Biodegradable polymeric gene delivering nanoscale hybrid micelles enhance the suppression effect of LRIG1 in breast cancer
Abstract
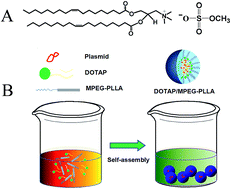
To increase the incorporation efficiency and improve the release kinetics of the LRIG1 gene from monomethoxy-poly(ethylene glycol)–poly(L-lactic acid) (MPEG–PLLA) micelles, a flexible method for the fabrication of N-(2,3-dioleoyloxy-1-propyl)trimethylammonium methyl sulfate (DOTAP)-embedded MPEG–PLLA (MPDT) nanoscale hybrid micelles was developed. The MPDT nanoscale hybrid micelles produced according to the optimal formulation were spherical in shape when observed by transmission electron microscopy (TEM), with a mean particle size of 23.5 ± 2.6 nm, which increased to 32.73 ± 3.4 nm after binding the plasmid. Compared with PEI25K, MPDT nanoscale hybrid micelles exhibited higher transfection efficiency and lower cytotoxicity. We also used MPDT nanoscale hybrid micelles to deliver the LRIG1 gene to treat breast cancer. MPDT delivered the LRIG1 gene (MPDT/LRIG1) and inhibited tumor cell proliferation, reducing the growth of 4T1 breast cancer cells in vitro. In vivo studies show that MPDT nanoscale hybrid micelles injected through the tail vein were able to deliver the LRIG1 gene efficiently and inhibited the growth of 4T1 breast cancer cells. These results indicate that MPDT nanoscale hybrid micelles delivering the LRIG1 gene might be valuable in treating breast cancer in humans.

 Please wait while we load your content...
Please wait while we load your content...