Bead beating-based continuous flow cell lysis in a microfluidic device
Abstract
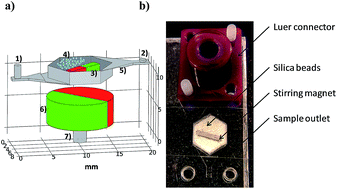
This paper describes a bead beating-based miniaturized cell lysis device that works in continuous flow allowing the analysis of large volumes of samples without previous treatment. A permanent magnet along with zirconium/silica beads were placed inside a lysis chamber fabricated with cyclo-olefin polymer (COP) by a fast prototyping technique, and the actuation of an external magnetic field caused the motion of the beads within the chamber. Characterisation of the lysis process was carried out using Staphylococcus epidermidis as the target cell and showed that both small bead size and large volume, along with the presence of Tween 20 and low flow rate, influenced significantly the device performance. Taking into account the compromise between time consumption and efficiency, 60 μL min−1 lysis flow rate was chosen as optimum yielding 43% lysis efficiency relative to off chip bead beating. Compatibility with injection moulding manufacturing techniques and capability of working in continuous flow make this device a potential DNA extraction method suitable for lab-on-a-chip applications.

 Please wait while we load your content...
Please wait while we load your content...