalyst was proven to be an effective and robust catalyst for preparation of 1,2,3-triazoles in a green way. The catalyst was easily prepared in large scale amount with high loading level of copper ion. The catalyst was recovered and reused several times without significant loss of activity. Because of high activity of catalyst in ppm amount the present protocol and catalyst can be used in large scale industrial applications.References
- M. Meldal, C. W. Tornne and C. Christensen, J. Org. Chem., 2002, 67, 3057–3064 CrossRef PubMed.
- V. V. Rostovtsev, L. G. Green, V. V. Fokin and K. B. Sharpless, Angew. Chem., Int. Ed., 2002, 114, 2708–2711 CrossRef.
- V. V. Rostovtsev, L. G. Green, V. V. Fokin and K. B. Sharpless, Angew. Chem., Int. Ed., 2002, 41, 2596–2599 CrossRef CAS.
- J. E. Hein and V. V. Fokin, Chem. Soc. Rev., 2010, 39, 1302–1315 RSC.
- M. S. Costa, N. Boechat, E. A. Rangel, F. D. C. Da Silva, A. M. T. De Sousa, C. R. Rodrigues, H. C. Castro, I. N. Junior, M. C. S. Lourenco, S. M. S. V. Wardell and V. F. Ferreira, Bioorg. Med. Chem., 2006, 14, 8644–8653 CrossRef CAS PubMed.
- Z. Y. Cheng, W. J. Lie, F. He, J. M. Zhou and Z. F. Zhu, Bioorg. Med. Chem., 2007, 15, 1533–1538 CrossRef CAS PubMed.
- M. J. Giffin, H. Heaslet, A. Brik, Y. C. Lin, G. Cauvi, C. H. Wong, D. E. McRee, J. H. Elder, C. D. Stout and B. E. Torbett, J. Med. Chem., 2008, 51, 6263–6270 CrossRef CAS PubMed.
- P. Wu, A. K. Feldman, A. K. Nugent, C. J. Hawker, A. Scheel, B. Voit, J. Pyun, J. M. J. Frechet, K. B. Sharpless and V. V. Fokin, Angew. Chem., Int. Ed., 2004, 43, 3928–3932 CrossRef CAS PubMed.
- D. D. Diaz, S. Punna, P. Holzer, A. K. McPherson, K. B. Sharpless, V. V. Fokin and M. G. Finn, J. Polym. Sci., Part A: Polym. Chem., 2004, 42, 4392–4403 CrossRef CAS.
- R. Huisgen, Proc. Chem. Soc., 1961, 357–369 Search PubMed.
- R. Huisgen, Angew. Chem., Int. Ed., 1963, 75, 565–598 CrossRef.
- R. Huigen, 1,3-Dipolar Cycloaddition Chemistry, ed. A. Padwa, Wiley, New York, 1984 Search PubMed.
- C. Zhang, B. Huang, Y. Chen and D. M. Cui, New J. Chem., 2013, 37, 2606–2609 RSC.
- P. Veerakumar, M. Velayudham, K. L. Lub and S. Rajagopal, Catal. Sci. Technol., 2011, 1, 1512–1525 CAS.
- E. A. Karakhanov, A. L. Maximov, Y. S. Kardasheva, V. A. Skorkin, S. V. Kardashev, V. V. Predeina, M. Y. Talanova, E. Lurie-Luke, J. A. Seeley and S. L. Cron, Appl. Catal., A, 2010, 385, 62–72 CrossRef CAS PubMed.
- J. L. Gole and M. G. White, J. Catal., 2001, 204, 249–252 CrossRef CAS.
- M. Nandi, P. Roy, H. Uyama and A. Bhaumik, Dalton Trans., 2011, 40, 12510–12518 RSC.
- H. Li, M. Yang, X. Zhang, L. Yan, J. Li and Y. Qi, New J. Chem., 2013, 37, 1343–1349 RSC.
- A. Megia-Fernandez, M. Ortega-Munoz, J. Lopez-Jaramillo, F. Hernandez-Mateo and F. Santoyo-Gonzalez, Adv. Synth. Catal., 2010, 352, 3306–3320 CrossRef CAS.
- R. Hudson, C. J. Li and A. Moores, Green Chem., 2012, 14, 622–624 RSC.
- R. B. Nasir Baig and R. S. Varma, RSC Adv., 2014, 4, 6568–6572 RSC.
- J. M. Pérez, R. Cano and D. J. Ramón, RSC Adv., 2014, 4, 23943–23951 RSC.
- S. Chassaing, A. S. S. Sido, A. Alix, M. Kumarraja, P. Pale and J. Sommer, Chem.–Eur. J., 2008, 14, 6713–6721 CrossRef CAS PubMed.
- P. Kuhn, A. Alix, M. Kumarraja, B. Louis, P. Pale and J. Sommer, Eur. J. Org. Chem., 2009, 3, 423–429 CrossRef.
- I. S. Park, M. S. Kwon, Y. Kim, J. S. Lee and J. Park, Org. Lett., 2008, 10, 497–500 CrossRef CAS PubMed.
- L. Bonami, W. V. Camp, D. Van Rijckegem and F. E. Du Prez, Macromol. Rapid Commun., 2009, 30, 34–38 CrossRef CAS PubMed.
- T. R. Chan and V. V. Fokin, QSAR Comb. Sci., 2007, 26, 1274–1279 CAS.
- U. Sirion, Y. J. Bae, B. S. Lee and D. Y. Chi, Synlett, 2008, 2326–2330 CAS.
- M. Chtchigrovsky, A. Primo, P. Gonzalez, K. Molvinger, M. Robitzer, F. Quignard and F. Taran, Angew. Chem., Int. Ed., 2009, 48, 5916–5920 CrossRef CAS PubMed.
- A. Coelho, P. Diz, O. Caamao and E. Sotelo, Adv. Synth. Catal., 2010, 352, 1179–1192 CrossRef CAS.
- C. H. Gary and T. Olsson, Org. Lett., 2004, 6, 3079–3082 CrossRef PubMed.
- A. Ouali, R. Laurent, A. M. Caminade, J. P. Majoral and M. Taillefer, J. Am. Chem. Soc., 2006, 128, 15990–15991 CrossRef CAS PubMed.
- Q. Zhao, P. Zhang, M. Antonietti and J. Yuan, J. Am. Chem. Soc., 2012, 134, 11852–11855 CrossRef CAS PubMed.
- Y. M. A. Yamada, S. M. Sarkar and Y. Uozumi, J. Am. Chem. Soc., 2012, 134, 9285–9290 CrossRef CAS PubMed.
- K. D. Karlin, R. W. Cruse, Y. Gultneh, A. Farooq, J. C. Hayes and J. Zubieta, J. Am. Chem. Soc., 1987, 109, 2668–2673 CrossRef CAS.
- D. Sadava, D. M. Hillis, H. C. Heller and M. R. Berenbaum, Life: The Science of Biology: Evolution, Diversity, and Ecology, WH Freeman & Co., New York, 2009 Search PubMed.
- A. Pourjavadi, S. H. Hosseini, F. Matloubi Moghaddam, B. Koushki Foroushani and C. Bennett, Green Chem., 2013, 15, 2913–2919 RSC.
- A. Pourjavadi, S. H. Hosseini and Z. S. Emami, Chem. Eng. J., 2013, 232, 453–457 CrossRef CAS PubMed.
- A. Pourjavadi, S. H. Hosseini and R. Solyman, J. Mol. Catal. A: Chem., 2012, 365, 55–59 CrossRef CAS PubMed.
- A. Pourjavadi, S. H. Hosseini, M. Doulabi, S. M. Fakoorpoor and F. Seidi, ACS Catal., 2012, 2, 1259–1266 CrossRef CAS.
- A. Pourjavadi, S. H. Hosseini, N. Zohreh and C. Bennett, RSC Adv., 2014, 4, 46418–46426 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra00127g |
| This journal is © The Royal Society of Chemistry 2015 |