Effect of pulsed electric field with variable frequency on coalescence of drops in oil
Abstract
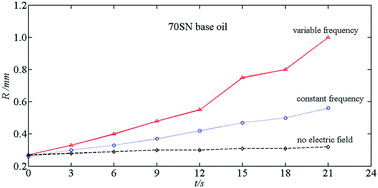
Under a pulsed electric field in oil, drops coalesce and become bigger constantly, leading to a change in the optimal coalescence frequency. A pulsed electric field with variable frequency can resolve this problem. To determine the effect of frequency variation of a pulsed electric field on coalescence, a nonlinear vibration kinetic model of a drop in oil is built to obtain the parametric excitation resonance frequency of the drop, which provides a theoretical basis for studying the effect of the change of the drop size on the optimal coalescence frequency. By utilizing a simple model of coalescence of double drops, the coalescence time is obtained, and then the frequency variation and operating parameters of the pulsed electric field is calculated. Experimental results show that the pulsed electric field with variable frequency has a better effect than that with constant frequency on the coalescence of emulsion drops. This finding has significance for engineering applications.

 Please wait while we load your content...
Please wait while we load your content...