Gold nanoparticle decorated single walled carbon nanotube nanocomposite with synergistic peroxidase like activity for d-alanine detection†
Abstract
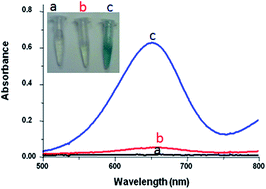
In this report, a gold nanoparticle decorated single walled carbon nanotube (SWCNTs) nanocomposite was shown to possess synergistic intrinsic peroxidase like activity and enhanced affinity towards H2O2 oxidation. The gold nanoparticle decorated SWCNTs nanocomposite was characterized by high catalytic activity, enhanced stability from the gold nanoparticles and improved dispersion from the SWCNTs. Subsequently, this nanocomposite was proved to be a novel peroxidase mimetic with great potential to catalyze the oxidation of 3,3′,5,5′-tetramethylbenzidine (TMB) in the presence of H2O2 to yield a blue colored product. As a proof of concept, the gold nanoparticle decorated SWCNTs composite was used as a robust nanoprobe for the detection of D-alanine with improved analytical characteristics. Taking into account the valuable intrinsic peroxidase activity of the nanohybrid, the present work may find widespread applications in the field of sensors and biosensors for diverse applications.

 Please wait while we load your content...
Please wait while we load your content...