Polystyrene resin supported palladium(0) (Pd@PR) nanocomposite catalyzed synthesis of β-aryl and β,β-diaryl unsaturated scaffolds following tandem approaches†‡
Arun K. Shilab and
Pralay Das*ab
aNatural Product Chemistry and Process Development, CSIR-Institute of Himalayan Bioresource Technology, Palampur-176061, HP, India. E-mail: pdas@ihbt.res.in; pdas_nbu@yahoo.com; Fax: +91-1894-230433
bAcademy of Scientific & Innovative Research, New Delhi, India
First published on 3rd March 2015
Abstract
A one pot general tandem procedure is described for β-aryl and β,β-diaryl alkenes synthesis following an alternative to the classical approaches by using aryl aldehyde as one of the starting materials. The developed polystyrene resin supported palladium(0) (Pd@PR) nanocomposite has been applied in an unprecedented sequential condensation–decarboxylation–Heck (CDH) and condensation–Heck (CH) strategies to generate the substituted alkenes (C6(C6)C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–C, C6–C
C–C, C6–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–C6, and C6(C6)C
C–C6, and C6(C6)C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CO–C6 units) under ligand, and additive free milder basic reaction conditions. The added momentous benefit over the classical methodologies is in terms of its multi-component approach to achieve the desired products without tedious step wise purifications.
C–CO–C6 units) under ligand, and additive free milder basic reaction conditions. The added momentous benefit over the classical methodologies is in terms of its multi-component approach to achieve the desired products without tedious step wise purifications.
1. Introduction
In recent times, β-aryl or β,β-diaryl unsaturated compounds such as cinnamates,1 stilbenoids,2 chalcones3 and neoflavonoids4 have attracted huge attention due to their extremely privileged biological profile and natural abundance. The conventional synthesis procedures of these compounds consists of acid or base catalyzed condensation,5a,b Wittig reaction5c and Lewis acid mediated cyclization5d with tedious work up, and step wise purifications. To surmount the limitations, the introduction of tandem or one pot sequential strategies is more beneficial and has been shown significant interest in the area of contemporary organic synthesis, especially methodology development5e,f and target oriented synthesis. In recent years, the transition metal catalyzed tandem processes have been highly nourished due to its minimal atom wastage, economic feasibility and efficiency.6 In this respect heterogeneous palladium(0) nanocomposite catalyzed domino-Heck approach might be an attractive alternative to furnish the valuable alkene scaffolds. Although the employment of transition metal nanocomposite as heterogeneous catalyst in such reactions is a challenging task mainly due to catalytic stability, reactivity and by-product prone side reactions in a variety of combined substrate/reagent conditions.7Synthesis of β,β-diarylalkenes through double Heck coupling is much difficult owing to the presence of less reactive β-hydrogen in 1,2-disubstituted olefin. Often palladium–phosphine and carbene complexes have shown good results, but unfortunately they are expensive, required additives and inert reaction conditions.8 Recently, a one pot CDH sequence has been attempted for the synthesis of 4-hydroxy stilbenes under the additive and homogeneous palladium–phosphine complex catalyzed condition (Scheme 1).9 In this report authors took electro-labile 4-hydroxy aromatic aldehyde as starting material which enabled an easy decarboxylation of the intermediate condensate. The choice of the substrate has greatly restricted the wider scope of this process. Moreover, use of much higher equivalents of active methylene component (14 equiv. malonic acid) and secondary nitrogenous base (15 equiv. piperidine) which might have participated in by-product formation, have limited the outreach of the methodology. But still design based one pot condensative approaches for Pd-catalyzed β-arylation starting from arylaldehyde remains untouched. To counteract the existing problems there is an intense urge to develop an efficient and facile single pot strategy for the synthesis of functionalized β-aryl or β,β-diaryl alkenes.
 | ||
| Scheme 1 (a) Previously reported method for the synthesis of 4-hydroxystilbenoids utilizing CDH strategy; (b) present work. | ||
In this context herein, we describe ligand free Pd@PR nanocomposite (formerly reported as SS-Pd, prepared from commercially available Amberlite IRA 900 resin (chloride form))10 catalyzed expeditious one pot sequential CDH and CH approaches using aromatic aldehyde, methylene compound and aryl halide to cinnamate, stilbene, chalcone and neoflavonoid synthesis.
2. Results and discussion
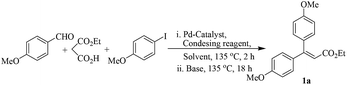
The Pd@PR nanocomposite was prepared following our earlier reports (ESI‡) through in situ reduction-deposition strategy using palladium(II) salt as Pd precursor.10a The heterogeneous Pd@PR nanocomposite was morphologically characterized by transmission electron microscopy (TEM) analysis before its application (Fig. 1). Inspired by our recent results,10,11 we applied Pd@PR catalyst for one pot sequential three component reactions for the synthesis of diaryl substituted olefin scaffolds following CDH and CH strategies. To select the best reagents and condition several optimization studies were performed to achieve the highest yield of the products (Table 1). 4-Methoxybenzaldehyde (150 mg), monoethyl malonate (1.2 equiv.), 4-iodoanisole (1.2 equiv.), Pd@PR (3 mol% Pd), and NH4OOCH (1 equiv.) followed by K2CO3 (2 equiv.) in DMF at 135 °C was found to be the optimum condition to give 1a in 72% yield (Table 1, entry 9). Considering other reagents (specially NH4OAc), NH4OOCH was found to be performing dual roles for facile condensation–decarboxylation reaction and as a reducing agent to facilitate conversion of Pd(II) to Pd(0), and in situ repairing/regeneration of Pd@PR catalyst which further confirmed by ICP-AES analysis (overall Pd leaching 0.14 ppm after three cycles, ESI‡).| Entry | Condensing reagent [equiv.] | Pd catalyst [mol% of Pd] | Base [equiv.] | Solvent | 1a % yielda |
|---|---|---|---|---|---|
| a Yields of isolated products; reaction condition: a mixture of substrates (4-methoxybenzaldehyde (150 mg.), monoethyl malonate (1.2 equiv.) and 4-iodoanisole (1.2 equiv.)) and palladium catalyst were magnetically stirred at 135 °C in presence of condensing agent for 2 hours and then base was added into the reaction mixture.b Yield of isolated by-product, 4,4′-dimethoxybiphenyl.c Both NH4OOCH (1 equiv.) and K2CO3 (2 equiv.) were added simultaneously into the reaction mixture. | |||||
| 1 | NH4OAc [2] | Pd@PR [3] | K2CO3 [2] | DMF | 47 |
| 2 | NH4OOCH [1] | Pd@PR [3] | K2CO3 [2] | DMF | 66 |
| 3c | NH4OOCH [1] | Pd@PR [3] | K2CO3 [2] | DMF | Trace, 39b |
| 4 | NH4OOCH [1] | Pd@PR [4] | K2CO3 [3] | DMF | 68 |
| 5 | K2CO3 [3] | Pd@PR [3] | – | DMF | Trace, 42b |
| 6 | KOOCH [2] | Pd@PR [3] | K2CO3 [2] | DMF | Trace, 31b |
| 7 | Et3N [2] | Pd@PR [2] | K2CO3 [2] | DMF | Trace, 37b |
| 8 | NH4OOCH [2] | Pd@PR [2] | K2CO3 [2] | DMF | 60 |
| 9 | NH4OOCH [2] | Pd@PR [2] | K2CO3 [2] | DMF | 72 |
| 10 | NH4OOCH [2] | Pd@PR [2] | K2CO3 [2] | Dioxane | 52 |
| 11 | NH4OOCH [2] | Pd@PR [2] | K2CO3 [2] | Toluene | 53 |
| 12 | NH4OOCH [2] | Pd@PR [2] | K2CO3 [2] | PEG-400 | 46 |
| 13 | NH4OOCH [2] | Pd(OAc)2 [3] | K2CO3 [2] | DMF | 30 |
| 14 | NH4OOCH [2] | Pd(allyl)2Cl2 [3] | K2CO3 [2] | DMF | 37 |
| 15 | NH4OOCH [2] | Pd2dba3 [3] | K2CO3 [2] | DMF | 41 |
The optimized condition was further applied to investigate the broader substrates scope as outlined in Table 2. Under the standard reaction condition, both aryl aldehydes and aryl iodides containing electron releasing substituents (–OMe, –OH and –Me) participated in CDH sequence to afford corresponding β-aryl ethylcinnamates (C6(C6)C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–C unit) (1b–g) in good to moderate yields. No significant effect of steric hindrance was observed for 2-OMe substituted aryl aldehydes or iodides for the same reaction and gave corresponding CDH products 1h–1k in good yields. More challenging electron withdrawing 2-NO2 and 3-NO2 substituted benzaldehydes were smoothly reacted with both mono ethyl malonate and malonic acid respectively in combination with 4-iodoanisole to furnish 1l and 1m in moderate yields. Similarly, 3,3-bis(4-methoxyphenyl)acrylonitrile 1n was obtained in 56% when 4-anisaldehyde was treated with cyanoacetic acid and 4-iodoanisole under the same reaction condition. Most of the products in Table 2 were found to be 100% E-configuration (confirmed by NMR studies and the reason might be the E-configured dehydro-decarboxylation (I) as described in mechanism section).
C–C unit) (1b–g) in good to moderate yields. No significant effect of steric hindrance was observed for 2-OMe substituted aryl aldehydes or iodides for the same reaction and gave corresponding CDH products 1h–1k in good yields. More challenging electron withdrawing 2-NO2 and 3-NO2 substituted benzaldehydes were smoothly reacted with both mono ethyl malonate and malonic acid respectively in combination with 4-iodoanisole to furnish 1l and 1m in moderate yields. Similarly, 3,3-bis(4-methoxyphenyl)acrylonitrile 1n was obtained in 56% when 4-anisaldehyde was treated with cyanoacetic acid and 4-iodoanisole under the same reaction condition. Most of the products in Table 2 were found to be 100% E-configuration (confirmed by NMR studies and the reason might be the E-configured dehydro-decarboxylation (I) as described in mechanism section).
| a All are isolated yields; reaction conditions: aryl aldehyde (150 mg), active methylene carboxylic acid (1.2 equiv.), aryl iodide (1.2 equiv.), Pd@PR (3 mol% Pd), NH4OOCH (2 equiv.), K2CO3 (2 equiv.), DMF (2 mL), 135 °C, the reaction was continued for 2 h before addition of K2CO3 followed by 18 h; E/Z ratio was determined by 1H NMR spectra. |
|---|
 |
In continuation, we applied the set tandem protocol for the synthesis of hydroxystilbenes which are among the most privileged scaffolds owing to their diversified biological and unrivalled physicochemical activities. The cascade Knoevenagel condensation–double decarboxylation–Heck process resulted hydroxy stilbene derivatives (1o and 1p) in 61% and 65% yields (E/Z = 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) respectively through the formation of corresponding styrene intermediates (Scheme 2).
0) respectively through the formation of corresponding styrene intermediates (Scheme 2).
 | ||
| Scheme 2 Pd@PR nanocomposite catalyzed tandem synthesis of hydroxy stilbenoids (E/Z ratio determined by 1H NMR spectra). | ||
Mechanistically it was presumed that the aromatic aldehyde first undergoes Knoevenagel condensation with α-carboxy active methylene component leading to the formation of most favourable E-configuration of the cinnamyl intermediate (II) after dehydro-decarboxylation of (I). The intermediate (II) is then coupled with aryl iodide in presence of Pd@PR catalyst through the oxidative addition, insertion and reductive elimination steps, to afford the desired β,β-diaryl alkene (V) (Scheme 3).
To our great interest, the set reaction condition was further extended for one pot tandem approach for the synthesis of β-arylchalconoids (C6(C6)–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CO–C6 unit) (Scheme 4) and neoflavonoids (C6(C6)–C
C–CO–C6 unit) (Scheme 4) and neoflavonoids (C6(C6)–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–C unit) (Scheme 5). The sequential aldol condensation of acetophenone with aromatic aldehydes and Heck reaction afforded the desired β-aryl chalcones 2a and 2b in moderate yields (Scheme 4).
C–C unit) (Scheme 5). The sequential aldol condensation of acetophenone with aromatic aldehydes and Heck reaction afforded the desired β-aryl chalcones 2a and 2b in moderate yields (Scheme 4).
 | ||
| Scheme 4 Pd@PR nanocomposite catalyzed tandem synthesis of β-aryl chalcones following CH approach (E/Z ratio determined by 1H NMR spectra). | ||
Whereas, the palladium catalyzed Heck arylation at β-position of in situ produced ethyl cinnamate intermediate with 2-iodophenol followed by cyclization gave neoflavonoids 3a and 3b in comparable yields (Scheme 5).
3. Conclusions
In summary, we have developed Pd@PR nanocomposite catalyzed proficient multicomponent strategies for the synthesis of β-aryl cinnamates, stilbenes, chalcones and neoflavonoids. The additive free one pot sequential process for the synthesis of β-aryl substituted alkene derivatives employing combined substrate/reagent compatible heterogeneous palladium nanocatalyst have imparted its potential future practical utility. In addition, we have explored the dual roles of NH4OOCH which governs the efficiency of condensation and stabilization of palladium(0) nanoparticles over the solid surface. Overall, the developed methodology comprises of the application of heterogeneous transition metal catalyst in multicomponent reactions, additive and ligand free process, and catalyst recyclability.4. Experimental section
General experimental procedure for one pot sequential condensation–decarboxylation–Heck (CDH) approach
To a mixture of arylaldehyde (150 mg), aryl iodide (1.2 equiv.), active methylene carboxylic acid (1.2 equiv.), ammonium formate (1.5 equiv.) and Pd@PR (3 mol% Pd) in 40 mL reaction vial was added 2 mL of dry DMF. The reaction mixture was stirred in preheated silicone oil bath at 135 °C for 2 hours and then K2CO3 (2 equiv.) was added into it. After addition of K2CO3 the reaction was again continued at the same temperature. The progress of the reaction was monitored by TLC. On completion, the cooled reaction mixture was extracted with ethyl acetate (3 × 5 mL) by addition of 2 mL of water and dried over anhydrous Na2SO4. Evaporation of the combined organic layer followed by column chromatography (Hexane and ethyl acetate gradient) over silica gel (mesh 60–200) afforded the desired products (1a–1p).Acknowledgements
This work was financially supported by the CSIR (project ORIGIN-CSC0108). We also gratefully acknowledge the Director CSIR-IHBT, Palampur, for providing necessary facilities during the course of work. The authors thank AIRF, JNU-New Delhi, for TEM and SAIF, IIT Bombay for ICP-AES analysis. AKS thanks CSIR for fellowship.Notes and references
- (a) Kirk Othmer Encyclopedia of chemical Technology; ed. W.F. Ringk, Wiley, New York, N.Y, 1981; vol. 6, pp. 143–149 Search PubMed; (b) J. Z. Deng, D. J. Newman and S. M. Hecht, J. Nat. Prod., 2005, 68, 465–467 CrossRef CAS PubMed; (c) N. Koolaji, A. Abu-Mellal, V. H. Tran, R. K. Duke and C. C. Duke, Eur. J. Med. Chem., 2013, 63, 415–422 CrossRef CAS PubMed.
- (a) Y. Q. Li, Z. L. Li, W. J. Zhao, R. X. Wen, Q. W. Meng and Y. Zeng, Eur. J. Med. Chem., 2006, 41, 1084–1089 CrossRef CAS PubMed; (b) H. F. Kung, S. R. Choi, W. Qu, W. Zhang and D. Skovronsky, J. Med. Chem., 2010, 53, 933–941 CrossRef CAS PubMed.
- (a) J. Wu, J. Li, Y. Cai, Y. Pan, F. Ye, Y. Zhang, Y. Zhao, S. Yang, X. Li and G. Liang, J. Med. Chem., 2013, 56, 7134–7134 CrossRef CAS; (b) Z. Nowakowska, Eur. J. Med. Chem., 2007, 42, 125–137 CrossRef CAS PubMed.
- (a) D. M. X. Donnelly and G. M. Boland, Nat. Prod. Rep., 1995, 12, 321–338 RSC; (b) C.-J. Wang, Y.-J. Hsieh, C.-Y. Chu, Y.-L. Lin and T.-H. Tseng, Cancer Lett., 2002, 183, 163–168 CrossRef CAS.
- (a) K. Wadhwa and J. G. Verkade, J. Org. Chem., 2009, 74, 4368–4371 CrossRef CAS PubMed; (b) B. P. Bandgar, S. S. Jalde, L. K. Adsul, S. N. Shringare, S. V. Lonikar, R. N. Gacche, N. A. Dhole, S. H. Nile and A. L. Shirfule, Med. Chem. Res., 2012, 21, 4512–4522 CrossRef CAS; (c) A. S. Saiyed, K. N. Patel, B. V. Kamath and A. V. Bedekar, Tetrahedron Lett., 2012, 53, 4692–4696 CrossRef CAS PubMed; (d) F. Boeck, M. Blazejak, M. R. Anneser and L. Hintermann, Beilstein J. Org. Chem., 2012, 8, 1630–1636 CrossRef CAS PubMed; (e) Y. Li, Z. Qi, H. Wang, X. Fu and C. Duan, J. Org. Chem., 2012, 77, 2053–2057 CrossRef CAS PubMed; (f) M. Khoobi, M. Alipour, S. Zarei, F. Jafarpour and A. Shafiee, Chem. Commun., 2012, 48, 2985–2987 RSC.
- (a) I. P. Beletskaya and A. V. Cheprakov, Chem. Rev., 2000, 100, 3009–3066 CrossRef CAS PubMed; (b) S. S. Ng and T. F. Jamison, J. Am. Chem. Soc., 2005, 127, 14194–14195 CrossRef CAS PubMed; (c) D. I. Chai and M. Lautens, J. Org. Chem., 2009, 74, 3054–3061 CrossRef CAS PubMed; (d) F. Felpin, O. Ibarguren, L. Nassar-Hardy and E. Fouque, J. Org. Chem., 2009, 74, 1349–1352 CrossRef CAS PubMed; (e) J.-P. Leclerc, M. Andr and K. Fagnou, J. Org. Chem., 2006, 71, 1711–1714 CrossRef CAS PubMed; (f) S. Yahiaoui, A. Fardost, A. Trejos and M. Larhed, J. Org. Chem., 2011, 76, 2433–2438 CrossRef CAS PubMed.
- (a) M. J. Climent, A. Corma, S. Iborra and M. J. Sabater, ACS Catal., 2014, 4, 870–891 CrossRef CAS; (b) F.-X. Felpin and E. Fouquet, ChemSusChem, 2008, 1, 718–724 CrossRef CAS PubMed.
- (a) K. Itami, K. Mitsudo, T. Kamei, T. Koike, T. Nokami and J.-I. Yoshida, J. Am. Chem. Soc., 2000, 122, 12013–12014 CrossRef CAS; (b) K. Itami, T. Nokami, Y. Ishimura, K. Mitsudo, T. Kamei and J.-I. Yoshida, J. Am. Chem. Soc., 2001, 123, 11577–11585 CrossRef CAS PubMed; (c) P. Nilsson, M. Larhed and A. Hallberg, J. Am. Chem. Soc., 2001, 123, 8217–8225 CrossRef CAS PubMed; (d) A. Svennebring, P. Nilsson and M. Larhed, J. Org. Chem., 2004, 69, 3345–3349 CrossRef CAS PubMed; (e) G. Zou, W. Huang, Y. Xiao and J. Tang, New J. Chem., 2006, 30, 803–809 RSC; (f) S. B. Park and H. Alper, Org. Lett., 2003, 5, 3209–3212 CrossRef CAS PubMed.
- N. Sharma, A. Sharma, A. Shard, R. Kumar, Saima and A. K. Sinha, Chem.–Eur. J., 2011, 17, 10350–10356 CrossRef CAS PubMed.
- (a) P. Das, D. Sharma, A. K. Shil and A. Kumari, Tetrahedron Lett., 2011, 52, 1176–1178 CrossRef CAS PubMed; (b) D. Sharma, S. Kumar, A. K. Shil, N. R. Guha, Bandna and P. Das, Tetrahedron Lett., 2012, 53, 7044–7051 CrossRef CAS PubMed; (c) D. Sharma, C. B. Reddy, A. K. Shil, R. P. Saroach and P. Das, Mol. Diversity, 2013, 17, 651–659 CrossRef CAS PubMed; (d) A. K. Shil, N. R. Guha, D. Sharma and P. Das, RSC Adv., 2013, 3, 13671–13676 RSC; (e) C. B. Reddy, A. K. Shil, N. R. Guha, D. Sharma and P. Das, Catal. Lett., 2014, 144, 1530–1537 CrossRef PubMed.
- (a) N. R. Guha, C. B. Reddy, N. Aggarwal, D. Sharma, A. K. Shil, Bandna and P. Das, Adv. Synth. Catal., 2012, 354, 2911–2915 CrossRef CAS; (b) A. K. Shil and P. Das, Green Chem., 2013, 15, 3421–3428 RSC.
Footnotes |
| † IHBT communication no. 3686. |
| ‡ Electronic supplementary information (ESI) available: Typical experimental procedures, recyclability experiment, Hg poisoning experiment, spectral data and copies of 1H, 13C NMR and ESIMS spectra of synthesised products. See DOI: 10.1039/c5ra00228a |
| This journal is © The Royal Society of Chemistry 2015 |