Cotton flame retardancy: state of the art and future perspectives
Jenny Alongi
* and
Giulio Malucelli
Dipartimento di Scienza Applicata e Tecnologia, Politecnico di Torino, Alessandria campus and INSTM Local Unit, Viale Teresa Michel 5, 15121 Alessandria, Italy. E-mail: jenny.alongi@polito.it; Fax: +39 0131229399; Tel: +39 0131229337
First published on 2nd March 2015
Abstract
As clearly reported in the scientific literature, the history of cotton flame retardancy is very old: this is easily attributable to the chemical and physical properties of this fibre that made it predominant during the 20th century; at present, it is only exceeded in volume by polyester. As a consequence, the huge number of papers published in the scientific literature so far covers an extended period and, sometimes, it could be easy to forget some of the knowledge already developed in the past and to focus the attention on the recent highlights only. Therefore, the present work is aimed to review the most significant scientific and technological results about the flame retardancy of cotton, merging the past experience and the current efforts, trying also to foresee a possible scenario for the next future. After a historical excursus on the achievements up to 2010, the review will summarize the recent developments reached in the so-called “era of nanotechnology” for cotton, as a consequence of the setup of new approaches like nanoparticle adsorption, Layer by Layer assembly and sol–gel processes. Finally, a possible alternative development route indicated by the potential exploitation of bio-macromolecules as green flame retardant systems will be briefly discussed.
1. Introduction
Cotton fibres are the purest form of cellulose, the most abundant polymer in nature: nearly 90% of the cotton fibres consist of cellulose. Although all plants are cellulose-based, the content of this latter may range from about 75% for flax, jute, ramie and kenaf (stalks of the plants), to 40–50% for both coniferous and deciduous wood, even to a much lower content in the case of other plant species or parts.1 The cellulose in cotton fibres is also of the highest molecular weight among all plant fibres and shows the highest structural order: for this reason, cotton is considered as a premier fibre and biomass. The economic impact of cotton in the global market is demonstrated by its majority share (over 50%) among the fibres employed for apparel and textile goods. Both the market value and the quality of cotton products are directly related to the fibre quality.The fundamental understanding of fibres (on a physico-chemical point of view) can mean significant improvements in their quality as well as in production. In this context, for a possible industrial exploitation in different application fields like automotive and transports, cotton thermal degradation, and thus its flammability, have been the main focus of several scientific studies, also aimed at finding potential strategies to limit or solve these issues. The huge interest in understanding cotton thermal degradation in air and flammability is due to the incessant market demand for natural textiles that intrinsically possess high features like transparency, comfort, hand and, at the same time, outstanding performances (including flame retardancy and/or UV resistance). In particular, both industrial and academic efforts are continuously focused on the possibility of reducing cotton ignitability, and thus its combustion, by modifying its thermal properties. Indeed, flaming combustion is a gas-phase oxidative process that requires oxygen (or air) from the atmosphere. Thus, prior undergoing combustion, cotton first degrades, giving rise to combustible species, which can mix together with atmospheric oxygen, hence fuelling a flame. Because of the exothermicity of the flame, when the heat transferred to the material surface is sufficient, it may cause further degradation, and a self-sustaining combustion cycle can occur. Thus, most of the flame retardants (FRs) commonly employed are designed for modifying cotton thermal degradation, by promoting the formation of a thermally stable carbonaceous structure (called char), instead of releasing volatile species able to further fuel the degradation in air and the combustion of the material. By this way, although a significant improvement of cotton thermal stability is not achieved, a strong modification of its degradation mechanism takes place.2
The present work is aimed to historically review the development of the synthetic strategies carried out up-to-now by industrial and academic researchers, through which cotton combustion has been tackled in the last century. The experience of past and current efforts will be thoroughly described on the basis of the results collected mainly by the scientific community. One of the most important issues when a new FR is usually designed, and more specifically for cotton, is to exploit its chemical structure for covalently linking the FR to the substrate: this process should not modify the physical properties of the fabric, with particular attention to the mechanical features. For this reason, the first paragraphs of this review will be devoted to the description of the chemical structure, reactivity/chemistry and physical properties of cotton.
1.1. Chemical structure of cotton
Cotton fibres are composed of mostly α-cellulose (88.0–96.5%);1 the other constituents, commonly called “non-cellulosics”, include proteins (1.0–1.9%), waxes (0.4–1.2%), pectins (0.4–1.2%), inorganics (0.7–1.6%), and other substances (0.5–8.0%) and are located either on the outer layers (cuticle and primary cell wall) or inside the lumens of the fibres; in reverse, the secondary cell wall is purely cellulose (Fig. 1). The specific chemical compositions of cotton fibres change by their varieties, growing environments (soil, water, humidity, temperature, pest, etc.) and maturity.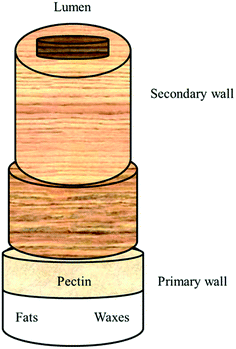 | ||
| Fig. 1 Cotton fibre structure. Data taken from ref. 1. | ||
The primary cell walls of cotton fibres contain less than 30% cellulose, non-cellulosic polymers, neutral sugars, uronic acid and various proteins. Here, cellulose has a lower molecular weight, with a degree of polymerization (DP) between 2000 and 6000. The secondary wall of the cotton fibre is nearly 100% cellulose with a higher DP (about 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000). The high molecular weight cellulose characteristic of mature cotton has been detected in fibres as young as eight days old. Among the non-cellulosic components in the cotton fibres, waxes (namely, carnauba derivatives consisting mostly of aliphatic esters (40 wt%), diesters of 4-hydroxycinnamic acid (21.0 wt%), ω-hydroxycarboxylic acids (13.0 wt%), and fatty acid alcohols (12 wt%) and pectins (poly(β-1,4-polygalacturonic acid)), rhamnose, arabinose, galactose, 2-O-methylfucose, 2-O-methylxylose and apiose) are primarily responsible for the hydrophobicity or low water wettability of raw cotton. Alcohols and higher fatty acids, hydrocarbons, aldehydes, glycerides, sterols, acyl components, resins, cutin, and suberin are found in the wax portion of the cuticle in different quantities, as well. Although proteins are located primarily in the lumen, small amounts of hydroxyproline-rich proteins are present on the fibre surface. Among the inorganic substances, the presence of phosphorus in the form of organic and inorganic compounds is of great importance for the scouring process employed for preparing the fibres for dyeing. These phosphorus compounds are soluble in hot water, but become insoluble in the presence of alkali earth metals.1
000). The high molecular weight cellulose characteristic of mature cotton has been detected in fibres as young as eight days old. Among the non-cellulosic components in the cotton fibres, waxes (namely, carnauba derivatives consisting mostly of aliphatic esters (40 wt%), diesters of 4-hydroxycinnamic acid (21.0 wt%), ω-hydroxycarboxylic acids (13.0 wt%), and fatty acid alcohols (12 wt%) and pectins (poly(β-1,4-polygalacturonic acid)), rhamnose, arabinose, galactose, 2-O-methylfucose, 2-O-methylxylose and apiose) are primarily responsible for the hydrophobicity or low water wettability of raw cotton. Alcohols and higher fatty acids, hydrocarbons, aldehydes, glycerides, sterols, acyl components, resins, cutin, and suberin are found in the wax portion of the cuticle in different quantities, as well. Although proteins are located primarily in the lumen, small amounts of hydroxyproline-rich proteins are present on the fibre surface. Among the inorganic substances, the presence of phosphorus in the form of organic and inorganic compounds is of great importance for the scouring process employed for preparing the fibres for dyeing. These phosphorus compounds are soluble in hot water, but become insoluble in the presence of alkali earth metals.1
1.2. Cotton cellulose chemistry
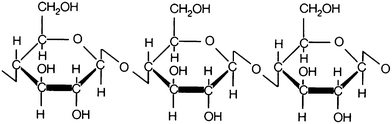
Cotton cellulose is highly crystalline and oriented; cellulose, (C6H10O5)n, is an organic compound consisting of a linear chain made up from several hundred to over ten thousand β(1 → 4) linked D-glucose units or, in simpler terms, it is made of repeated units of the monomer glucose (Fig. 2). Cellulose from plants is usually oriented in β crystalline form with a long and rigid molecular structure.3The steric effects prevent free rotation of the anhydrogluco-pyranose C–O–C link. Each anhydroglucose contains three hydroxyl groups, one primary on C(6) and two secondary on C(2) and C(3). The abundant hydroxyl groups and the chain conformation allow extensive inter-molecular and intra-molecular hydrogen bonding to further enhance the rigidity of the cellulose structure. Chemical reactions and heating effects on cotton cellulose depends on the supra-molecular structure as well as on the activity of the C(2), C(3) and C(6) hydroxyl groups.
Heat or reactions begin in the more accessible amorphous regions and the surfaces of crystalline domains. Chemical reactivity of the cellulose hydroxyl groups is similar to that of aliphatic hydroxyl groups, i.e. it is higher for the C(6) primary than the secondary on the C(2) and C(3). Etherification and esterification are the two main reactions that may take place: more specifically, the esterification reactions (e.g. nitration, acetylation, phosphorylation, and sulphation), are usually carried out under acidic conditions, while etherifications are favoured in an alkaline medium.
Among the esterification reactions, the post-treatments of fibres known as finishing processes capable of conferring superior features to cotton fibres are the most representative; in particular, the flame retardant treatments (quite often phosphorylation reactions) are carried out exploiting this route.4
Cellulose is readily attacked by oxidizing agents, such as hypochlorites, chlorous, chloric, and perchloric acids, peroxides, dichromates, permanganates, periodic acid and nitrogen tetroxide.1
1.3. Thermal stability of cotton cellulose
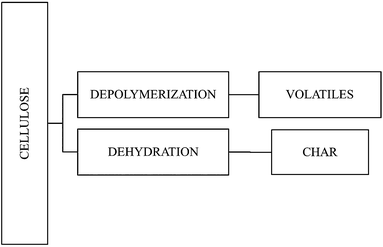
From an overall point of view, heating causes dehydration and decomposition (more specifically, depolymerization) of cellulose5 (Fig. 3). These reactions are influenced by the presence of impurities as well as by temperature and heating rate. Dehydration reactions are favoured in the presence of acid catalysts whereas depolymerization reactions are privileged in alkaline media. When a polymer is heated, several thermal decomposition steps may occur at different transition temperatures, thus affecting its ultimate flammability.Heating at low rates favours dehydration and enhances the subsequent formation of the char (carbonaceous multi-lamellar structure).6 Higher heating rates cause depolymerization with consequent rapid volatilization via the formation of levoglucosan, forming more gaseous combustible products. Greater dehydration also reduces the yield of levoglucosan and subsequently lowers the volatile species.5
In a simple way, it is possible summarise the thermal decomposition of cotton cellulose in air as follows:
• Up to 120 °C heating drives off moisture without affecting strength.
• Beyond 150 °C heating causes a significant reduction of solution viscosity, molecular weight and tensile strength.
• Between 200 °C and 300 °C, volatile products and liquid pyrolysate, mainly 1,6-anhydro-β-D-glucopyranose (levoglucosan) evolve.
• At 450 °C, only char remains.
Of the total amount of pyrolytic products, 20% belong to the gaseous phase (CO, CO2, CH4), 65% to the liquid phase (of which 80% is levoglucosan and furan derivatives) and 15% forms the solid char. The heating rate can affect the amount of char formation:7 indeed, the lower is the heating, the higher is the char amount left by cellulose.
1.4. Physical structure of cotton
Cotton was the dominant fibre of the 20th century; at present it is only exceeded in volume by polyester. Partly the dominance of cotton textiles is due to the economics of production, distribution and manufacture, but it also results from the combination of structure and physical properties. From a physical viewpoint, cellulose molecule is a ribbon-like structure of linked six-membered rings with hydroxyl groups projecting out in the plane of the ribbon (Fig. 2). The covalently bonded chain molecule is further stiffened by internal hydrogen bonds, which, parallel the oxygen bridges between the rings. Under a tensile stress, the molecule exhibits high modulus and high strength, and it has high rigidity for bending in the plane; however, it can easily twist or bend out of the plane.1Exact determination of molecular weight is difficult, because of the problems of dissolving cellulose; however, the distribution of molecular weights is usually centred at about 2 × 105 (MW distribution ranges between 104 and 5 × 106), which corresponds to a chain length of 0.5 mm with a molecular aspect ratio of 1000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (average values). Cellulose crystallises with lattices, in which hydrogen bonds between hydroxyl groups link the molecules into sheets, showing weak van der Waals interactions. Natural cellulose fibres, including cotton, have a crystal lattice known as cellulose I, which differs from cellulose II in cellulose regenerated from solution or treated with a strong swelling agent.1 X-ray diffraction studies proposed a unit cell for cellulose I, with 1.03 nm for two repeats along the chain axis, 0.835 nm for spacing between neighbouring chains in the sheets, and 0.79 nm between two equivalent sheets, which are separated by a staggered sheet of anti-parallel chains. However, the question of whether the chains are parallel or anti-parallel is controversial.
1 (average values). Cellulose crystallises with lattices, in which hydrogen bonds between hydroxyl groups link the molecules into sheets, showing weak van der Waals interactions. Natural cellulose fibres, including cotton, have a crystal lattice known as cellulose I, which differs from cellulose II in cellulose regenerated from solution or treated with a strong swelling agent.1 X-ray diffraction studies proposed a unit cell for cellulose I, with 1.03 nm for two repeats along the chain axis, 0.835 nm for spacing between neighbouring chains in the sheets, and 0.79 nm between two equivalent sheets, which are separated by a staggered sheet of anti-parallel chains. However, the question of whether the chains are parallel or anti-parallel is controversial.
The density of the cell wall of cotton is 1.55 g cm−3 (dry material), 1.52 g cm−3 (cotton conditioned at 65% room humidity) and 1.38 g cm−3 (wet material).1
A standard half-change period of 12 h applies to a slab of cotton fibres 2.5 cm thick, with a density of 0.5 g cm−3 when dry, at a regain of 7% and a temperature of 18 °C. The time increases linearly with (volume/surface area);2 it increases with package density from 1/6 of standard value at 0.09 g cm−3 to 1.33 times at 0.7 g cm−3. Due to hysteresis, the equilibrium regain is higher when humidity is increased than when humidity decreases. Initially, from the dry state, there is an increase of density due to water molecules occupying ‘empty space’ within the fibre. The density reaches a maximum value at about 2% regain, and then decreases since the density of water is less than that of the fibre. Above 20% regain, the volume increase equals the equivalent volume of liquid water. The swelling of cotton fibres is predominantly in the transverse direction. It has been demonstrated that, moving from the dry to the wet state, the increase in length is 1.2% but the increase in diameter is 14%. However, these quantities are difficult to measure because of the lumen, the cross-sectional shape of cotton fibres, and changes in helix angles and twist angle of convolutions as a result of swelling. This aspect is really important for the finishing processes, in particular when some chemicals like FRs should be absorbed within cotton fibres as in the case of metal salts. In addition, cotton combustion can be deeply affected by the presence of water or humidity, as recently demonstrated.8,9
| Cotton type | Fineness [dtex] | Modulus [N per tex] | Tenacity [N per tex] | Work of rupture [mN per tex] | Elongation at break [%] |
|---|---|---|---|---|---|
| St Vincent, Sea Island | 1.00 | 7.3 | 0.452 | 15.0 | 6.80 |
| Uppers, American | 1.84 | 5.0 | 0.323 | 10.7 | 7.10 |
| Bengals, Indian | 3.24 | 3.9 | 0.185 | 5.0 | 5.60 |
The weakest cottons, such as those grown in India, now have been replaced by improved varieties. Cotton falls in the category of weak and less extensible general textile fibres. The toughness (work of rupture) values are low, in comparison with many other textile fibres. Usually, longer and finer fibres show greater tenacity, which is a measure of strength, modulus and stiffness.
Stress–strain curves of cotton derived from different varieties and of the same cotton sample conditioned at different relative humidity are shown in Fig. 4a and b.
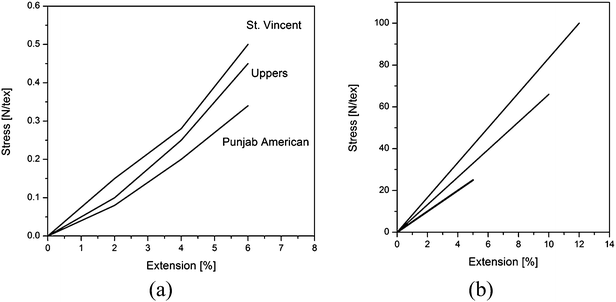 | ||
| Fig. 4 Stress–strain curves of cotton derived from different varieties and of the same cotton sample conditioned at different relative humidity1 | ||
During finishing treatments, unfortunately, the use of chemicals like FRs can affect cotton tensile properties and elastic recovery, in particular when these molecules have been deposited on fibres via impregnation and exhaustion, padding or applied as coatings.
1.5. Cotton production and consumption
Cotton is the most important natural fibre of the 20th century. In a development context, cotton is crucial for income and employment provided in its production and processing. A consistent part of the growth of cotton production since the end of the Second World War was ascribed to improved yield (output per hectare more than quadrupled between 1945/46 and 2006/07, from 0.2 tons per hectare (t ha−1) to 0.8 tons per hectare, according to the International Cotton Advisory Committee – ICAC), rather than to expanded area (the cultivated land increased by only 35% over the 1945/46-2006/07 period, expanding from 22.3 million hectares to 34.8 millions). The development of the cultivated area mainly occurred at the end of the 1940s and remained relatively unchanged since then.10In 2007, cotton was grown in 90 countries. In 2006/07, the four main producing countries were China, India, USA and Pakistan and accounted for approximately three quarters of world output. Including also Uzbekistan and Brazil, these six countries would account for 83% of world cotton production. This concentration in cotton production, which appears to increase for several years, has to be put into perspective by considering the impact of domestic policy reforms in the largest cotton producing countries, as well as climatic and sanitary contingencies. As an example, global output increased by 30% between the seasons 1983/84 and 1984/85, rising to 19.2 million tonnes. Most of the growth came from China, where increases in production (Chinese production edged upward from 4.6 million tonnes in 1983/84 to 6.3 million tonnes in the 1984/85 season) were prompted by incentive measures taken by the Government. Cotton production increased by 3.7 million tonnes in the 1992/93 season to 4.34 million tonnes in 1993/94 (16.1% increase). Up to now, 27.2 million tonnes are produced.
Since the beginning of the 1940s, world cotton consumption has augmented at an average annual growth rate of about 2% (roughly the same as its production). Growth in the demand for cotton was comparatively higher in the 1950s and 1980s, with an average growth rate of 5% a year during the 1950s and 3% in the 1980s. Developing countries have absorbed much of global cotton output since the end of Second World War. Their share in global consumption has become even more significant since the beginning of 2000s. Developing countries accounted for approximately 78% of global cotton consumption between 1981 and 1999; since 2000, their ratio has been above 80 and now 94% of global cotton output.
The main cotton producing economies also account for a large part of consumption. According to ICAC data, China, USA, India, and Pakistan as a whole have accounted for approximately more than 55% of global cotton consumption over the period 1980 to 2008. Their overall consumption has risen considerably in volume (3 times in China and more than 3 times in India). Pakistan has undergone the largest increase in volume (which multiplied by 6 between 1980 and 2008) for responding to export-driven demand for textiles.
Cotton production and consumption are due to its widely use for different applications. More specifically, for what concerns its employment in flame retardancy, protective garments (coveralls, uniforms, lab coats, etc.), furniture (bed linen, mattresses, curtains, armrests, etc.) and transports (seat blanket, curtains, etc.) are the most important uses.
2. Cotton flammability
What is a flammable material? A material is said to be flammable if it is susceptible to easy ignition and rapid flaming combustion on the basis of the ASTM E176 definition (1981).11 Subsequently, it was maintained and included in the ISO 13943 standard (2008).12When a polymer is heated, several thermal decomposition steps may occur at different transition temperatures, thus affecting its ultimate flammability, as already described for cotton (see Section 1.3). Table 2 presents the comparison among some thermal properties of cotton with those of commonly available fibres, in terms of degradation or pyrolysis temperatures (Td or Tp) and ignition flaming combustion (Tc) values.13 The combustion enthalpy (ΔHc) is reported, as well. Usually, the lower is Tc (and usually Td), the more flammable is the fibre. This generalisation is typical of natural cellulosic fibres such as cotton, viscose and flax, as well as of some synthetic fibres like acrylics. Furthermore, Table 2 collects the Limiting Oxygen Index (LOI) values, which represent the minimum volumetric concentration of oxygen, expressed as a percentage that will support the polymer combustion.14 LOI has been considered a good flammability index for a long time both by industrial and academic researchers, although it is questionable that the collected results can be correlated with those of any other test and in particular with those of a real fire scenario. Indeed, it is common opinion that LOI can be considered only a useful tool for defining candle-like ignition, and is of no value for real world fire safety.15
| Fibre | Td [°C] | Tc [°C] | ΔHc [kJ g−1] | LOI [%] |
|---|---|---|---|---|
| Wool | 245 | 600 | 27 | 25 |
| Cotton | 350 | 350 | 19 | 18.4 |
| Polyamide 6 | 431 | 450 | 39 | 20–21.5 |
| Polyester | 420–447 | 480 | 24 | 20–21 |
| Modacrylic | 273 | 690 | — | 29–30 |
| meta-Aramid (e.g. Nomex®) | 410 | >500 | 30 | 29–30 |
| para-Aramid (e.g. Kevlar®) | >590 | >550 | — | 29 |
However, generally speaking, fibres like cotton that exhibit LOI ≤ 21.0% (the standard oxygen content in air) are considered very flammable; they turn to be moderately flammable when LOI is within 21.0 and 25.0% (wool, polyamide and polyester) and show a limited flammability when LOI is beyond 25.0% (modacrylics, meta- and para-aramids), so that they start to pass various national and international standard tests adopted for flame retardant textiles.
2.1. Cotton thermo-oxidation and combustion
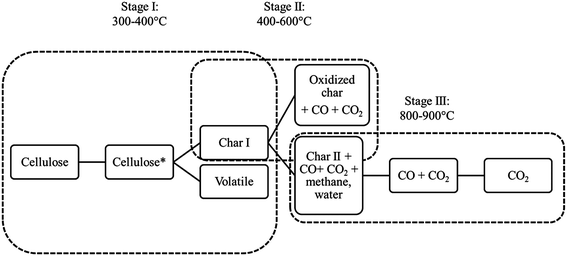
Before a polymer undergoes flaming combustion, it must first decompose releasing flammable volatiles. Referring to cellulose, its decomposition has been extensively studied. Whilst other more detailed mechanisms have been reported in the scientific literature,16 the basic proposed processes agree with that firstly described by Shafizadeh and Bradbury, who suggested the formation of an ‘activated’ cellulose species (‘Cellulose*’), during the early degradation stages. This activated species undergoes further reactions depending on the temperature regime, as presented in Stage I of the reaction scheme, Fig. 5.While the existence of this Cellulose* species is controversial, the experimental evidence of a free radical for the Cellulose* species was assessed by Price et al.17 At low temperatures, oxygen plays a dominant role in cellulose degradation, as pyrolysis has been shown to be faster in an oxidative atmosphere than in inert conditions; nonetheless, at higher temperatures, degradation products are slightly affected by the chosen atmosphere.16 Oxygen catalyses the evolution of volatiles as well as of char-promoting reactions7 (sometimes called charring). This mechanism has been recently shown to be heating rate dependent at very high heating rates (100–300 °C min−1).5 In particular, the balance between the two subsequent competitive processes at 300–400 °C in Stage I, namely depolymerisation and dehydration, schematised in Fig. 5, determines the ease of ignition. Condensed phase flame retardants primarily influence this stage, as they usually enhance the dehydration, charring reactions at the expense of volatile fuel formation. Depolymerisation is initiated by the scission of acetal bonds of the glycosidic units, followed by splitting of volatile fuel-forming levoglucosan, the cyclic monomer of cellulose, from ensuing chain ends. Competing dehydration reactions lead to thermally stable aliphatic structures (char I), which subsequently are converted via Stage II into aromatic structures (char II), with the evolution of water, methane, carbon mono and dioxide (400–600 °C). Char II (ca. 18%) is thermally stable at least up to 800 °C (Fig. 5). As a consequence, the overall degradation process is the result of several competing reactions; furthermore, the yield of volatiles and the yield and thermal stability of the final char depend on the kinetic control exerted by the chemical degradation reactions and therefore on the adopted heating rate. Thus, the char produced by cotton degradation in nitrogen at 10 °C min−1 is thermally stable up to 800 °C, while, at very high heating rates, it decomposes at much lower temperatures. When the degradation occurs in the presence of atmospheric oxygen, a further heating rate effect appears, depending on the sensitivity to oxygen of the chemical degradation reactions occurring in the polymer condensed phase, in which oxygen diffusion is heating rate dependent. As an example, cotton heated at low heating rates in air gives a larger char yield than at high heating rates with variable thermal stability. These findings suggest that cotton possesses intrinsic features for protecting itself toward the thermo-oxidation, inhibiting the production and release of volatile species that can further favour its degradation and hence its combustion. On the other hand, the tendency of cotton to form a thermally stable residue (char) represents the most promising way, through which it is possible to make it flame retarded. Indeed, the most valuable commercially-available FRs (ammonium salts, Proban®, Pyrovatex®) act in the condensed phase favouring the char formation: this approach seems to be the only possibility also for polymers that are not intrinsically char formers like polyolefins.
2.2. Thermal tests
Thermal stability represents one of the most important issues in the design of a new flame retardant for fabrics, more and more for a very flammable fibre like cotton. More specifically, before ignition, any polymeric material undergoes thermo-oxidation (i.e. thermal degradation in air) and then starts to burn. Thus, in order to develop a new FR, it is necessary to thoroughly investigate its behaviour toward thermo-oxidation. Usually, this issue is studied by thermogravimetry (TGA) that is considered the most widely employed tool for assessing the thermal and thermo-oxidative degradation of polymers. Indeed, it supplies quantitative results regarding weight loss of a sample and corresponding rate, as functions of temperature or heating time (in isothermal conditions), provided that thermal effects and temperature gradients across the sample are taken into account and properly corrected. These measurements are often carried out at lower heating rates (namely 2–20 °C min−1) as compared with those occurring during the thermo-oxidation triggered in combustion tests. Only recently, more sophisticated instrumentations (like fast TGA, flash pyrolysis, …) are becoming available for mimicking what really happens during combustion. Notwithstanding this, a good agreement between standard TGA performed at low heating rates and combustion tests can be found, as reported in the scientific literature.5,182.3. Regulatory and testing requirements for flame retardant textile applications
Very recently, Horrocks thoroughly reviewed the regulatory and testing requirements for flame retardant textiles on the basis of their applications:13 in the following, the complex scenario regarding the flame retardancy of cotton will be summarized.Most of the fire regulations relating to textiles (and thus cotton) are designed:
• To prevent facile ignition of textiles in the first instance,
• To offer potential victims more time to escape, or
• To provide protection of the body or parts of the body from fire.
Those responsible for issuing fire regulations fall into a number of categories including:
• National governments: typically all national governments within the European Union (EU) issue their own fire regulations, which can now fall, if relevant, within an overarching EU directive and its requirements. Related standards may be issued also by national standards organisations such as American Society for Testing and Materials (ASTM), BS (British Standard) and Deutsche Industrial Norms (DIN) as well as International organisations such as European Standards (CEN) and International Organization for Standardization (ISO). In the USA, standards are also issued by the Consumer Product Safety Commission (CPSC) and the National Fire Protection Association (NFPA); federal regulations may specify them as appropriate.
• State or provinces: in the US, states such as California issue their own fire regulations, which may differ from national or federal regulations.
• International organisations: they are responsible for transports such as civilian air and marine transport.
While textile-related fire regulations among different countries may offer an overall confusing picture in terms of the items regulated and the applications covered by them, in general, regulations fall into different categories depending on whether they apply to the normal consumer living in a domestic environment, a member of the public in a public environment (hotel, airport, public building including hospitals and prisons), in the workplace for worker protection, for personal protection in the emergency and defence services or in transport, where escape by passengers and personal is restricted. Thus regulations generally cover:
• Nightwear (domestic environment).
• Protective clothing (workplace, civil emergency and defence).
• Bedding (domestic and contract or public).
• Upholstered furnishings (domestic and contract or public).
• Transports (land, marine and air).
As it is clear, the number of regulations and thus tests/standards is enough wide and depends on the application field. Although this review is not addressed to deeply describe them (as recently reported in ref. 13 by the same authors), some examples for nightwear, protective clothing, bedding and transports will be briefly shown hereafter. As an example, Table 3 lists selected international nightwear fire regulations and related standards prior to 2000.
| Country | Test standard | Face (F)/edge (E) | Flame height [mm] | Testing type | Ignition time [s] |
|---|---|---|---|---|---|
| Germany/France | EN ISO 6940 | F–E | 40 | Ignitability | 0–20 |
| EN ISO 6941 | F–E | 40 | Flame spread | 10 | |
| EN 1103 | F | 40 | Flame spread/flash/flaming debris | 10 | |
| Netherlands | Normen Europäischer Modellbahnen 1722 based on EN/ISO 6941 | F–E | 40 | Flame spread | |
| Ireland | ISO 148 | F | 45 | Flame spread | 10 |
| UK | BS 5722 | F | 45 | Flame spread | 10 |
| AS/NZS | AS/NZS 1249 | F–E | 40 | Flame spread | 5–15 |
| Denmark, Finland, Norway and Sweden | Nordtest FIRE 029 | F | 16 | Flame spread | 1–20 |
| ASTM 1230 | 40 | Flame spread | 1 | ||
| ASTM 1320 | 40 | Flame spread | 1 | ||
| Germany | Dutch covenant | F | 40 | Flame spread | 5 spread 1 flash |
| Flash | |||||
| Flaming debris | |||||
| USA, Norway and Sweden | ASTM D1230 | F | 16 | Flame spread | 1 |
Most of the national test methods listed in Table 3 were and are still based on existing national standards, which outside of the respective country of origin do not have acceptance. As a consequence, the new standard EN 14878 was promulgated in 2007.19 This standard covers all types of nightwear, including nightdresses, nightshirts, pyjamas, dressing gowns, and bath robes. It provides requirements for an absence of surface flash and a maximum burn rate acceptable for different categories of nightwear garments. It also places responsibility on the manufacturer to ensure that any used flame retardant chemicals are effective throughout the life of the garment and do not present health hazards. This standard defines the following classes:
• Class A – all nightwear (except pyjamas): there shall be no surface flash and the 3rd marker thread (520 mm) must not be severed in less than 15 s.
• Class B – pyjamas: there shall be no surface flash and either the 3rd marker thread (529 mm), in combination with certain required design criteria (including hem circumference, sleeve cuff, and bottom trouser width dimension), must not be severed in less than 10 s, or the burn rate from Class A is applicable without the design criteria.
• Class C – babies' nightwear (up to 6 months): NO criteria.
This standard does not override existing legislation in any EU country, which must still be complied with (e.g., UK, Ireland) and which are more stringent with regards to burn rate. However, additional aspects covered by this standard include no surface flash and requirements for terry towelling bathrobes and pyjamas and children's nightwear from ages 13–14.
As far as protective garments are concerned, the Directive on Personal Protective Equipment (PPE) 89/686/EEC (European Economic Community) of 1989 belongs to the family of directives under Article 114 of the Treaty on the functioning of the EU. In the case of protective clothing, these directives harmonise products to ensure a high level of protection for citizens throughout Europe. National regulations incorporate the requirements of the relevant directive for the various protective clothing types. As an example, in the UK need for protective clothing is regulated by the Health and Safety at Work Act of 1974.13 In the main, protective clothing falls into one of two groups:
• Protective clothing for work-wear, hazardous industrial occupations, fire-fighters and defence personnel.
• Extreme hazard protection, e.g., furnace operators' aprons to protect against hot metal splash, fire entry suits, racing car drivers' suits.
A number of CEN testing methods for heat and fire protective clothing are included within:13
• ISO 11613 – protective clothing for fire-fighters – laboratory test methods and performance requirements.
• BS EN 469 – protective clothing for fire-fighters – performance requirements for protective clothing for fire-fighting.
• BS EN IS0 11611 – protective clothing for welders; a unique aspect is that it contains a note as to the dangers of ultraviolet radiation from welding processes in the context of potential skin cancer.
• BS EN ISO 11612 – protective clothing – clothing to protect against heat and flame. This is a complex performance specification providing a choice of several main performance levels to a variety of heat sources including molten metal splash protection plus one extreme level of heat protection. It also sets design criteria for garments and seams.
• BS EN ISO 14116 – protection against heat and flame – limited flame spread materials, material assemblies and clothing. This classifies clothing materials subjected to ISO 15025, which allows determining the extent of damage to a fabric sample subjected to a small flame. The final index gives a measure of both flame resistance and durability.13
In the USA, there are no federal organizations dealing with protective clothing but several regulatory agencies such as the Occupational Safety and Health Administration (OSHA) and the US Departments of Defence and of Transportation (which use a standard vertical flame test like ASTM D6413). This requirement is essentially regulated as a mandatory standard, since most legislative requirements of the regulatory agencies include a ‘general duty clause’: as an example, under OSHA an employer is required to maintain ‘a safe and healthful workplace’.13
Referring to upholstered furniture, furnishing and bedding regulations, Table 4 lists the current regulations relating to furnishings and furniture in Europe and the USA. This area has been very recently reviewed in detail by Nazaré and Davis20 and thus will not be reviewed here.
| Country | Test method | Domestic/contract or public |
|---|---|---|
| UK | BS 5852 Part 1 (bedding) | Domestic |
| BS 5852 Part 2 and BS 7176 (seating) and BS 7177 (bedding) | Contract/public | |
| France | EN ISO 12953 Parts 1 and 2 | Domestic |
| EN 597 Parts 1 and 2 | Public | |
| Italy | CSE RF/4/83 | Public |
| Finland | EN 1021 Part 1 | Domestic |
| EN 1202 Part 1 and EN 597 Part 1 | Domestic/public | |
| Germany | DIN 4102 | |
| USA California Federal | Technical Bulletin 116 | Domestic |
| Technical Bulletin 117 | ||
| CPSC 16 CFR Parts 1632, 1633 and 1634 |
As far as regulations and tests relating to transports are considered, textiles are generally associated with seating, floor-coverings and other furnishings within the vehicle or vessel interior. Within the defence, civil emergency and industrial sectors, similar associations may be set, although protective clothing and other safety/protection-related equipment will comprise textile components. In most of these transport applications where safety is an issue, there are national or international regulations that govern their fire performance requirements. Cars may be included here because of their many textile components employed in internal passenger compartments (seating, carpet and internal side and roof lining fabrics), which require a defined level of flame resistance.
In aircraft, all the internal textiles comprising seating, internal decor and blankets require specific levels of flame or fire resistance defined on the basis of internationally recognised standards.
In any surface marine vessels, the involved fire safety issues require solutions similar to those adopted for aircraft interior textiles. A detailed description of the different standards referring to transports is reported in ref. 13.
2.4. Flammability tests
It is probably correct to state that nearly every developed country has its own set of textile fire testing standard methods, which, together with those defined by other national and international bodies (i.e. air, land, and sea transport authorities, insurance organisations and governmental departments relating to industry, defence and health), show a very complex picture. Table 5 attempts to give a glimpse of tests currently available for textile products. All these tests can be categorizes as follows:13| Test type | Nature of test | Textile type | Standard | Ignition source |
|---|---|---|---|---|
| Simple fabric strip tests | Vertical strip method | Curtains and drapes | BS 5867 Part 2 | Small flame |
| Nightwear | BS 5722 | Small flame | ||
| Vertical strip method | Vertical fabrics | BS EN ISO 6940 Part 1 | Small flame | |
| ISO 15025 | ||||
| US strip tests | Vertical fabrics | ASTM D6413 | Small flame | |
| Horizontal fabrics | FMVSS 302 | Small flame | ||
| Textile composite and product tests | Apparel not for protective clothing | Vertical clothing fabrics | BS EN 1103 | Small flame |
| UK small-scale composite test for furnishing fabric/fillings | Furnishing fabrics | BS 5852 Parts 1 and 2 | Cigarette and simulated match flame | |
| BS 5852 or ISO 8191 Parts 1 and 2 | Small flames and wooden cribs applied to small and full scale tests | |||
| BS EN 1021 Parts 1 and 2 | Cigarette and simulated match flame | |||
| Bedding (mattresses) | BS 6807 | Cigarette and match ignition tests | ||
| BS EN 597 Parts 1 and 2 | ||||
| BS EN ISO 12952 Parts 1 and 2 | International standard for cigarette and match ignition testing | |||
| Carpets | BS 6307 and ISO 6925 | Methenamine pill ignition source | ||
| BS EN ISO 9239 Part 1 | Reaction to fire test using a radiant source at 30° (∼10 kW m2) | |||
| Curtains | BS EN 13772 | Uses EN ISO 6941 fitted with an additional radiator source | ||
| BS 5867 Part 2 | Uses BS EN ISO 6941 for domestic and ISO 15025 for contract fabrics | |||
| BS EN 1102 | Uses EN ISO 6941 with 10 s ignition time | |||
| Tests undertaken with the addition of radiant heat including reaction to fire tests | Use of radiant flux plus specified ignition | Carpets: fabrics/composites often for use in seatings | EN ISO 9239 Part 1 | Irradiate with 30° gas heated panel (∼10 kW m−2) with small burner |
| NF 92503, French ‘M test’ | ||||
| Aircraft seat assemblies, so-called ‘Boeing’ test | ASTM E906 and FAR 25.853 Part 4 | Irradiate under 35 kW m−2 with small flame ignite | ||
| Thermal protection (including protective clothing and manikin tests) | Protective clothing: general requirements | BS EN 340 | Design, comfort, durability and labelling requirements. | |
| Resistance to radiant heat | — | BS EN ISO 6942 | Exposure to radiant source | |
| Resistance to convective heat (flame) | — | BS EN 367 | Determine heat transfer index | |
| Resistance to molten metal splash | — | BS EN ISO 9185 | Molten metal | |
| Gloves against thermal risks | — | BS EN 407 | Composite standard (including fire-fighters' and welders' gloves) | |
| Fire-fighters clothing | — | BS EN 469 | Composite standard | |
| Welders' and allied industrial clothing | — | ISO 11611 | Composite standard | |
| Protective clothing against heat and flame | — | BS EN ISO 11612 | Composite standard | |
| Protection against limited heat and flame | — | BS EN ISO 15025 | Small flame | |
| Protective clothing – protection from limited flame spread | — | BS EN ISO 14116 | Damage definition enables fabric classification | |
| Contact heat transmission | — | BS EN 702 | Contact temperatures 100–500 °C | |
| Fire-fighters' hoods | — | EN 131911 | — | |
| Instrumented manikin testing of whole garments | — | BS ISO 13506 | Prediction of burn injury in terms of 1st, 2nd and 3rd degree burn propensity | |
| Durability tests | Cleansing and wetting procedures for use in flammability tests | All fabrics | BS 5651 | Used on fabrics prior to submitting for standard ignition tests |
| Commercial laundering | BS EN ISO 10528 | — | ||
| Domestic laundering and dry cleaning | BS EN ISO 12138 | — | ||
| BS EN ISO 6330 |
• Simple fabric strip tests.
• Textile composite tests.
• Tests undertaken with the addition of radiant heat (including reaction to fire tests).
• Thermal protection tests (including protective clothing and manikin tests).
Many of these tests require samples to have undergone some “durability” tests, as well.
The complexity of the burning process for a textile material (indeed, this latter is a ‘thermally thin’ system, but, at the same time, it possesses a high specific volume and oxygen accessibility as compared to other polymeric materials) is difficult to quantify, particularly regarding its ignition and post-ignition behaviour. Most of the common textile flammability tests are currently based on ease of ignition and/or burning rate behaviour, which can be simply quantified for different geometries of fabrics and composites. However, few tests yield quantitative and fire science-related data, unlike the often underestimated LOI.15 This value, while it proves to be a very effective indicator of ease of ignition, has not achieved the status of an official test within the textile arena. For instance, it is well known that in order to provide a fabric with a flame retardant degree sufficient to pass a typical vertical strip test, a LOI value of at least 26% is required, which must be measurable in a reproducible fashion. However, since the sample ignition occurs at its top, giving rise to a particular vertically downward burning geometry, LOI cannot be associated with the most typical ignition geometries. Furthermore, being constant the fibre type, the exact LOI value is influenced by fabric structural variables and is not single-valued for a given fibre type or blend. However, it finds a significant use within the design, development and application of novel flame retardants for fibres and fabrics.
2.5. Flammability tests discussed in the scientific literature
In order to assess the efficiency of FRs, different approaches have been carried out and published in the scientific literature; it is worthy to note that sometimes they do not perfectly correspond to current regulatory tests. Usually, two types of tests can be exploited, i.e. the resistance of the flame retarded fabrics to a flame application (in horizontal or vertical configuration) or to an irradiative heat flux, generated by an electrical resistance like a cone calorimeter21,22 or a radiating panel.In the former case, rectangular specimens of different sizes are ignited by a flame, the characteristics of which (length, gas type, …) as well as its application times are chosen on the basis of the employed standard that depends on the application field. Two types of tests can be carried out: more specifically, the specimen can be placed in horizontal or vertical configuration; usually, a methane flame (25 mm length) is applied on the short site of the specimen for at least 3 s. Important parameters like total burning time and rate, as well as the final residue are measured. An efficient flame retardant must be able to suppress the fabric combustion, leading to the self-extinguishment of the material; on the other hand, if the flame retardant is capable of significantly reducing the burning time and rate, while strongly increasing the final residue, it can be considered as a valuable and suitable choice for several applications.
An alternative method for assessing the resistance of a treated fabric to a flame application is represented by LOI tests. This standard is very simple and rapid, but at the same time very useful as any molecule able to increase the fabric LOI achieving 26% as minimum value13,15 can be classified as a FR. Obviously, the higher is LOI value, the better are the performances of the FR. As already mentioned, however, it is only a useful tool for defining candle-like ignition, and is of no value for real world fire safety.15
When a flame retarded fabric is subjected to an irradiative heat flux (e.g. generated by a cone calorimeter or a radiating panel), an efficient FR must reduce its total heat release, accordingly modifying all the related parameters. To this aim, different tests can be used: quite recently, our research group has set up an optimised procedure employing a cone calorimeter for studying the resistance of a flame retarded fabric.21,22 The measurements are usually carried out under a 35 kW m−2 irradiative heat flux (that can be related to the early stages of a developing fire) in horizontal configuration. Such parameters as Time To Ignition (TTI), Total Heat Release (THR), peak of Heat Release Rate (pkHRR) are evaluated. Other important parameters concerning smokes like Total Smoke Release (TSR), and CO and CO2 release are assessed, as well. From an overall point of view, a valuable FR should increase fabric TTI, reduce THR and pkHRR, and strongly limit the smokes evolved during combustion (TSR, CO and CO2), lowering their optical density.
Very recently, a new instrumentation defined pyrolysis-flow combustion calorimetry (PCFC) has been employed for investigating the flammability of polymers23 and fabrics.24,25 In this context, the pyrolysis products of a certain material (collected generally heating the sample at 60 °C min−1) are further burnt at 900 °C. Also in this case, as in the cone calorimetry, THR and pkHRR are measured as main parameters.
3. Past, present and future perspectives of cotton flame retardancy
The following heading is entirely devoted to historically summarize past experience, current efforts and future perspectives carried out by industrial and academic researchers in the flame retardancy of cotton.3.1. Past: from 1783 to 2010
| Type of additive | Additive amount [phr] |
|---|---|
| Ammonium chloride | 4.2 |
| Ammonium phosphate | 4.5 |
| Ammonium sulphate | 4.5 |
| Zinc chloride | 4.0 |
| Calcium chloride | 4.5 |
| Magnesium chloride | 4.5 |
| Aluminium hydroxide | 3.8 |
| Zinc sulphate | 4.5 |
| Sodium borate | 8.5 |
| Boric acid | 10.0 |
| Magnesium sulphate | 15.0 |
| Sodium chloride | 35.0 |
| Sodium silicate | 50.0 |
| Silicic acid | 30.0 |
| Potassium chloride | 45.0 |
| Sodium phosphate | 30.0 |
| Aluminium borate | 24.0 |
| Aluminium phosphate | 30.0 |
| Calcium phosphate | 30.0 |
| Magnesium phosphate | 30.0 |
| Zinc borate | 20.0 |
| Tungstic acid | >15.0 |
| Sodium tungstate | >15.0 |
| Ammonium tungstate | >10.0 |
| Clay | >5.0 |
Perkin's experience turned out to be very useful for the further developments of new FRs for cotton. However, the older methods for imparting flame retardancy to this material involve the addition of metal salts, which are soluble in water and non-durable, so that they require periodic replacement owing to the action of laundering, rain and perspiration. Thus, non-durable FRs can be divided into three main categories:
(i) Low-melting foam-producing boron-containing compounds.
(ii) Inorganic acids and their salts, which, upon heating, release acids that esterify cotton cellulose hydroxyl groups (mainly C(6)). Relevant compounds include phosphoric, sulphuric and sulphamic acids and their salts, as well as chlorides of tin(IV), zinc(II) and copper(II).
(iii) Inorganic compounds that, upon heating, decompose giving rise to non-flammable gases like carbonates, ammonium salts and certain hydrated oxides and salts.26
Only more recently, semi-durable and durable treatments, which have to withstand usual weathering and at least a limited amount of laundering, have been designed.
Semi-durable FRs are insoluble salts and oxides; other semi-durable systems include chromium(III), tungsten(VI), and tin(IV) hydroxides, which are added as the corresponding salts in the presence of either acids or alkalis in a two-bath technique originally developed at the beginning of this century. A good penetration into the fibre is, however, difficult to attain and a considerable afterglow tends to be encountered. This depends on the typical water absorption and swelling of cotton fibres, as already discussed in Section 1.4.
In the same period, Shafizadeh and coworkers6,7,16 tried to understand the real mechanism, through which cotton cellulose decomposes with and without FRs, with particular attention to the effect of metal salts (already observed by Perkin at the beginning of the century),29–33 phosphorus and phosphorus–nitrogen pairs.33 Indeed, referring to the latter system, the discussion about the effect of phosphorus–nitrogen combination was not clearly defined as antagonism4 or synergism.34 Analogously, Hendrix et al. focused their attention on the effects of triphenyl phosphate in the presence of nitrogenous bases35 on cotton cellulose pyrolysis and flammability. To this aim, some model molecules have been used, as well.36,37
This period was particularly interesting for the development of two FRs still currently used and that represent the two major and commercially dominant generic types of durable flame retardants for cotton and cotton-rich blends, namely those based on tetrakis(hydroxymethyl) phosphonium salt (THPX) condensates and those based on N-methylol dimethylphosphonopropionamide derivatives. The former group is typified by Proban® (Rhodia) product which is based on tetrakis(hydroxymethyl) phosphonium–urea condensate: after padding onto cloth, it is cross-linked by ammonia gas and followed by peroxide oxidation to stabilise the resulting polymeric matrix.27,38 Commercial evidence, that some formaldehyde may be released during use but at much lower levels than in cured N-methylol dimethylphosphonopropionamide (N-MDMPA) derivatives, exists. On the other hand, N-MDMPA derivatives are typified by the former Ciba (and now Huntsman) product Pyrovatex CP®, which is available in various modifications, like the dimethylol derivative (claimed to improve durability and/or reduce formaldehyde release during application and in use). These N-MDMPA derivatives require the presence of a methylolated crosslinking agent in order to ensure the required durability, through the formation of chemical bonds with the cellulose hydroxyl groups. Unlike Proban®, which uses a patented ammonia curing process and requires specialised plant, Pyrovatex® and its many alternative versions may be applied by a conventional pad-dry-cure process.
This period can be considered as the time of unsolved problems and unanswered questions in flame retardance of polymers and in particular of cotton, as stated by Lewin, stressing on the durability of the FR systems available on the market.77 Indeed, in order to replace Proban® and Pyrovatex®, any durable FR suitable for cotton has to fulfil the following conditions, as pointed out by Horrocks:27
• A new FR should have equivalent or superior ease of application,
• A new FR should not release formaldehyde during application or service,
• A new FR should guarantee comparable textile service-life properties in terms of durability, effect on handle and tensile properties,
• A new FR should have an overall comparable cost-effectiveness and preferably be cheaper,
• A new FR should have equivalent or lower toxicological and environmental impacts.
Analogously, Lewin individuated the most stringent requirements that a durable system should have in order to replace the consolidated Proban® and Pyrovatex® systems, that can be summarised as follows:77
• A new FR should be resist to 50 hot alkaline launderings, both in soft, as well as in hard water;
• Cotton fabrics should not lose much of their tensile, tear, and burst strengths as well as their abrasion resistance;
• Cotton fabrics should not lose their high air permeability due to high deposits of the chemicals needed to impart flame retardancy;
• Cotton fabrics should keep their typical soft handle;
• A new FR should not bring any change in the outward appearance and aesthetics of the fabric;
• A new FR should not bring any change in the hue of the dye and/or dyeability of the fabric.
Considering Horrocks and Lewin opinions, it is clear that all these requirements are not easy to fulfil, especially by modifying cotton chemical structure for conferring such a high level of flame retardancy.
Recently, Horrocks briefly summarised the attempts carried out during this period:27 in order to avoid a useless repetition, we listed the most efficient FR systems in Table 7; furthermore, this table collects some other results more recently reported in the literature. In order to better understand the differences among them, a further classification among durable, semi-durable and non-durable treatments has been proposed.
| FR system | FR element | Notes | Reference |
|---|---|---|---|
| Aminomethyl phosphonic acid diamide | Phosphorus–nitrogen pair | Methylolation using formaldehyde is an essential feature for their subsequent reactivity with cellulose anhydroglucopyranose –OH groups. Application with methylolated melamine is required in order to achieve desired durability | 78 |
| Triethylamino phosphine oxides | Phosphorus–nitrogen pair | ||
| Phosphate–phosphonate oligomeric structure | Phosphorus | Fyrol® 51 | 79 |
| Hydroxyl-functional organo-phosphorus oligomer and a multifunctional carboxylic acid | Phosphorus–carboxylic acid pair + nitrogen | Application requires the presence of methylolated resin like dimethyloldihydroxyethylene urea (DMDHEU) or methylated formaldehyde-urea. Resistance up to 12 launderings if the correct cross-linker is chosen although the problem of formaldehyde release will still remain | 80 and 81 |
| Hydroxyalkyl organophosphorus oligomer (Fyroltex®)/trimethyl amine/DMDHEU combination | Phosphorus–nitrogen | Resistance up to 50 laundering durability when applied to nylon (6 or 6.6)/cotton blends (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) 50) |
82 |
| Butyl tetracarboxylic acid (BTCA) phosphorylated with hydroxyalkyl organophosphorus oligomer (Fyroltex®) | Char-forming polycarboxylated coupled with phosphorus | BTCA forms bridge between the phosphorus oligomer and cellulose molecules. Durability is limited as a consequence of calcium salt formation | 83 |
| BTCA phosphorylated with hydroxyalkyl organophosphorus oligomer (Fyroltex®)-triethanolamine (TEA) | Char-forming polycarboxylated coupled with phosphorus + nitrogen | Reduction of calcium salts formation by using a Fyroltex®/BCTA/TEA combination applied to a cotton/Nomex® blend (35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 65). Acceptable durability after 30 home launderings 65). Acceptable durability after 30 home launderings |
83 |
| Maleic acid–sodium hypophosphite | Phosphorus | Acceptable durability after 20 home launderings | 84 |
| Succinic, malic or tartaric acids–sodium hypophosphite | Phosphorus | Acceptable durability after 20 home launderings | 85 |
| Phosphorus-containing maleic acid oligomers–sodium hypophosphite–TEA | Phosphorus–nitrogen | Class 1 passes to 16 CFR 1610 (US Federal Standard for the flammability of clothing textiles) to be achieved | 86 |
| Alkyl phosphoramidate is stabilized as a salt adduct with ammonium chloride | Phosphorus–nitrogen | It is a formaldehyde-free system. It may be applied either in a resin binder or cross-linked using a methylolated resin. It is effective on cotton and cotton–polyester blends with reasonable levels of durability | 79 |
| Diammonium phosphate (DAP), phosphoric acid (PA), tributyl phosphate (TBP), triallyl phosphate (TAP) and triallyl phosphoric triamide (TPT) | Phosphorus | With 4 wt% phosphorus, the highest LOI values were achieved: 35.5, 31.0, 25.0, 25.0 and 26.0% for DAP, PA, TBP, TAP and TPT, respectively | 87 |
| Tributyl phosphate (TBP)–nitrogen bases (urea, guanidine carbonate, and melamine formaldehyde) | Phosphorus–nitrogen | LOI of cotton treated with only TBP is always increased by the presence of nitrogen-bases, regardless of type | 88 |
| Triethyl phosphate (TEP)–diethyl phosphoramidate, –phosphoramidic acid, N(2-hydroxy ethyl) diethylester, –diethyl ethyl phosphoramidate or –diethyl 2-methoxyethylphosphoramidate | Phosphorus–nitrogen | LOI of cotton treated with only TEP is always increased by the presence of phosphoramidate, regardless of type | 89 |
In this period, an alternative development toward the use of phosphorus only or phosphorus coupled with nitrogen involved the exploitation of metal ions: indeed, they may influence the thermal oxidative degradation of cotton cellulose since they are able to catalyse the cellulose dehydration, favouring the formation of high amounts of char. As a consequence, the flame resistance of cotton is strongly affected. Usually, this effect can be observed either when the metal ions are singularly used30,33,57,59,90,91 or in combination with phosphorus-based FRs.51,92–97 Just in '80s, Shafizadeh observed a similar effect on cellulose degradation exerted by metals: in particular, he investigated the role of inorganic additives on cellulose degradation due to their ability to enhance or inhibit smouldering combustion in cotton fabrics.30,33 Thermal analysis showed that the rate of oxidation of chars prepared by pyrolysis of fabrics treated with metal salts enhancing smouldering is higher than that of chars prepared from fabrics treated with additives like phosphates or boric acid, which inhibit smouldering. Definitely, the combustion of cellulosic materials proceeds through the pyrolysis of the substrate by two alternative pathways, which involve decomposition of glycosyl units to form a char and their depolymerization to volatiles containing anhydrosugar derivatives. Oxidation of the volatiles in the gas phase gives flaming combustion and oxidation of the char in the solid phase produces smouldering or glowing combustion. It was observed that the addition of inorganic acid compounds, such as (NH4)2HPO4 or ZnCl2, promotes the decomposition reactions and forms more char than combustible volatiles, and thus suppresses flaming combustion.55 Rather surprisingly, also the addition of NaCl has turned out to be somehow efficient for this purpose.59
As an example, Soares et al. have found that these organometallic additives as Zn-ethylhexanoate, K-ethylhexanoate and Co-ethylhexanoate, strongly modified the decomposition process of cellulose, enhancing the char and reducing the tar formation.90 Furthermore, it was observed that Zn2+ and Co2+ are able to increase the formation of an aromatic char, whereas K+ ions favour the aliphatic char. The action mechanism of metal ions changes when they are combined with phosphorus-based species, since they are able to significantly modify the thermal stability of these latter. Indeed, the volatility of the phosphorus oxides formed during the pyrolysis is reduced and thus these species are still available to phosphorylate the cellulose and induce the char formation. In particular, Horrocks and coworkers51 have demonstrated that Mn2+ and Zn2+ shift the thermal degradation of ammonium polyphosphate toward lower temperatures, so that flame retardancy is induced at lower temperatures. Similar results were observed also employing other transition metal ions, such as Cu(II), Zn(II), Fe(II), Co(II), Cr(III), Ce(IV), La(III), Y(III) and Ho(III) or Mn(II), Pb(II), Bi(III), in combination with cellulose ammonium phosphate, as reported by Tian et al.92,94 Indeed, the temperature of cellulose decomposition was lowered in the presence of metal complexes of cellulose ammonium phosphate with respect to the samples not treated by metal ions; furthermore, the values of char yield were greater for samples treated with Ho3+ and Ce3+ (ref. 92) and Mn2+, Pb2+, Bi3+ (ref. 94) as compared to the untreated counterparts.
It is important to highlight that, during last years of this period, the exigency to have computational models for better understanding the behaviour of cotton under heating is emerged for the first time. The models proposed by Bourbigot and coworkers98–102 represent the only studies currently available in the scientific literature that can be considered worthy to be mentioned.
3.2. Present: from 2010 to today
Nowadays, the approach adopted by researchers is being slightly changed; indeed, although the durability of a new FR remains mandatory, the novel approaches developed in last five years are quite far from this goal. In the continuous seeking of more eco-friendly systems, the attention of the scientific community has been focused on exploiting the “amazing” features of nanotechnology.13,103,104 It is common consensus that the use of nano-objects able to create a very thin layer on the fabric surface could be the pivotal key for new FRs.105,106 Among the nanotechnology approaches, nanoparticle adsorption and the derived Layer by Layer (LbL) assembly as well as sol–gel and dual-cure processes seem to deserve further investigations in order to achieve the best performances described above.We have recently published accurate reviews on these topics (not exclusively devoted to cotton) and thus any duplication should be avoided. However, in order to make the present review on cotton flame retardancy for the best comprehension, the results currently updated regarding nanoparticle adsorption, LbL and sol–gel have been summarised in Tables 8–12.
| Nanoparticle | Formula | Results | Reference |
|---|---|---|---|
| Sodium cloisite | Mx[Al4−xMgx](Si)8O20(OH)4 | TTI increase, pkHRR reduction | 109 |
| Carbonate hydrotalcite | Mg6Al2(CO3)(OH16)·4(H2O) carbonate salt | TTI increase, pkHRR reduction | 110 |
| Sulphonate bohemite | AlO(OH) p-toluenesulphonate salt | TTI increase, pkHRR reduction | 111 |
| Titania (anatase form) | TiO2 | pkHRR reduction | 110 |
| Silica | SiO2 | pkHRR reduction | 110 |
| Octapropylammonium POSS® | R(SiOx) | TTI increase, pkHRR reduction | 111 |
| Positive counterpart | Negative counterpart | Results | Reference |
|---|---|---|---|
| LbL inorganic coatings deposited by dipping | |||
| Polyethylenimine | Laponite | Afterglow of 10BL-coated cotton fabrics occurred 10 s earlier with respect to untreated fabrics | 121 |
| Polyethylenimine | Sodium montmorillonite | Final residue of 10BL-coated cotton fabrics after vertical flame spread tests is consistent | 122 |
| Alumina-coated silica | Silica | 10BL-coated fabrics exhibit a 20% pkHRR reduction, assessed by cone calorimetry | 123 |
| Octa-3-ammoniumpropyl chloride POSS® | Octakis(tetramethylammonium) pentacyclo[9.5.1.13.9,15,15.17,13]octasiloxane 1,3,5,7,9,11,13,15-octakis(cyloxide)hydrate POSS® | Afterglow time is reduced and fabric texture is preserved | 124 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| LbL inorganic coatings deposited by spray | |||
| Alumina-coated silica | Silica | Applying the horizontal spray, 40% increase of TTI and 30 and 20% reduction of pkHRR and TSR were assessed by cone calorimetry (35 kW m−2 heat flux) | 125 |
| Positive Counterpart | Negative counterpart | Results | Reference |
|---|---|---|---|
| Chitosan | Ammonium polyphosphate | 20BLs reduced cotton-rich (70%) blend THR (−22%) and pkHRR (−25%), as assessed by cone calorimetry (35 kW m−2 heat flux) | 126 |
| Alumina-coated silica nanoparticles | Ammonium polyphosphate | 10BLs increased cotton-rich (70%) blend TTI (+40%) and reduced THR (−15%), as assessed by cone calorimetry (35 kW m−2 heat flux) | 126 |
| Poly(allylamine) | Poly(sodium phosphate) | 10BLs reduced cotton THR and pkHRR (−80 and −60%, respectively), as assessed by PCFC | 127 |
| Chitosan | Phytic acid | 30BLs blocked the flame propagation on cotton and reduced pkHRR (−50%), as assessed by PCFC | 128 |
| An aminoderivative of poly(acrylic acid) | Sodium montmorillonite | 20BLs increased cotton TTI (40%) and reduce THR and pkHRR (−50 and −18%, respectively), as assessed by combustion calorimetry (35 kW m−2 heat flux) | 129 |
| A derivative of polyacrylamide | Graphene oxide | 20BLs increased cotton TTI (56%) and reduced pkHRR (−50%), as assessed by combustion calorimetry (35 kW m−2 heat flux) | 130 |
| Chitosan | Poly(sodium phosphate) | 17 BLs made cotton consistently passed vertical flame testing, reduced pkHRR and THR (−73 and −81%, respectively), as assessed by PCFC | 131 |
| Branched poly(ethylene imine) | Poly(sodium phosphate) | Layer by Layer is proposed in alternative approach as “One-pot” for imparting flame retardancy to cotton, mimicking nanoparticle adsorption | 132 |
| Branched poly(ethylene imine)–urea-diammonium phosphate | Kaolin | Only case in which a semi-industrial roll-to-roll plant is employed for depositing LbL assemblies in order to enhance cotton flame retardancy. The final add-ons as well as the combustion results by PCFC (significant reductions of pkHRR and THR) turned out to be functions of the cotton texture (namely, print cloth, mercerized print cloth, and twill cotton fabrics) | 133 |
| Architecture | Sol–gel precursor | Results | Reference |
|---|---|---|---|
| Silica | Tetraethylorthosilicate (TEOS) | With respect to untreated fibres, the temperatures of TEOS-treated fabric first degradation and of flame combustion of volatile products were found increased by 20 °C, while the temperature rise of glowing combustion of the residue was higher than 40 °C | 138 |
| Silica | TEOS | The formation of a continuous silica film deposited on cotton and cotton-rich blend exerted a protective role toward their thermal degradation in air and significantly reducing pkHRR during combustion, as assessed by cone calorimetry (35 kW m−2 heat flux) | 139 |
| Silica | TEOS | Investigation on the effect of dibutiltindiacetate as condensation catalyst in order to promote the oxidic phase formation during sol–gel processes | 140 |
| Silica | Tetramethylorthosilicate (TMOS) | Precursor to water molar ratio, temperature and time of the thermal treatment, moisture presence turned out to affect the resulting cotton flame retardant properties | 141 and 142 |
| Silica | TMOS, TEOS and TBOS (tetrabutylorthosilicate) | Chain length of alkoxysilane precursor turned out to affect the resulting cotton flame retardant properties: shorter the chain length, higher is the protective role of coating | 143 |
| Silica | Alkoxysilane precursors with different number of hydrolysable groups | The number of hydrolysable groups alkoxysilane precursor turned out to affect the resulting cotton flame retardant properties: higher the number, higher is the protective role of coating | 143 |
| Titania | Tetraethylorthotitanate | Among silica, titania, zirconia and alumina, silica exhibited the highest FR performances: the slowest burning rate and highest residue in vertical flame spread tests and highest TTI, lowest pkHRR, THR and TSR in cone calorimetry tests (35 kW m−2 heat flux) | 144 |
| Zirconia | Tetraethylorthozirconate | ||
| Alumina | Aluminium isopropylate | ||
| Silica–alumina particles | TMOS + alumina particles with micrometric or nanometric sizes | The combination of silica–alumina was more effective in protecting the underlying fabric substrates (cotton and cotton–linen blends) than pure silica or alumina phases, regardless of alumina size: the slowest burning rate and highest residue were achieved in vertical flame spread tests | 145 |
| Silane precursor | Doping agent | Doped oxidic phase | Results | Reference |
|---|---|---|---|---|
| (i) Mixing the alkoxysilane precursor with a phosphoric-acid source | ||||
| TMOS | Aluminium phosphinate | P-doped silica | High amounts of (30 and 50 wt%) generates high concentrations of flat smokes. It was found that 5 and 15 wt% were the most promising concentrations of phosphorus compounds with respect to sol–gel precursor to strongly increase TTI, as assessed by cone calorimetry (35 kW m−2 heat flux) | 146 |
| A mixture of aluminium phosphinate, melamine poly(phosphate) and zinc and boron oxide | P-, P- and N-, Zn- and B-doped silica | The presence of both species increased the resistance to a flame applications, reduced the total burning rate and increased the final residue, as assessed by vertical flame spread tests | 146 | |
| α-Zirconium phosphate nano-platelets | P-doped silica | 4 flame applications were necessary to ignite cotton fabrics. The above system increased the final residue from 14 to 62% in vertical flame spread tests. This treatment turned out to be stable after a washing cycle of 1 h at 60 °C | 146 | |
| TEOS | H3PO4 or ethyldichlorophosphate | P-doped silica | Flammability tests in vertical configuration have shown that cotton does not burn when phosphoric acid acts synergistically with the silica coating | 147 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| (ii) Using an alkoxysilane precursor bearing both silane and phosphate functionalities | ||||
| DPTES | 1, 3 or 6 layers | P-doped silica | If DPTES is not pre-hydrolyzed, 6 layers gave the best performances in terms of pkHRR and TSR reductions (−40 and 80%, as assessed by cone calorimetry (35 kW m−2). CO and CO2 were strongly reduced, as well. If DPTES is pre-hydrolyzed in acid, 1 layer gave the best results in both resistance to a flame application and heat flux. The synergistic effect between phosphate group in DPTES molecule and silica network was demonstrated by comparing the resulting flame retardant level of cotton when treated with DPTES or TEOS | 148–150 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| (iii) Mixing an alkoxysilane precursor bearing both silane and phosphate functionalities with P- and N-containing chemicals | ||||
| DPTES | APTES or APTES and melamine-based resin | P- and N-doped silica | Flammability tests (according to ASTM D1230) have shown a significant enhancement of char-forming properties and thus of flame retardancy of cotton, as a consequence of the synergistic effects between phosphorus and nitrogen with the silica phase. Indeed, a char yield of 9, 42 and 38 wt% for APTES-, APTES/DPTES- and APTES/DPTES/melamine-treated samples, respectively, has been found by thermogravimetric analysis in air | 151 |
| DPTES | APES or N,N,N′,N′,N′′,N′′-hexakis-methoxymethyl-[1,3,5] triazine-2,4,6-triamine | P- and N-doped silica | These coatings turned out to be char-former architecture, promoting high residues during vertical flame spread tests (ca. 50 and 70 wt% for APTES-DPTES and MF-DPTES, respectively) | 152 |
| DPTES | 1-Hydroxyethane 1,1-diphosphonic acid | P-doped silica | The calculation of synergism parameters demonstrated synergism between DPTES and 1-hydroxyethane 1,1-diphosphonic acid for further increasing flame retardancy of DPTES-treated cotton, as assessed by cone calorimetry (35 kW m−2) | 148 |
| N,N,N′,N′,N′′,N′′-hexakis-methoxymethyl-[1,3,5] triazine-2,4,6-triamine | P- and N-doped silica | The calculation of synergism parameters demonstrated cumulative effect between N,N,N′,N′,N′′,N′′-hexakis-methoxymethyl-[1,3,5] triazine-2,4,6-triamine urea and DPTES and for further increasing flame retardancy of DPTES-treated cotton, as assessed by cone calorimetry (35 kW m−2) | 148 | |
| Urea | ||||
In addition, Table 13 reports the main results achieved depositing new green coatings consisting of bio-macromolecules like proteins and nucleic acids. The latter approach is very similar to what recently reviewed about enzyme immobilization.107
| Bio-macromolecule | Results | Ref. |
|---|---|---|
| Deoxyribonucleic acid (DNA) | Cotton treated with 19% of DNA add-on do not burn when a 2.5 cm methane flame is applied in horizontal configuration as well as under a heat flux of 35 kW m−2. It burns very slowly under a heat flux of 50 kW m−2 that represents a very severe condition. 10% Of DNA add-on protected cotton with comparable performances to 19%. The coatings showed intumescent behaviour | 18, 155 and 156 |
| DNA-chitosan | In the LbL assembly, DNA layers promoted the char formation of chitosan counterparts, by releasing phosphoric and polyphosphoric acid. The obtained char was thermally stable, and thus it is able to impart a remarkable flame retardant character to cotton. 20BLs reduce pkHRR and THR (−40 and −30%, respectively), as assessed by cone calorimetry and PCFC, as well as self-extinguishment during horizontal flame spread tests. With respect to only DNA, DNA chitosan allowed reaching these results with a total add-on of 14% | 157 |
| Whey proteins | The protein coating, irrespective of its structure (folded or unfolded) significantly sensitized cotton degradation, ensuring very high final residues. Features like good oxygen barrier properties and great water vapour adsorption justified the reduced burning rate and high final residue during horizontal flame spread tests | 158 |
| Caseins and hydrophobins | The protein coating significantly sensitized cotton degradation, ensuring very high final residues. A significant reduction of burning rate and increase of final residue during horizontal flame spread tests were found. Both proteins reduced cotton pkHRR (−27 and −45% for caseins and hydrophobins, respectively), as assessed by cone calorimetry (35 kW m−2) | 159 and 160 |
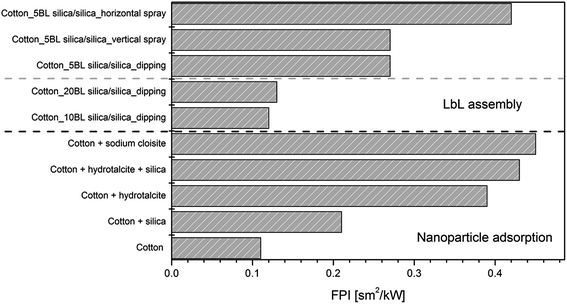
Table 8 summarises the collected data for cotton treated with different nanoparticles in terms of resistance to 35 kW m−2 heat flux assessed by cone calorimetry.109 From an overall consideration, all the nanoparticles under study showed similar behaviour in that they improve cotton fire performance to varying degrees; in particular, they promoted an increase of TTI (like in the case of sodium cloisite, hydrotalcite,110 boehmite and POSS®111), a reduction of pkHRR (all nanoparticles cited in Table 8) or both these events.
As a result, the Fire Performance Index (FPI, calculated as TTI![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) pkHRR ratio) of nanoparticle-treated cotton is always higher than that of pure cotton, hence showing a good flame retardant effect. Based on an increasing value for FPI, the next ranking has been recently established109 (Fig. 6), taking into account the corresponding value for cotton (FPI = 0.11 s m2 kW−1):
pkHRR ratio) of nanoparticle-treated cotton is always higher than that of pure cotton, hence showing a good flame retardant effect. Based on an increasing value for FPI, the next ranking has been recently established109 (Fig. 6), taking into account the corresponding value for cotton (FPI = 0.11 s m2 kW−1):
| Silica (0.21 s m2 kW−1) < POSS® (0.24 s m2 kW−1) < hydrotalcite (0.39 s m2 kW−1) < boehmite (0.44 s m2 kW−1) ≈ cloisite (0.45 s m2 kW−1) |
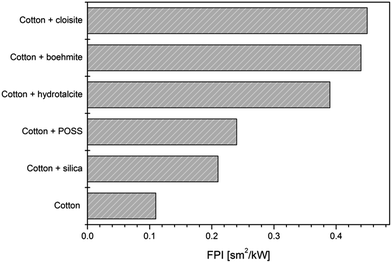 | ||
| Fig. 6 FPI values for different nanoparticle-treated cotton fabrics. Data taken from ref. 109. | ||
In conclusion, Layer by Layer assembly can be considered an evolution of the nanoparticle adsorption process. Exploiting this approach, two types of architectures can be deposited on a certain substrate: inorganic or intumescent coatings.116 In the former case, analogously to what occurs by using only nanoparticle adsorption, the flame retardancy effect is due to the thermal shielding promoted by the LbL architecture, which is able to modify the thermal degradation in air, and thus the flammability, of the substrate. On the contrary, intumescent coatings can strongly influence the combustion due to their own FR features. As it is well-known,117 an intumescent formulation is a complex system, in which three components synergistically cooperate to create an expanded carbonaceous protective layer on the surface, thanks to the cooperation of an acid, a blowing agent and carbon source. If these species are combined in a LbL assembly, the protection may be very efficient in the case of cotton fabrics.105
Tables 9 and 10 show the collected data hitherto, on the basis of the coating type deposited on cotton (namely, inorganic and intumescent architectures). All the systems here described consist in the alternation of positive and negative counterparts forming bi-layers (BLs) architecture. More complex architectures (quad-layers or BLs + BLs118–120) have been recently prepared by our group and applied to cotton and cotton-rich blends. In these cases, cotton self-extinguishment has been achieved both in horizontal and vertical flame spread tests118 due to high intrinsic intumescent features of the deposited architectures.120
First of all, Fig. 8 shows the FPI values derived from cone calorimetry tests for cotton treated with different nanoparticles (namely, silica, hydrotalcite and sodium cloisite) and with the LbL approach. It is noteworthy that the best results are achieved in the presence of sodium cloisite, notwithstanding that the combination of hydrotalcite and silica still gives good FPI result. In this latter case, since the nanoparticles have been adsorbed on cotton through a two-step deposition, the obtained assembly could be considered as an “odd” bilayer, in which chemical or physical interactions may occur.
Comparing these results with those obtained by Grunlan's group employing an LbL approach123 (namely, cotton_10BL_silica/silica_dipping and cotton_20BL_silica/silica_dipping assemblies), it is clear that these latter are less performing than those obtained by nanoparticle adsorption, regardless of the nanoparticle type or aspect ratio. Conversely, 5BL of silica/silica deposited by dipping (or vertical spray) on cotton turned out to be more performing than simple silica adsorption.125 Finally, the same architectures, deposited via horizontal spray, have shown the same results achieved by sodium cloisite and hydrotalcite/silica pair adsorption.
As far as the resistance to a 35 kW m−2 heat flux is concerned, nanoparticle adsorption turned out to be the best system for reaching the highest performances with a simple, fast and cheap method.
On the other hand, LbL assembly has proven to be more efficient in terms of resistance to flame exposure. Indeed, cotton treated with simple nanoparticle adsorption does not generally pass vertical flame spread tests (like the ISO 15025 standard134). Conversely, LbL-treated systems are able to strongly enhance cotton flame resistance, as already shown in Tables 9 and 10. The most plausible explanation can be referred to the coating nanostructure created through the Layer by Layer deposition that is able to homogeneously and finely cover cotton fibres. In the case of cotton, this controlled growing cannot be performed through nanoparticle adsorption, unlike polyester fabrics, previously subjected to a plasma treatment.135
As already mentioned, no significant improvements in the scientific literature with respect to those already described in our recent reviews105,137 on the use of sol–gel for cotton flame retardancy have been recently published; however, for completeness reasons, Tables 11 and 12 show the most significant data achieved and updated, once again dividing them on the basis of the chosen type of architecture: inorganic or phosphorus-doped systems. More specifically, in the latter case, a further classification of these architectures on the basis of the doping procedure is proposed as follows:
(i) Mixing the alkoxysilane precursor with a phosphoric-acid source.
(ii) Using an alkoxysilane precursor bearing both silane and phosphate functionalities: in this case, DPTES (diethylphosphatoethyltriethoxysilane) is commonly employed.
(iii) Mixing an alkoxysilane precursor bearing both silane and phosphate functionalities with P- and N-containing chemicals.
In all these cases, silica is the oxidic phase employed for creating the ceramic barrier and thus the thermal shield: the only difference among the systems reported in Table 11 is the silane precursor used.
3.3. Past and present durability issues
Durability is one of the mandatory features that a FR system should exhibit. Historically, all the attempts carried out during 1950–1980s were aimed to find new durable solutions for cotton: the solution was found in the design of Proban® and Pyrovatex® that showed all the requirements desired by the market. Then, alternative solutions were developed in 1980–2010s, launching Fyrol®, Fyroltex® and their derivatives on the market. All these systems are based on phosphorus–nitrogen-based products applied in the presence of a methylolated resin (e.g. dimethyloldihydroxyethylene urea or methylated formaldehyde-urea). The durability is achieved by chemically grafting the FR onto cellulose C(6) hydroxyl groups.The required durability level strictly depends on the application fields and specific international standards. One of the most common adopted for assessing the durability of a FR system is the ISO 6330 (ref. 161) standard, which defines domestic washing and drying procedures for textile testing.
As far as the novel nanotechnological approaches are concerned, the durability issue has not been fulfilled yet. Indeed, only few attempts have been made and some quite interesting results have been achieved employing sol–gel142 and dual-cure processes.162 In particular, in the former, a silica coating turned out to be resistant to washing treatments (1 h at 60 or 100 °C) and its effect on the flame resistance disappeared only when critical conditions were adopted (5 washing cycles for 1 h at 60 °C or 1 washing cycle for 5 h at 100 °C). On the other hand, very recently, we have found promising results by coupling LbL with UV curing. Indeed, by this way, it was possible to deposit a silica-based coating on polycarbonate films that is stable to treatments in hot water (60 or 90 °C) or ammonia (1 M) for 1 h, without threatening the flame retardant performances. This route could deserve further investigation for solving the durability of LbL assemblies that is intrinsic of such treatments as based on ionic interactions.163 Concerning the biomacromolecules-based coatings on cotton, the durability of these treatments still represents a challenging issue: indeed, because of their waterborne character, these coatings come off from the cotton fabrics even when subjected to mild washing processes (i.e. at a low temperature e.g., 30 °C and without using surfactants).148 Thus, this limitation deserves further investigation, keeping in mind that an acceptable balance between the green features of the biomacromolecules and the use of chemical products for permanently linking the biomacromolecules to cotton should be achieved. In other words, the chemical finishing processes that could be suitable for this purpose should be designed in order to keep the “green” character of the proposed treatments with the biomacromolecules.
4. Conclusions and future perspectives
The world of cotton flame retardancy has undergone and still undergoes several changes and evolutions, which seem to strongly depend on different “driving forces”: first of all, it is worthy to mention the efforts exerted by the scientific community, which are directed toward the design of novel FR products showing effectiveness, scalability to an industrial use and, of course, durability. Regulations and legislations are, at the same time, pushing the research in this topic toward the fulfillment of products having a very low environmental impact that should not threaten their effectiveness as flame retardants. This dynamism is obviously very useful in order to take on new challenges and seems to be able to save unexpected surprises for the next future.From a perspective point of view, as clearly indicated in the review, phosphorus-containing products seem to be the most promising, currently efficient and industrializable flame retardants for cotton. For all these reasons, it could be desirable that new flame retardants would be designed from suitable phosphorus-containing biosources, possibly coming from a waste stream or by-products from agriculture, thus further achieving a high-added value. Obviously, durability – in terms of resistance to washing – should be mandatory, notwithstanding that a green approach as far as both the recovery from wastes or by-products and the subsequent application of the obtained flame retardants to cotton has to be fulfilled.
Abbreviation list
| APTES | Aminopropryltriethoxysilane |
| ASTM | American society for testing and materials |
| BL | Bi-layer |
| BS | British standard |
| BTCA | Butyl tetracarboxylic acid |
| CEN | European standard |
| CPSC | Consumer product safety commission |
| DAP | Diammonium phosphate |
| DIN | Deutsche industrial norms |
| DMDHEU | Dimethylol dihydroxyethylene urea |
| DP | Degree of polymerization |
| DPTES | Diethylphosphatoethyltriethoxysilane |
| EEC | European economic community |
| EU | European union |
| FR | Flame retardant |
| ICAC | International cotton advisory committee |
| ISO | International standards organisation |
| LbL | Layer by Layer |
| LOI | Limiting oxygen index |
| NFPA | National fire protection association |
| OSHA | Occupational safety and health administration |
| PA | Phosphoric acid |
| PCFC | Pyrolysis-flow combustion calorimetry |
| pkHRR | Peak of heat release rate |
| PPE | Personal protective equipment |
| RH | Room humidity |
| TAP | Triallyl phosphate |
| TBOS | Tetrabutylorthosilicate |
| TBP | Tributyl phosphate |
| TEOS | Tetraethylorthosilicate |
| TEP | Triethyl phosphate |
| TGA | Thermogravimetry |
| THPX | Tetrakis(hydroxymethyl) phosphonium salt |
| THR | Total heat release |
| TMOS | Tetramethylorthosilicate |
| TPT | Triallyl phosphoric triamide |
| TSR | Total smoke release |
| TTI | Time to ignition |
Acknowledgements
The authors thank the European COST Action FLARETEX (MP1105) “Sustainable flame retardancy for textiles and related materials based on nanoparticles substituting conventional chemicals”.References
- S. Gordon and Y. L. Hsie, Cotton: Science and Technology, Woodhead Publishing Limited and CRC Press, Boca Raton, FL, 2007 Search PubMed
.
- D. Klemm, B. Philipp, T. Heinze, U. Heinze and W. Wagenknecht, in Comprehensive Cellulose Chemistry: Fundamentals and Analytical Methods, Volume 1, ed. I., John Wiley & Sons, 2004, vol. 1, pp. 9–29 Search PubMed
.
- D. Battegazzore, S. Bocchini, J. Alongi, A. Frache and F. Marino, Cellulose, 2014, 21, 1813–1821 CrossRef CAS
.
- D. Davies and A. R. Horrocks, J. Appl. Polym. Sci., 1986, 31, 1655–1662 CrossRef CAS
.
- J. Alongi, G. Camino and G. Malucelli, Carbohydr. Polym., 2013, 92, 1327–1334 CrossRef CAS PubMed
.
- F. Shafizadeh and Y. L. Fu, Carbohydr. Res., 1973, 29, 113–122 CrossRef CAS PubMed
.
- F. Shafizadeh, R. H. Furneaux, T. G. Cochran, J. P. Scholl and Y. Sakai, J. Appl. Polym. Sci., 1979, 23, 3525–3539 CrossRef CAS
.
- O. Ceylan, J. Alongi, L. Van Landuyt, A. Frache and K. De Clerck, Fire Mater., 2013, 37, 482–490 CrossRef CAS
.
- O. Ceylan, L. Van Landuyt, H. Rahier and K. De Clerck, Cellulose, 2013, 20, 1603–1612 CrossRef CAS
.
- ICAC, Nations Conference on Trade and Development based on International ICAC statistics, 2013, http://www.unctad.info/en/Infocomm/Agricultural_Products/Cotton/Market/United.
- ASTM E176, Standard Terminology of Fire Standards, West Conshohocken, USA, 1981 Search PubMed
.
- ISO 13943, Fire safety-Vocabulary, Geneva, 2008 Search PubMed
.
- J. Alongi, F. Carosio, A. R. Horrocks and G. Malucelli, Update on Flame Retardant textiles: State of the art, Environmental Issues and Innovative Solutions, Smithers Rapra, Shawbury, Shrewsbury, Shropshire, UK, 2013 Search PubMed
.
- A. R. Horrocks, D. Davies and M. Greenhalgh, Fire Mater., 1985, 9, 57–64 CrossRef
.
- E. Weil, M. Hirschler, N. Patel, M. Said and S. Shakir, Fire Mater., 1992, 16, 159–167 CrossRef CAS
.
- F. Shafizadeh and A. G. W. Bradbury, J. Appl. Polym. Sci., 1979, 23, 1431–1442 CrossRef CAS
.
- D. Price, A. R. Horrocks, M. Akalin and A. A. Faroq, J. Anal. Appl. Pyrolysis, 1997, 40–41, 511–524 CrossRef
.
- J. Alongi, J. Milnes, G. Malucelli, S. Bourbigot and B. Kandola, J. Anal. Appl. Pyrolysis, 2014, 108, 212–221 CrossRef CAS
.
- EN 14878, Textiles – Burning behaviour of Children's nightwear-Specification, Geneva, 2007 Search PubMed
.
- S. Nazarè and R. Davis, in Handbook of Fire Resistant Textiles, ed. F. Selcen Kilinc, Woodhead Publishing Ltd, Cmbridge, UK, 2013, pp. 456–498 Search PubMed
.
- J. Tata, J. Alongi and A. Frache, Fire Mater., 2012, 36, 527–536 CrossRef CAS
.
- J. Tata, J. Alongi, F. Carosio and A. Frache, Fire Mater., 2011, 35, 397–409 CrossRef CAS
.
- R. Lyon and R. Walters, J. Anal. Appl. Pyrolysis, 2004, 71, 27–46 CrossRef CAS
.
- C. Yang and Q. He, Fire Mater., 2011, 36, 127–137 CrossRef
.
- C. Yang, Q. He, R. Lyon and Y. Hu, Polym. Degrad. Stab., 2010, 95, 108–115 CrossRef CAS
.
- C. F. Cullis and M. Hirschler, The combustion of organic
polymers, Clarendon Press, Oxford, UK, 1981 Search PubMed
.
- A. R. Horrocks, Polym. Degrad. Stab., 2011, 96, 377–392 CrossRef CAS
.
- E. Weil and S. V. Levchik, J. Fire Sci., 2008, 26, 243–281 CrossRef CAS
.
- F. Shafizadeh and Y. Sekiguchi, Combust. Flame, 1984, 55, 171–179 CrossRef CAS
.
- Y. Sekiguchi and F. Shafizadeh, J. Appl. Polym. Sci., 1984, 29, 1267–1286 CrossRef CAS
.
- F. Shafizadeh and Y. Sekiguchi, Carbon, 1983, 21, 511–516 CrossRef CAS
.
- Y. Sekiguchi, J. S. Frye and F. Shafizadeh, J. Appl. Polym. Sci., 1983, 28, 3513–3525 CrossRef CAS
.
- F. Shafizadeh, A. G. W. Bradbury, W. F. DeGroot and T. W. Aanerud, Ind. Eng. Chem. Prod. Res. Dev., 1982, 21, 97–101 CrossRef CAS
.
- D. Bakos, M. Kosik, K. Antos, M. Karolyova and I. Vyskocil, Fire Mater., 1982, 6, 10–12 CrossRef CAS
.
- J. E. Hendrix, J. E. Bostic, E. S. Olson and R. H. Barker, J. Appl. Polym. Sci., 1970, 14, 1701–1723 CrossRef CAS
.
- J. E. Hendrix, G. L. Drake and R. H. Barker, J. Appl. Polym. Sci., 1972, 16, 41–59 CrossRef CAS
.
- J. E. Hendrix, G. L. Drake and R. H. Barker, J. Appl. Polym. Sci., 1972, 16, 257–274 CrossRef CAS
.
- A. R. Horrocks, Rev. Prog. Color. Relat. Top., 1986, 16, 40–101 Search PubMed
.
- A. R. Horrocks, B. K. Kandola, P. J. Davies, S. Zhang and S. A. Padbury, Polym. Degrad. Stab., 2005, 88, 3–12 CrossRef CAS
.
- P. J. Davies, A. R. Horrocks and A. Alderson, Polym. Degrad. Stab., 2005, 88, 114–122 CrossRef CAS
.
- S. Zhang and A. R. Horrocks, J. Appl. Polym. Sci., 2003, 90, 3165–3172 CrossRef CAS
.
- D. Price, Y. Liu, T. R. Hull, G. J. Milnes, B. K. Kandola and A. R. Horrocks, Polym. Degrad. Stab., 2002, 77, 213–220 CrossRef CAS
.
- A. R. Horrocks and S. Zhang, Fire Mater., 2002, 26, 173–182 CrossRef CAS
.
- A. R. Horrocks, Polymer, 2001, 42, 8025–8033 CrossRef CAS
.
- D. Price, G. V. Coleman and A. R. Horrocks, J. Therm. Anal., 1993, 40, 649–656 CrossRef CAS
.
- A. R. Horrocks, S. C. Anand and D. Sanderson, Polymer, 1996, 37, 3197–3206 CrossRef CAS
.
- B. K. Kandola and A. R. Horrocks, Text. Res. J., 1999, 69, 374–381 CrossRef CAS
.
- B. K. Kandola, A. R. Horrocks, D. Price and G. V. Coleman, J. Macromol. Sci., Rev. Macromol. Chem. Phys., 1996, C36, 721–794 CrossRef CAS
.
- D. Davies, A. R. Horrocks and M. Greenhalgh, Thermochim. Acta, 1983, 63, 351–362 CrossRef CAS
.
- A. R. Horrocks, Polym. Degrad. Stab., 1996, 54, 143–154 CrossRef CAS
.
- J. Bhagwan, K. Lal, A. R. Horrocks and D. Price, Polym. Int., 1993, 30, 33–45 CrossRef CAS
.
- B. K. Kandola and A. R. Horrocks, Polym. Degrad. Stab., 1996, 54, 289–303 CrossRef CAS
.
- A. R. Horrocks, D. Price and M. Akalin, Polym. Degrad. Stab., 1996, 52, 205–213 CrossRef CAS
.
- A. A. Faroq, D. Price, G. J. Milnes and A. R. Horrocks, Polym. Degrad. Stab., 1991, 33, 155–170 CrossRef CAS
.
- A. R. Horrocks, J. Soc. Dyers Colour., 1983, 99, 191–197 CrossRef CAS
.
- C. Morterra and M. J. D. Low, Carbon, 1985, 23, 301–310 CrossRef CAS
.
- C. Morterra and M. J. D. Low, Carbon, 1985, 23, 335–341 CrossRef CAS
.
- C. Morterra and M. J. D. Low, Mater. Chem. Phys., 1985, 12, 207–233 CrossRef CAS
.
- M. J. D. Low and C. Morterra, Carbon, 1985, 23, 311–316 CrossRef CAS
.
- C. Morterra, M. J. D. Low and A. G. Severdia, Carbon, 1984, 22, 5–12 CrossRef CAS
.
- C. Morterra and M. J. D. Low, Mater. Lett., 1984, 2, 289–293 CrossRef CAS
.
- M. J. D. Low and C. Morterra, Carbon, 1983, 21, 275–281 CrossRef CAS
.
- C. Morterra and M. J. D. Low, Carbon, 1983, 21, 283–288 CrossRef CAS
.
- A. M. Emsley, Cellulose, 2007, 15, 187–192 CrossRef
.
- M. Ali, A. M. Emsley, H. Herman and R. J. Heywood, Polymer, 2001, 42, 2893–2900 CrossRef CAS
.
- R. J. Heywood, A. M. Emsley and M. Ali, IEE Proc.: Sci., Meas. Technol., 2000, 147, 86–90 CrossRef CAS
.
- A. M. Emsley, X. Xiao, R. J. Heywood and M. Ali, IEE Proc.: Sci., Meas. Technol., 2000, 147, 110–114 CrossRef CAS
.
- A. M. Emsley, X. Xiao, R. J. Heywood and M. Ali, IEE Proc.: Sci., Meas. Technol., 2000, 147, 115–119 CrossRef CAS
.
- A. M. Emsley, X. Xiao, R. J. Heywood and M. Ali, IEE Proc.: Sci., Meas. Technol., 2000, 147, 285–290 CrossRef CAS
.
- A. Emsley, Polymer, 2000, 41, 8513–8521 CrossRef CAS
.
- A. M. Emsley, R. J. Heywood, M. Ali and C. M. Eley, Cellulose, 1997, 4, 1–5 CrossRef CAS
.
- M. Ali, D. C. Apperley, C. D. Eley, A. M. Emsley and R. K. Harris, Cellulose, 1996, 3, 77–90 CrossRef CAS
.
- A. M. Emsley, Institute of Electrical and Electronics Engineers (IEEE) Eletrical Insulation Methods, 1996, 28–34 Search PubMed
.
- A. M. Emsley and R. J. Heywood, Polym. Degrad. Stab., 1995, 49, 145–149 CrossRef CAS
.
- A. M. Emsley and G. C. Stevens, Cellulose, 1994, 1, 26–56 CrossRef CAS
.
- A. M. Emsley, Polym. Degrad. Stab., 1994, 44, 343–349 CrossRef CAS
.
- M. Lewin, Polym. Degrad. Stab., 2005, 88, 13–19 CrossRef CAS
.
- F. Abdel-Mohdy, J. Appl. Polym. Sci., 2003, 89, 2573–2578 CrossRef CAS
.
- E. D. Weil, US Pat. no. 3,746,572, July, 1970US Pat. no. 3695925, October, 1972US Pat. no. 3855359, December, 1974US Pat. no. 4404313, September, 1983
.
- C. Yang and W. Wu, Fire Mater., 2003, 27, 239–251 CrossRef CAS
.
- W. Wu and C. Yang, J. Fire Sci., 2004, 22, 125–143 CrossRef CAS
.
- H. Yang and C. Yang, Polym. Degrad. Stab., 2005, 88, 363–370 CrossRef CAS
.
- H. Yang and C. Yang, J. Fire Sci., 2007, 25, 425–446 CrossRef CAS
.
- X. Wu and C. Yang, J. Fire Sci., 2008, 26, 351–368 CrossRef CAS
.
- C. Yang and X. Wu, J. Fire Sci., 2009, 27, 431–444 CrossRef
.
- X. Cheng and C. Yang, J. Fire Sci., 2009, 27, 583–600 CrossRef CAS
.
- S. Gaan and G. Sun, Polym. Degrad. Stab., 2007, 92, 968–974 CrossRef CAS
.
- S. Gaan, G. Sun, K. Hutches and M. H. Engelhard, Polym. Degrad. Stab., 2008, 93, 99–108 CrossRef CAS
.
- S. Gaan, P. Rupper, V. Salimova, M. Heuberger, S. Rabe and F. Vogel, Polym. Degrad. Stab., 2009, 94, 1125–1134 CrossRef CAS
.
- S. Soares, G. Camino and S. Levchik, Polym. Degrad. Stab., 1998, 62, 25–31 CrossRef CAS
.
- M. D. C. C. Lucena, A. E. V. de Alencar, S. E. Mazzeto and S. D. A. Soares, Polym. Degrad. Stab., 2003, 80, 149–155 CrossRef CAS
.
- C. M. Tian, H. Z. Guo, H. Y. Zhang, J. Z. Xu and J. R. Shi, Thermochim. Acta, 1995, 253, 243–251 CrossRef CAS
.
- C. M. Tian, Z. H. Shi, H. Y. Zhang, J. Z. Xu, J. R. Shi and H. Z. Guo, J. Therm. Anal. Calorim., 1999, 55, 93–98 CrossRef CAS
.
- C. M. Tian, J. X. Xie, H. Z. Guo and J. Z. Xu, J. Therm. Anal. Calorim., 2003, 73, 827–834 CrossRef CAS
.
- X. M. Fang, C. F. Sun and M. Gao, Combust. Sci. Technol., 2013, 185, 1044–1055 CrossRef CAS
.
- L. Wang, T. Zhang, H. Yan, M. Peng and Z. Fang, J. Appl. Polym. Sci., 2013, 129, 2986–2997 CrossRef CAS
.
- S. Hu, Y. Hu, L. Song and H. Lu, J. Therm. Anal. Calorim., 2011, 109, 27–32 CrossRef
.
- V. Mamleev, S. Bourbigot, M. Le Bras and J. Yvon, J. Anal. Appl. Pyrolysis, 2009, 84, 1–17 CrossRef CAS
.
- V. Mamleev, S. Bourbigot and J. Yvon, J. Anal. Appl. Pyrolysis, 2007, 80, 151–165 CrossRef CAS
.
- V. Mamleev, S. Bourbigot and J. Yvon, J. Anal. Appl. Pyrolysis, 2007, 80, 141–150 CrossRef CAS
.
- V. Mamleev, S. Bourbigot, M. Le Bras, J. Yvon and J. Lefebvre, Chem. Eng. Sci., 2006, 61, 1276–1292 CrossRef CAS
.
- S. Bourbigot, S. Chlebicki and V. Mamleev, Polym. Degrad. Stab., 2002, 78, 57–62 CrossRef CAS
.
- J. Alongi, A. Frache, G. Malucelli and G. Camino, in Handbook of Fire Resistant Textiles, ed. F. Selcen Kilinc, Woodhead Publishing Ltd, Cmbridge, UK, 2013, pp. 68–93 Search PubMed
.
- T. Harifi and M. Montazer, Carbohydr. Polym., 2012, 88, 1125–1140 CrossRef CAS
.
- G. Malucelli, F. Carosio, J. Alongi, A. Fina, A. Frache and G. Camino, Mater. Sci. Eng., R, 2014, 84, 1–20 CrossRef
.
- S. Liang, N. M. Neisius and S. Gaan, Prog. Org. Coat., 2013, 76, 1642–1665 CrossRef CAS
.
- M. Gulrajania and D. Gupta, Indian J. Fibre Text. Res., 2011, 36, 388–397 Search PubMed
.
- J. Alongi, F. Carosio and G. Malucelli, Polym. Degrad. Stab., 2014, 106, 138–149 CrossRef CAS
.
- J. Alongi, J. Tata, F. Carosio, G. Rosace, A. Frache and G. Camino, Polymers, 2015, 7, 47–68 CrossRef CAS
.
- J. Alongi, J. Tata and A. Frache, Cellulose, 2011, 18, 179–190 CrossRef CAS
.
- J. Alongi, G. Brancatelli and G. Rosace, J. Appl. Polym. Sci., 2012, 123, 426–436 CrossRef CAS
.
- K. Iler, J. Colloid Interface Sci., 1966, 21, 569–594 CrossRef
.
- G. Decher and J. Schlenoff, Multilayer Thin Films Sequential Assembly of Nanocomposite Materials, 2nd edn, Wiley-VCH, Weinheim, 2012 Search PubMed
.
- G. Decher, J. Hong and J. Schmitt, Thin Solid Films, 1992, 210/21, 831–835 CrossRef
.
- G. Rydzek, P. Schaaf, J. Voegel, L. Jierry and F. Boulmedais, Soft Matter, 2012, 8, 9738–9755 RSC
.
- L. A. Lowden and T. R. Hull, Fire Sci. Rev., 2013, 2, 4–19 CrossRef
.
- S. Bourbigot and S. Duquesne, in Fire retardancy of polymeric materials, ed. C. A. Wilkie and A. Morgan, CRC Press, Boca Raton, FL, 2010, pp. 163–206 Search PubMed
.
- F. Carosio, J. Alongi and G. Malucelli, Polym. Degrad. Stab., 2013, 98, 1626–1637 CrossRef CAS
.
- J. Alongi, F. Carosio and G. Malucelli, Cellulose, 2012, 19, 1041–1050 CrossRef CAS
.
- J. Alongi, F. Carosio and G. Malucelli, Polym. Degrad. Stab., 2012, 97, 1644–1653 CrossRef CAS
.
- Y. C. Li, J. Schulz and J. C. Grunlan, ACS Appl. Mater. Interfaces, 2009, 1, 2338–2347 CAS
.
- Y. C. Li, J. Schulz, S. Mannen, C. Delhom, B. Condon, S. Chang, M. Zammarano and J. C. Grunlan, ACS Nano, 2010, 6, 3325–3337 CrossRef PubMed
.
- G. Laufer, F. Carosio, R. Martinez, G. Camino and J. C. Grunlan, J. Colloid Interface Sci., 2011, 356, 69–77 CrossRef CAS PubMed
.
- Y. C. Li, S. Mannen, J. Schulz and J. C. Grunlan, J. Mater. Chem., 2011, 21, 3060–3069 RSC
.
- J. Alongi, F. Carosio, A. Frache and G. Malucelli, Carbohydr. Polym., 2013, 92, 114–119 CrossRef CAS PubMed
.
- F. Carosio, J. Alongi and G. Malucelli, Carbohydr. Polym., 2012, 88, 1460–1469 CrossRef CAS
.
- Y. C. Li, S. Mannen, A. Morgan, S. Chang, Y. Yang, B. Condon and J. C. Grunlan, Adv. Mater., 2011, 23, 3926–3931 CrossRef CAS PubMed
.
- G. Laufer, C. Kirkland, A. Morgan and J. C. Grunlan, Biomacromolecules, 2012, 13, 2843–2848 CrossRef CAS PubMed
.
- G. Huang, L. Liang, X. Wang and J. Gao, Ind. Eng. Chem. Res., 2012, 51, 12299–12309 CAS
.
- G. Huang, J. Yang, J. Gao and X. Wang, Ind. Eng. Chem. Res., 2012, 51, 12355–12366 CAS
.
- T. Guin, M. Krecker, A. Milhorn and J. C. Grunlan, Cellulose, 2014, 21, 3023–3030 CrossRef CAS
.
- A. Cain, S. Murray, K. Holder, C. Nolen and J. C. Grunlan, Macromol. Mater. Eng., 2014, 299, 1180–1187 CrossRef CAS
.
- S. Chang, R. Slopek, B. Condon and J. C. Grunlan, Ind. Eng. Chem. Res., 2014, 53, 3805–3812 CrossRef CAS
.
- ISO 15025, Protective clothing – protection against heat and flame – method of test for limited flame spread, Geneva, 2000 Search PubMed
.
- F. Carosio, J. Alongi and A. Frache, Eur. Polym. J., 2011, 47, 893–902 CrossRef CAS
.
- S. Sakka, Sol-gel Science and Technology. Topics and Fundamental Research and Applications, Kluwer Academic Publishers, Norwell, 2003 Search PubMed
.
- J. Alongi and G. Malucelli, J. Mater. Chem., 2012, 22, 21805–21809 RSC
.
- S. Hribernik, M. Smole, K. Kleinschek, M. Bele, J. Jamnik and M. Gaberscek, Polym. Degrad. Stab., 2007, 92, 1957–1965 CrossRef CAS
.
- J. Alongi, M. Ciobanu, J. Tata, F. Carosio and G. Malucelli, J. Appl. Polym. Sci., 2011, 119, 1961–1969 CrossRef CAS
.
- C. Colleoni, I. Donelli, G. Freddi, E. Guido, V. Migani and G. Rosace, Surf. Coat. Technol., 2013, 235, 192–203 CrossRef CAS
.
- J. Alongi and G. Malucelli, J. Therm. Anal. Calorim., 2013, 111, 459–465 CrossRef CAS
.
- J. Alongi, M. Ciobanu and G. Malucelli, Cellulose, 2011, 18, 167–177 CrossRef CAS
.
- J. Alongi, M. Ciobanu and G. Malucelli, Carbohydr. Polym., 2012, 87, 627–635 CrossRef CAS
.
- J. Alongi, M. Ciobanu and G. Malucelli, Carbohydr. Polym., 2012, 87, 2093–2099 CrossRef CAS
.
- J. Alongi and G. Malucelli, Polym. Degrad. Stab., 2013, 98, 1428–1438 CrossRef CAS
.
- J. Alongi, M. Ciobanu and G. Malucelli, Carbohydr. Polym., 2011, 85, 599–608 CrossRef CAS
.
- A. Cireli, N. Onar, M. Ebeoglugil, I. Kayatekin, B. Kutlu, O. Culha and E. Celik, J. Appl. Polym. Sci., 2007, 105, 3747–3756 CrossRef CAS
.
- J. Alongi, C. Colleoni, G. Rosace and G. Malucelli, Polym. Degrad. Stab., 2014, 99, 92–98 CrossRef CAS
.
- J. Alongi, C. Colleoni, G. Rosace and G. Malucelli, Cellulose, 2013, 20, 525–535 CrossRef CAS
.
- J. Alongi, C. Colleoni, G. Malucelli and G. Rosace, Polym. Degrad. Stab., 2012, 97, 1334–1344 CrossRef CAS
.
- G. Brancatelli, C. Colleoni, G. Rosace and M. Massafra, Polym. Degrad. Stab., 2011, 96, 483–490 CrossRef CAS
.
- J. Alongi, C. Colleoni, G. Rosace and G. Malucelli, J. Therm. Anal. Calorim., 2012, 110, 1207–1216 CrossRef CAS
.
- G. Malucelli, F. Bosco, J. Alongi, F. Carosio, A. Di Blasio, C. Mollea, F. Cuttica and A. Casale, RSC Adv., 2014, 4, 46024–46039 RSC
.
- J. Alongi, F. Bosco, F. Carosio, A. Di Blasio and G. Malucelli, Mater. Today, 2014, 17, 152–153 CrossRef
.
- J. Alongi, R. A. Carletto, A. Di Blasio, F. Cuttica, F. Carosio, F. Bosco and G. Malucelli, Carbohydr. Polym., 2013, 96, 296–304 CrossRef CAS PubMed
.
- J. Alongi, R. A. Carletto, A. Di Blasio, F. Carosio, F. Bosco and G. Malucelli, J. Mater. Chem. A, 2013, 1, 4779–4785 CAS
.
- F. Carosio, A. Di Blasio, J. Alongi and G. Malucelli, Polymer, 2013, 54, 5148–5153 CrossRef CAS
.
- F. Bosco, R. A. Carletto, J. Alongi, L. Marmo, A. Di Blasio and G. Malucelli, Carbohydr. Polym., 2013, 94, 372–377 CrossRef CAS PubMed
.
- J. Alongi, R. A. Carletto, F. Bosco, F. Carosio, A. Di Blasio, F. Cuttica, V. Antonucci, M. Giordano and G. Malucelli, Polym. Degrad. Stab., 2014, 99, 111–117 CrossRef CAS
.
- F. Carosio, A. Di Blasio, F. Cuttica, J. Alongi and G. Malucelli, Ind. Eng. Chem. Res., 2014, 53, 3917–3923 CrossRef CAS
.
- ISO 6330, Textiles – Domestic washing and drying procedures for textile washing, Geneva, 2000 Search PubMed
.
- J. Alongi, M. Ciobanu and G. Malucelli, Cellulose, 2011, 18, 1335–1348 CrossRef CAS
.
- J. Alongi, A. Di Blasio, F. Carosio and G. Malucelli, Polym. Degrad. Stab., 2014, 107, 74–81 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2015 |