Asphaltene: structural characterization, molecular functionalization, and application as a low-cost filler in epoxy composites†
Abstract
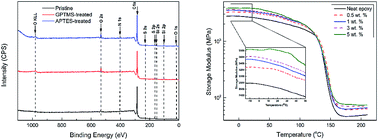
Asphaltene obtained by extraction from asphalt was investigated by different analytical techniques in order to characterize its composition, molecular structure and morphology. Then, the asphaltene molecules were successfully functionalized by 3-glycidyloxypropyltrimethoxysilane and 3-aminopropyltriethoxysilane as confirmed by thermogravimetric analysis, X-ray photoelectron spectroscopy, and Fourier transform infrared spectroscopy. Finally, asphaltene/epoxy composites at four different loading levels were prepared and their thermo-mechanical properties were examined. The thermal analysis results indicated that asphaltene as a novel reinforcing filler in epoxy resin caused a significant increase in storage modulus of both glassy and rubbery regions, slightly increased the glass transition temperature without negatively affecting thermal stability, and reduced the overall cost of the material.


 Please wait while we load your content...
Please wait while we load your content...