Characterization and screening of tight binding inhibitors of xanthine oxidase: an on-flow assay
M. V. N. Rodriguesab,
R. S. Corrêab,
K. L. Vanzolinib,
D. S. Santosc,
A. A. Batistab and
Q. B. Cass*b
aCentro Pluridisciplinar de Pesquisas Químicas, Biológicas e Agrícolas, Universidade Estadual de Campinas, Rua Alexandre Cazelatto, 999, Vila Betel, Paulínia 13140-000, SP, Brazil
bDepartamento de Química, Universidade Federal de São Carlos, Rodovia Washington Luiz, km 235, Cx Postal, 676, São Carlos, 13565-905, SP, Brazil. E-mail: quezia@pq.cnpq.br; Tel: +55 16 3351 8087
cInstituto Nacional de Ciência e Tecnologia em Tuberculose, Centro de Pesquisas em Biologia Molecular e Funcional, Pontifícia Universidade Católica do Rio Grande do Sul, Avenida Ipiranga, 6681, Porto Alegre, 90619-900, RS, Brazil
First published on 17th April 2015
Abstract
Xanthine oxidase (XO) is an enzyme in the purine salvage pathway that catalyzes the oxidation of hypoxanthine to xanthine with subsequent production of uric acid from the xanthine oxidation, and it has been considered an important target of newly developed inhibitors. Based on the advantages of using immobilized capillary enzyme reactors (ICERs) in a 2D LC system as a tool for screening new enzymatic ligands, this work validated an XO-ICER using allopurinol as a positive control. Despite the complex interaction between XO and allopurinol due its tight binding nature, it was possible to recognize the inhibitory kinetics parameters through Morrison's equation. The tight binding nature of inhibition was established by varying the IC50 values according to the substrate concentration. The kinetic inhibitory profile of allopurinol was used to validate the XO-ICER. Then, the XO-ICER was used to screen specific ruthenium derivatives. The selected compound, 4CBALO, an allopurinol ruthenium derivative, exhibited 100% inhibition at 200 μM compared to 86% inhibition from allopurinol at the same concentration. The inhibitory effect on the immobilized XO was reversible after the elution of the compound, with immediate recovery of the ICER activity. Additionally, 4CBALO behaved as a selective and competitive tight binder of xanthine oxidase with a true Ki value of 0.29 μM, which was obtained from the Morrison equation. This report describes the first on-flow characterization of tight binders of xanthine oxidase.
1. Introduction
Multidimensional high performance liquid chromatography (2D LC) has been a powerful tool for screening and characterization of enzymatic inhibitors in the search for new bioactive molecules.1–3 Among several approaches, the use of immobilized capillary reactors (ICERs) in the first dimension of the chromatographic system has guaranteed reliability of results when compared with off-line enzymatic assays, due to the fact that, among other applications, the generated product can be expressed using enzymatic activity.1 For classical binders this technique has been successfully used in their characterization,1,4 however, for compounds with more complex inhibition mechanism such as tight binders, the use of ICER has not yet been explored.Xanthine oxidase (XO) is an important target for the development of antihyperuricemic drugs, which help decrease uric acid production (Fig. 1). This enzyme also generates reactive oxygen species (ROS) that have been associated with certain pathophysiological processes, such as post-ischemic reperfusion injury, diabetes and chronic heart failure.5 The XO inhibitor, allopurinol, was approved by the Food and Drug Administration (FDA) in 1966 and, in spite of its side effects, has been used for gout treatment since then.
Allopurinol is not only an inhibitor of but also a substrate for XO, and its oxidation product, oxypurinol, is also an inhibitor of the enzyme. The nature of its inhibition, however, is quite different from that of allopurinol. Although the apparent inactivation of XO is caused by allopurinol in the absence or presence of xanthine, oxypurinol requires the substrate.6,7
For these compounds, which are known as tight-binding inhibitors, the steady state approximation model and the use of double reciprocal graphics are not valid. This is because, under equilibrium conditions, these inhibitors cause depletion of the non-bound form of the inhibitor by the formation of the enzyme-inhibitor complex.8,9
This type of inhibitors offers a special challenge to its characterization since it requires different methods of analysis.
The simplest way to determine that a tight binding inhibition is occurring is through an inhibitors dose-response curve. An IC50 value similar to the total enzyme concentration (within a factor of 10) is a good hint of this type of inhibition.8
Thus, a straightforward procedure for determining the Kappi for tight binder inhibitors is to measure the IC50 at a fixed concentration of the substrate and with a variety of enzyme concentrations and then plot the obtained IC50 versus total enzyme concentration. The results of these data can be fitted to a linear equation and the y-intercept provides an estimate of Ki. The major limitation of this approach is one's ability to accurately determine the y-intercept of a plot with a data containing typical levels of experimental error.5
Nevertheless, this well settled approach is not feasible when using an on-flow assay with an immobilized enzyme; therefore, the adopted procedure has been to determine IC50 values for these tight binders at a wide range of substrate concentrations with Kappi determined by the Morrison equation.8
This wide range of substrate concentrations is required to avoid misinterpretation of results, because the tight binding inhibitors exhibits double reciprocal plots similar to classical non-competitive inhibition pattern. The initial concentration of a double reciprocal plot, obtained only at extreme conditions (higher substrate concentrations and higher inhibitor concentrations), allows evidencing the nonlinearity portion of the double reciprocal plot and, thus, the selection of the best method for obtaining concentration-response data for tight binders. For this approach, the total enzyme concentration is needed. Fortunately, for tight binding inhibitor this is a feasible highly accurate measurement.8
In this context, to the best of our knowledge, this is the first report describing an on-flow assay for XO tight inhibitors using allopurinol as a reference binder. Furthermore, the developed 2D LC assay was used to screen and characterize five ruthenium complexes that were synthesized and characterized by the following techniques: elemental analysis, NMR (31P and 1H), UV-Vis, infrared, electrochemical and X-ray crystallography (Fig. 2).10
An approach to the discovery of new metallodrugs involves binding of an organic compound of known therapeutic activity to a metal-containing fragment; this results in a metal-drug synergism in which the metal acts as a carrier and stabilizer for the drug until it reaches its target. Such combined effects may result in an important enhancement of the activity of the drug. Thus, for the screening assay with the XO-ICER we selected a series of five ruthenium(II) complexes. All five complexes present in their structure a bisphosphonic ligand, dppb (1,4-bis(diphenylphosphino)butane), one chloride, one diimine ligand and a ligand L. The L ligands used were either allopurinol or imidazole or benzimidazole. As shown in Fig. 2, the complexes: CBALO, 4CBALO and 5CBALO, each has one molecule of allopurinol as the ligand. These allopurinol analogs differ with respect to the diimine ligands, 2,2′-bipyridine, 4,4′-dimethyl-2,2′-bipyridine and 5,5′-dimethyl-2,2′-bipyridine. Additionally, two ruthenium complexes with imidazole (CBIM) and benzoimidazole (CBZM) were also tested. The on-flow assay would furnish a comparison in the inhibition effect produced by the three series of allopurinol ruthenium(II) complexes and the imidazole (CBIM) or benzimidazole (CBZM) complexes. Furthermore, the influence regarding the position of in which the ruthenium coordinates with allopurinol would also be evaluated. This information is important for our ongoing research with respect to the mechanism of action in the inhibition process of the enzyme for the production of uric acid. Besides, Ru(II)/N-heterocyclic complexes are stable, both, in solid state, and in solution.
2. Experimental
2.1 Chemicals and materials
Xanthine oxidase (EC 1.17.3.2) grade I ammonium sulfate suspension from bovine milk, xanthine, uric acid, allopurinol, ammonium formate, 25% glutaraldehyde solution in water (grade II) and (3-aminopropyl) triethoxysilane (APTS) were purchased from Sigma (St. Louis, MO, USA). The 9-deazaguanine derivate (BCX-762) was synthesized and kindly supplied by scientists at BioCryst Pharmaceuticals. The expression and purification of purine nucleoside phosphorylase from human (HsPNP) were performed as reported by Semeraro et al.,11 and it was prepared at 2.1 mg mL−1 in Tris–HCl buffer (pH 7.0). Methanol (HPLC grade) was from J.T. Baker (Xalostoc, MC, Mexico). Formic acid from Fluka, solvents and all other chemicals not mentioned above were of analytical grade. The mobile phases were prepared daily with ultrapure water obtained from a Milli-Q system (Millipore, São Paulo, Brazil) and were filtered through a PVDF membrane (0.45 μm) purchased from Millipore. The silica-fused capillaries (30 cm × 0.364 mm, 0.07 mm i.d.) that were used for the preparation of the ICERs were provided by Polymicro Technologies (Phoenix, AZ, USA).2.2 Apparatus
XO-ICERs were produced using a syringe pump Harvard Apparatus 11 Plus.Analytical columns were packed using the ascending slurry method at 7500 psi using methanol for the slurry preparation (50 mL) and for packing. Afterwards, the column was conditioned with methanol at a 1.0 mL min−1 flow rate for 12 h.
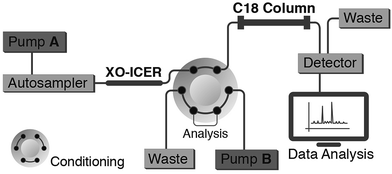
Chromatographic analyses were performed using a Shimadzu 2D LC system (Shimadzu, Kyoto, Japan), which consisted of two LC-20 AD pumps, a CBM-20A controller, an SIL-20 A autosampler, an SPD-20 A UV-Vis detector and a VICI six-way switching valve that was used to select the columns. Fig. 3 illustrates the 2D LC system used.
2.3 Chromatographic conditions
The xanthine oxidase enzymatic reaction was conducted on-flow with the XO-ICER using 10 mM ammonium formate, pH 7.4, at 0.05 mL min−1 (first dimension). The chromatographic separations were performed in the second dimension using a home-made packed Luna® C18 (150 × 4.6 mm; 10 μm) analytical column with 10 mM ammonium formate, pH 4.0/methanol (99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) at 0.6 mL min−1 as the mobile phase. Dual mode detection was used for uric acid and xanthine at 294 and 260 nm, respectively.
1 v/v) at 0.6 mL min−1 as the mobile phase. Dual mode detection was used for uric acid and xanthine at 294 and 260 nm, respectively.
The chromatographic conditions used during the selectivity assay with HsPNP were the same as previously published.12
2.4 ICERs preparation
The immobilization of XO and HsPNP were based on previously reported procedures4,12 with minor adjustments. The glutaraldehyde space linker was used at a concentration of 0.25%. Additionally, to improve the enzyme immobilization, the syringe pump flow rate was adjusted to only 50 μL min−1.For the immobilization of HsPNP, 1 mL of the enzyme solution (2.1 mg mL−1 protein in Tris–HCl buffer, pH 7.0) was dialyzed at 4 °C against phosphate buffer (50 mM, pH 7.0).
The ICERs were stored at 4 °C in the activity enzymatic buffer (first dimension eluents).
2.5 Method validation
Uric acid solutions were prepared, in triplicate, from 0.4 to 40 μM for the calibration curve. The solutions were transferred to a vial, and 10 μL of each solution was injected into an empty capillary connected to the first dimension of the chromatographic system. The intra- and inter-day precision and accuracy of the method were evaluated by analyzing quality control samples at three levels of concentration (0.7, 15 and 37 μM). Five samples of each concentration were prepared and analyzed on 3 nonconsecutive days.The acceptance criteria for the limit of quantification mandated that the precision of three samples had less than 20% variability, whereas the limit of detection was estimated as the concentration that produced a signal-to-noise ratio of 3.
2.6 Kinetics studies
The enzymatic activity was evaluated by quantifying the uric acid that was produced from the oxidation of xanthine by the XO-ICERs at 294 nm.Solutions with xanthine concentrations ranging from 1.0 to 500 μM were injected in duplicate. Michaelis–Menten plots were used to evaluate KM via nonlinear regression analysis using Origin software version 9.0.
ICER stability was determined from the ICER production of uric acid by injecting 10 μL of 200 μM xanthine, in duplicate, over a period of 60 days.
2.7 Screening study
Five ruthenium complexes (Fig. 2) synthesized and characterized in accordance with reported procedures10 were screened with the XO-ICERs. Stock solutions of each complex were first prepared in methanol at a concentration of 2.0 mM. Preliminary studies showed that the use of 10–20% of methanol had no significant effect on the XO-ICER activity; therefore, a solution containing 10% v/v of methanol was chosen for the final dilution.The compounds were evaluated at concentrations of 200 μM by injecting 10 μL of a solution containing 25 μM xanthine. The inhibition percentage was calculated by comparing the uric acid concentration obtained in the absence and presence of the complexes. Moreover, the reversibility of the XO inhibition was studied by determining the enzymatic activities of the XO-ICER before and after the elution of the solutions containing the complexes (200 μM).
2.8 Determination of mechanism of inhibition
The inhibition mechanism was determined by plotting the IC50 values against the substrate concentration, expressed as the [substrate]/KM. To determine each IC50, the fixed concentrations of xanthine were 32, 128, 320, 384 and 512 μM with varying concentrations of allopurinol in the range of 0.0075 to 4 μM. The series of IC50 values for 4CBALO were determined in the 0.01 to 2.56 M concentration range at the following fixed concentrations of xanthine: 32, 96, 128, and 256 μM.The IC50 values for allopurinol and 4CBALO were obtained by plotting the percentage inhibition versus the inhibitor concentration using Origin 9.0 software.
The Morrison equation (Eqn (1)) was used to calculate the inhibition constant Kappi through a dose-response curve that was calculated using GraphPad Prism software. To calculate the dose-response curve, the enzymatic activity was determined at 25 μM of xanthine and different concentrations of either allopurinol (0.3 to 1.8 μM) or 4CBALO (0.08 to 3.0 μM).
The total enzyme concentrations were estimated by extrapolating the linear portion of the dose-response curve to the (x) axis.8
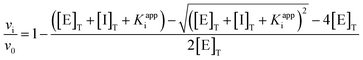 | (1) |
In the Morrison equation (Eqn (1)), vi/v0 represents the enzyme activity, whereas [E]T is the total enzyme concentration, [I]T is the total inhibitor concentration, and Kappi is the apparent inhibition constant. The true inhibition constant (Ki) is obtained using a different equation in accordance with the inhibitor type.
3. Results and discussion
3.1 XO-ICER preparation and kinetics parameters
Zonal bioaffinity chromatography has previously been successfully used for ICERs characterization through Michaelis–Menten parameters by means of a 2D LC system.1,2 Herein, an XO-ICER was prepared and used in the first dimension of a 2D LC system (Fig. 3) to not only determine its kinetic parameters but also fully characterize its tight-binding inhibitors. The validated LC method was used to quantify the production of uric acid by the ICERs. With regard to validation, the method provided a linear response (r = 0.9997) for uric acid production over a concentration range of 0.4 to 40 μM. Additionally, the intermediate precision, measured as the CV, was ≤5.1%, with results ranging from 97.3 to 114% accuracy. The limit of quantification and detection were 0.4 and 0.2 μM, respectively.The immobilization of XO is not trivial, and, although immobilization was achieved by means of the Schiff's base approach with good enzymatic performance, the immobilization conditions were carefully examined. Firstly, the glutaraldehyde (used as spacer) concentration was decreased from 1% to 0.25% v/v. This was done to prevent polymerization inside the capillaries, which would lead to flow obstruction and, the extensive crosslinking of the enzyme during the immobilization.
Additionally, the flow-rate of the syringe-pump was also evaluated as a mean to maximize reaction time. The flow-rate of 50 μL min−1 furnished the highest enzymatic activity for the XO-ICER.
For the 2D LC assay, the phosphate buffer used in the solution assay for XO1 was replaced, without impairing the enzymatic activity, by ammonium formate, without changing the pH of the solution, but at a lower molar concentration.
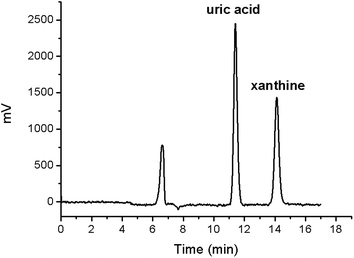
The activities of the XO-ICERs prepared over 2 months were reproducible with a CV = 2.18% (n = 7). Furthermore, The XO-ICERs activity was maintained over 60 days by storing them at 4 °C. The chromatogram in Fig. 4 illustrates the XO-ICERs production of uric acid under the activity assay conditions evaluated.
The experimental data of the XO-ICER activity obtained from xanthine concentrations ranging from 1 to 800 μM were fitted using a nonlinear regression analysis resulting in a KM value of 23.26 ± 3.88 μM. This value of KM shows a decrease in activity when compared to a reported KM value obtained from a solution assay of xanthine oxidase from bovine milk (KM = 8.00 ± 1).13 This is, however, of non-concern since the activity of the XO-ICER was maintained for a long period, allowing a series of experiments. It must be noticed that oxidation reaction occurs on flow, and in this the contact time between the enzyme and the substrate is shorter. Furthermore, allopurinol, a reference inhibitor, was characterized by the XO-ICER demonstrating that the molecular recognition was not affected.
3.2 XO-ICER validation in ligand screening assay
Allopurinol was selected as the reference inhibitor for the purposes of validating the XO-ICER on-flow screening assay for tight-binding inhibitors.6 Under the assay conditions, an 86% inhibition was achieved with 200 μM allopurinol. A graph of the IC50 value as a function of substrate concentration was plotted to characterize the inhibition mode (Fig. 5A).8 In agreement with published results,7 the data obtained indicated that allopurinol was a competitive inhibitor.Moreover, Morrison's plot (Fig. 5B) was used to obtain a Kappi of 3.25 ± 0.37 μM. Because allopurinol is a competitive tight-binding enzyme inhibitor, eqn (2) was used to furnish a Ki value of 1.55 μM. In eqn (2), Ki is the true inhibition constant, [S] is the substrate concentration and KM is the Michaelis–Menten constant.8 The active enzyme concentration value of 4.5 μM was estimated from the dose-response plot obtained with a 25 μM substrate concentration
 | (2) |
The Ki value obtained here (Fig. 5B) was in the same order of magnitude of that of a previously published work (6.3 μM),7 thus validating the 2D LC XO-ICER screening assay for tight-binding inhibitors.
3.3 Screening assay
Five ruthenium complexes (Fig. 2) were screened using the validated XO-ICER on-flow assay conditions, and the obtained results are graphically illustrated in Fig. 6.Because XO inhibitors can affect the purine pathway, and because selectivity plays a crucial role in selecting enzyme inhibitors, the three most XO-ICER-active ruthenium complexes were also evaluated with the HsPNP-ICER.12 With the HsPNP-ICER, compound BCX-762 was used as a reference inhibitor.14 The results demonstrated a high selectivity index of the tested complexes towards XO (Fig. 6).
Additionally, the reversibility of the XO inhibition by 4CBALO and 5CBALO was evaluated by determining the XO-ICER activity immediately before and after the injection of both inhibitors. The XO-ICER activity was readily recovered (97%) with 4CBALO, but the ICER activity did not recover with 5CBALO, suggesting, for the latter, an irreversible inhibition or a slow tight binder.15 For this reason, 4CBALO was selected for investigation of its mechanistic modality.
As expected for an allopurinol derivative, a tight binder behavior was found for 4CBALO towards XO-ICER. Reversible tight-binding inhibitors exert their effects on enzyme-catalyzed reactions at low concentrations, i.e., comparable to the concentration of the enzyme (within a factor of 10). Therefore, the results obtained for allopurinol ([E]T = 5.0 μM and IC50 = 0.32 μM) and 4CBALO ([E]T = 0.5 μM and IC50 = 0.07 μM) corroborated the assigned mechanistic modality.
From the obtained data, 4CBALO was characterized as a competitive inhibitor with Kappi and Ki values of 0.60 ± 0.07 and 0.29 μM, respectively.
4. Conclusions
The produced XO-ICER showed high stability with good reproducibility following preparation. The complete chromatographic separation of xanthine (the substrate) from uric acid (XO oxidation product) in the second dimension of a 2D LC system resulted in the development of a zonal bioaffinity chromatographic assay that was efficiently used to not only screen XO tight binder inhibitors but also fully characterize their inhibition modalities. From the developed assay, a series of five ruthenium allopurinol derivatives complexes were screened and the 4CBALO was identified as a competitive tight-binding inhibitor of XO with a tighter inhibition constant than the one found for allopurinol: K4CBALOi = 0.29 μM; Kallopurinoli = 1.55 μM.The great advantage of this fully automatized method is that not only the kinetics parameters is easily determined, but also the search for inhibitors are facilitated in as much as that the same chromatographic conditions can be used for screening and for the inhibition mechanism studies. With an assay condition of less than 17 min, 84 analyses can be carried out in a single day. Moreover, the same XO-ICER was continually used for about 2 months.
To our knowledge, this is the first time that a zonal bioaffinity chromatographic assay was used to mechanistically characterize tight-binding inhibitors, providing a comprehensive application of this assay model.
Acknowledgements
This work was supported by a research grant from the São Paulo Research Foundation (FAPESP 2013/01710-1). The Fellowships from FAPESP (2009/08131-1 and 2013/26559-4) and from the National Council for Scientific and Technological Development (CNPq 150954/2013-1) are acknowledged. The support from the National Institute of Science and Technology in Tuberculosis (INCT-TB) and National Institute of Science and Technology Controle Biorracional de Insetos Praga (CBIP) – is also acknowledged. The authors also thank Dr Marcela C. de Moraes for her helpful discussion concerning HsPNP immobilization.Notes and references
- M. C. de Moraes, C. L. Cardoso and Q. B. Cass, Anal. Bioanal. Chem., 2013, 405, 487 CrossRef PubMed.
- J. da Silva, M. de Moraes, L. Vieira, A. Correa, Q. Cass and C. Cardoso, J. Pharm. Biomed. Anal., 2013, 73, 44 CrossRef PubMed.
- Y. Fu, H. Mo, W. Gao, J. Hong, J. Lu, P. Li and J. Chen, Anal. Bioanal. Chem., 2014, 406, 4987 CrossRef CAS PubMed.
- K. Vanzolini, L. Vieira, A. Correa, C. Cardoso and Q. Cass, J. Med. Chem., 2013, 56, 2038 CrossRef CAS PubMed.
- P. Pacher, A. Nivorozhkin and C. Szabo, Pharmacol. Rev., 2006, 58, 87 CrossRef CAS PubMed.
- T. Spector and D. G. Johns, J. Biol. Chem., 1970, 26, 4 Search PubMed.
- G. Elion, Ann. Rheum. Dis., 1966, 25, 8 Search PubMed.
- R. A. Copeland, in Evaluation of Enzyme Inhibitors in Drug Discovery: A Guide for Medicinal Chemists and Pharmacologists, John Wiley & Sons, 2005, ch. 7, pp. 178–213 Search PubMed.
- S. Cha, Biochem. Pharmacol., 1975, 24, 2177 CrossRef CAS.
- R. S. Corrêa, Ph.D. thesis, Federal University of São Carlos, 2013.
- T. Semeraro, A. Lossani, M. Botta, C. Ghiron, R. Alvarez, F. Manetti, C. Mugnaini, S. Valensin, F. Focher and F. Corelli, J. Med. Chem., 2006, 49, 6037 CrossRef CAS PubMed.
- M. C. de Moraes, R. G. Ducati, A. J. Donato, L. A. Basso, D. S. Santos, C. L. Cardoso and Q. B. Cass, J. Chromatogr. A, 2012, 1232, 110 CrossRef CAS PubMed.
- H. Cao, J. M. Pauff and R. Hille, J. Biol. Chem., 2010, 285, 28044 CrossRef CAS PubMed.
- M. C. de Moraes, Ph.D. thesis, Federal University of São Carlos, 2012.
- J. F. Morrison, Trends Biochem. Sci., 1982, 7, 102 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |