Insights into the roles of two constituents CL-20 and HMX in the CL-20:HMX cocrystal at high pressure: a DFT-D study
Abstract
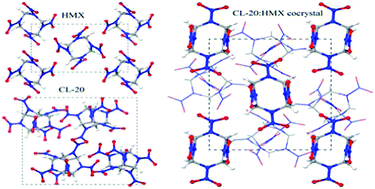
Density functional theory with dispersion corrections (DFT-D) was used to study pressure-induced effects in a novel energetic CL-20:HMX cocrystal and to understand what role its constituents CL-20 and HMX have. The structural, electronic, absorption, and mechanical properties of the cocrystal and its constituents were compared and analyzed in detail. The results indicate that the two constituents produce different effects on the crystal structure of the cocrystal in different directions. This distinct energy distribution in the cocrystal suggests that electron transitions may take place between the HMX and CL-20 molecules. The CL-20 in the cocrystal plays a leading role in the electronic structure of the cocrystal. The cocrystal has quite similar absorption spectra to ε-CL-20 but very different ones from β-HMX. Compared with the pure crystals, the mechanical properties of the cocrystal present a great anisotropy, which not only greatly strengthens the stiffness but also affects the preference of the stiffness towards different directions. Our results may provide fundamental insight into the roles of the two constituents in the cocrystal and may be helpful for developing new cocrystals with high energy and good safety.

 Please wait while we load your content...
Please wait while we load your content...