Fabrication and in vivo chondrification of a poly(propylene carbonate)/L-lactide-grafted tetracalcium phosphate electrospun scaffold for cartilage tissue engineering†
JiaoJiao Denga,
YueLong Wanga,
LiangXue Zhou*a,
MaLing Goua,
Na Luob,
HaiFeng Chena,
AiPing Tonga,
Chao Youa and
Gang Guo*a
aState Key Laboratory of Biotherapy and Cancer Center, Department of Neurosurgery, West China Hospital, Sichuan University and Collaborative Innovation Center for Biotherapy, Chengdu, 610041, PR China. E-mail: guogang1999@sina.com; liangxue_zhou@126.com; Fax: +86 28 85164060; Tel: +86 28 85164063
bNankai University School of Medicine, Tianjin, 300071, PR China
First published on 8th May 2015
Abstract
Regenerative therapies that utilize stem cell differentiation in three-dimensional porous scaffolds have attracted significant interest in recent years. In this study, fibrous poly(propylene carbonate)/poly(L-lactic acid)-grafted tetracalcium phosphate (PPC/g-TTCP) scaffolds were prepared using an electrospinning method. The characteristics of the fabricated scaffolds were investigated using scanning electron microscopy, differential scanning calorimetry, thermogravimetric analyses, Fourier transform infrared spectroscopy, X-ray diffraction analyses, water contact angle measurements and tensile tests. Due to the importance of biocompatibility, rat bone marrow-derived stem cells were cultured on the scaffolds, and the cell proliferation was investigated using 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide assays. Subsequently, chondrogenic differentiation was induced in these cells in vitro and in vivo. Fourteen days later, chondrocyte-like cells had developed on the PPC/g-TTCP scaffolds, as evidenced by the accumulation of glycosaminoglycan and type II collagen. After subcutaneous transplantation into nude mice, a typical cartilage cell morphology was observed on the scaffolds. These findings suggest that PPC/g-TTCP scaffolds can support cartilage development and are excellent candidate scaffolds for cartilage defect repair.
1. Introduction
Due to the absence of an abundant blood supply and its nearly acellular nature, cartilage lacks the innate ability to be restored to its normal function and structure after damage.1 The concept of cartilage repair promises healing of damaged tissues via the use of living, functional constructs. However, the only current therapeutic options for cartilage and related diseases are pain management and surgical intervention. In many cases, the outcome is the formation of fibrocartilaginous repair tissue that does not possess the full load-bearing properties and durability of healthy articular cartilage.2 Based on their respective advantages, disadvantages, and limitations, no single strategy, or even combination of strategies, provides researchers with approved outcomes. The significance of cartilage injury requires novel cartilage tissue engineering strategies.3To date, bone marrow-derived stem cells (BMSC) have been tested in clinical trials for several orthopedic applications, including articular cartilage repair.4–6 BMSC are an attractive resource for clinical applications due to their potential to differentiate into several kind of mesenchymal lineages including cartilage, bone, and fat.7,8 Their harvesting methods in humans are minimally invasive, and they proliferate more readily ex vivo than osteoblasts.9 However, the application of BMSC for cartilage production usually requires appropriate scaffolds that can serve as supports for new tissue formation.2 In order for a tissue-engineered construct to achieve functional properties, it must mimic the architecture of the native tissue. From a structural perspective, natural extracellular matrix (ECM) consists of various interwoven protein fibers with diameters ranging from tens to hundreds of nanometers. To this end, various techniques are being developed to design and prepare 3D scaffolds.10 Electrospinning is one of the most economical and advantageous techniques to fabricate fibrous scaffolds that mimic the nanoscale structures of the ECM. In addition, development of nanofibers has greatly improved the scope for preparing scaffolds that can imitate the architecture of natural cartilage tissues in the nanoscale. The large surface area of electrospun nanofibers as well as their porous structure favors cell adhesion, proliferation, migration, and differentiation.11,12 This technique can produce polymer fibers with diameters down to nanoscale dimensions with porous structure, allowing scaffolds to maintain spherical chondrocyte morphology and phenotype. Due to their suitable physical and biological properties, these electrospun fibers have been applied for many specific applications including cartilage engineering.13,14
Poly(propylene carbonate) (PPC) is a polymer which synthesized from propylene oxide and CO2. Due to its desirable mechanical properties, biodegradability and benign degradation by-products of CO2 and water, this kind of aliphatic polycarbonate has been utilized in many applications such as adhesives, photoresists, barrier materials and tissue engineering applications.15,16 The wide application of PPC material not only reduces the dependence on petroleum but also lowers the massive emission of CO2 that has been considered to be the main factor causing the greenhouse effect in the world. However, its hydrophobicity and poor biocompatibility impedes its use in more in vivo applications. Hydrophilic particles are commonly added to the polymer to solve these problems.17 Tetracalcium phosphate (TTCP, Ca4(PO4)2O) is known to be the only calcium phosphate with a Ca/P ratio greater than hydroxyapatite (HA) that elicits an excellent tissue response, can bioresorb and is osteoconductive.18 The interfacial adhesion between the calcium phosphate particles and polymer matrix is a key issue associated with the construction of nanocomposites.19 The surface functionalization of TTCP can play a significant role in producing well-dispersed TTCP/polymer composites. In our previous study, TTCP particles were modified with L-lactide via a ring-opening polymerization reaction on their surface to enhance their dispersibility, biocompatibility and interfacial interactions.20,21
The self-renewal of BMSC is the consequence of the cell division that occur within the stem cell niche of the microenvironment.22,23 Nanofibrous scaffolds can recapitulate both the structural features of the stem cell niche and the material features of the matrix via modification of the fiber surfaces to mimic biochemical cues.24
Therefore, PPC scaffolds containing different percentages of g-TTCP nanoparticles were successfully fabricated in this work using electrospinning. The physical and chemical properties of the scaffolds were analyzed with scanning electron microscopy (SEM), water contact angle measurements, differential scanning calorimetry (DSC), thermogravimetric analyses (TG), Fourier transform infrared (FTIR) spectroscopy and X-ray diffraction (XRD) analyses and tensile tests. The biocompatibility was investigated with a 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) assay in vitro. In addition, we successfully induced the differentiation of third-generation rat BMSC that were cultured on the scaffolds into cartilage cells with transforming growth factor-beta1 (TGF-β1). For further application in cartilage repair in vivo, the constructs were subcutaneously transplanted into nude mice for chondrification.
2. Materials and methods
2.1. Materials
The following materials were used to fabricate the fibrous scaffolds: poly(propylene carbonate) (PPC) (Henan Tianguan Co., Ltd. China), dichloromethane (DCM) (Kelong Chemical, Chengdu, China) and poly(L-lactic acid) (PLA)-grafted TTCP (g-TTCP) particles. To decrease the aggregation of TTCP (Ensail Beijing Co., Ltd. China) in composites and develop better cartilage substitute materials, a series of g-TTCP powders was prepared via a ring-opening polymerization with L-lactide (the monomer for synthesizing PLA) in the presence of the catalyst stannous octoate [Sn(Oct)2].25 All other used chemical agents used in this study were of analytical reagent grade.2.2. Preparation of PPC/TTCP and PPC/g-TTCP hybrid composite scaffolds
Solutions of PPC/g-TTCP were prepared using the electrospinning method. The PPC, TTCP and g-TTCP powder were dried in a vacuum oven at room temperature for 48 hours before use. The TTCP and g-TTCP particles (2, 5, 10 and 15 wt%) were initially dispersed in DCM to form a suspension. The PPC was then dissolved in TTCP/DCM or g-TTCP/DCM with vigorous stirring overnight to generate a 8% (w/v) homogeneous solution. The solution was continuously electrospun from a 20 ml syringe with a steel needle (inner diameter of 0.7 mm) at a rate of 7 ml h−1 with a programmable syringe pump (Smith Medical Instrument Company, Zhejiang, China). A voltage (18 kV) was applied to the tip of the needle by a high-voltage supply (High Voltage Technology Institute, Beijing, China) when the fluid jet was ejected at 40% humidity and room temperature (26 ± 2 °C). To obtain randomly arranged PPC fibrous scaffolds, a metal rotating plate (radius = 20 cm) was used as a collector at a distance of 15 cm from the tip of the needle. Neat PPC scaffolds were fabricated as a control. All obtained electrospun fibrous scaffolds were vacuumed for 24 h in a vacuum oven to eliminate any potential residual solvents for further characterization studies.2.3. Characterization
| θ = arctan(2h/b) |
XRD measurements were used to study the phase and crystallinity of the PPC/g-TTCP composite samples using an X'Pert Pro MPD DY1291 (PHILIPS, Netherlands) diffractometer. The X-ray diffraction was studied using graphite-monochromatized Cu K radiation (λ = 0.1542 nm; 40 kV; 40 mA) at a scanning rate of 4° per minute.
The mechanical behavior of the fibrous scaffolds containing different percentages of g-TTCP was also assessed using dynamic mechanical measurements (Instron 5567, Instron Corp., USA) at room temperature and a relative humidity of 60%. All samples were rectangular with dimensions of 50 × 5 mm2 and a thickness of 0.15 to 0.25 mm. The cross-head speed was 20 mm min−1, and the gauge length was 20 mm. The stress and strain were calculated from the machine-recorded force and displacement based on the initial cross-sectional area and gauge length, respectively.
2.4. In vitro biocompatibility
The cytotoxicity of the neat PPC and PPC/g-TTCP scaffolds containing different amounts of g-TTCP was also investigated. Briefly, the samples were fixed in each bottom of the 24-well cell culture plate and sterilized with ethylene oxide (ETO) steam for 24 h at room temperature. Subsequently, 3 × 103 293 T cells were seeded evenly onto each sample. The culture medium was changed every 3 days. After seeding for 1, 3, and 5 days, the cytotoxicity of the scaffolds was tested with a MTT assay.
The immunohistochemical staining procedures were performed in accordance with the instruction manual of the reagent kit (mouse anti-rabbit type II collagen monoclonal antibody (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250)). SABC-Cy3 was used for coloration, and the immunostained cells were then counterstained with DAPI (4′,6-diamidino-2-phenylindole).
250)). SABC-Cy3 was used for coloration, and the immunostained cells were then counterstained with DAPI (4′,6-diamidino-2-phenylindole).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2000 mouse anti-rabbit IgG was added. After 1 h of incubation, the PVDF membranes were washed three times with 10% TBST. The protein expression was quantitatively analyzed using enhanced chemiluminescence (ECL).
2000 mouse anti-rabbit IgG was added. After 1 h of incubation, the PVDF membranes were washed three times with 10% TBST. The protein expression was quantitatively analyzed using enhanced chemiluminescence (ECL).2.5. Foreign body response and cartilage formation in vivo
We performed a subcutaneous implant study to assess the inflammatory response to the fibers. The neat PPC and 10 wt% PPC/g-TTCP scaffolds were transplanted into SD rats. All animal care and experimental procedures were conducted according to institutional Animal Care and Use guidelines. After 4 weeks, animals from each material group were euthanized and the implants were harvested. The harvested samples were fixed, paraffin-embedded and sectioned (5 μm) according to standard protocols for hematoxylin and eosin (HE) staining.To assess cartilage formation in vivo, the neat PPC and 10 wt% PPC/g-TTCP scaffolds cultured with induced BMSC (21 days) were subcutaneously implanted into nude mice. After 8 weeks, all nude mice were sacrificed. The implanted scaffolds were excised and stained with HE and safranin o. Collagen II staining was performed following the manufacturer's instructions. All results were analyzed with a digital image analysis system (Eclipse E600 microscope with a DXM 1200 digital camera; Nikon Corporation, Tokyo, Japan).
2.6. Statistical analysis
The data collected from the MTT assays are expressed as the mean ± standard deviation. A statistical analysis was performed using the SPSS 17.0 software (Chicago, IL, USA), and the statistical significance of the difference between groups was determined using a one-way analysis of variance.3. Results and discussion
3.1. Morphological observation of the scaffolds
The morphology of the electrospun fibrous scaffolds was observed using SEM (Fig. 1A–E). Random, beadless, electrospun fibrous scaffolds with fiber diameters ranging from 220 nm to 3390 nm were formed under controlled conditions. The TTCP and g-TTCP particle sizes were about 470–1170 nm and 510–1110 nm, respectively (ESI 1†). The diameter positively correlated with the g-TTCP nanoparticle content, which suggested that the formation of fibers primarily is a function of the viscosity. The fibers were smooth and continuous when the g-TTCP particle content was low (<10 wt%), indicating that the g-TTCP nanoparticles were evenly dispersed in the scaffolds. However, the fibers thinned and roughened when the g-TTCP particle content increased to 15 wt%. Larger particles were observed to intercalate between the fibers, while smaller particles tended to adhere to the fiber surface, imparting a rough texture to the scaffolds. According to previous studies, rougher surfaces are desirable in electrospun scaffolds to improve cell attachment and growth and enhance the presence of functional groups and surface hydrophilicity.273.2. Water contact angle
The water contact angle of the PPC/TTCP hybrid scaffolds was found to gradually decrease as the TTCP concentration increased (Fig. 1F). However, the values increased slightly when the TTCP concentration increased to 10 wt%. The contact angle data also indicated that the incorporation of g-TTCP improved the hydrophilicity of the scaffolds compared to the neat PPC scaffolds, except for the scaffolds containing 2 wt% g-TTCP particles. This phenomenon may be due to the smaller surface pores of the 2 wt% composite scaffolds compared to the neat PPC scaffolds, which may prevent the water drop from penetrating into the pores.28,29 However, the contact angles improved as the g-TTCP loading increased when the g-TTCP content exceeded 5 wt%, and all contact angles were lower than that observed for the neat PPC scaffold. This observation may be due to the homogeneous incorporation of the nano-scale g-TTCP particles in the scaffolds, which improved their wettability compared to the neat PPC scaffold.303.3. FTIR and XRD analysis
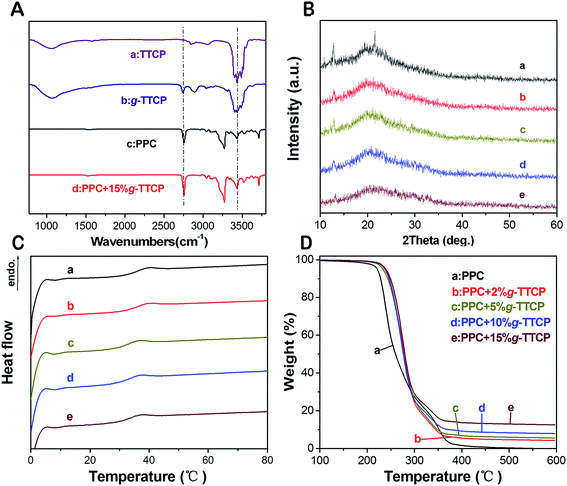
As shown in the FTIR spectra (Fig. 2A), the new bands of the g-TTCP at approximately 1062 cm−1 and 1750 cm−1 were attributed to the vibrations of the CH and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds, and the broad peak at 3200–3500 cm−1 was related to the OH– from the PLA and the absorbed water. Fig. 2A(a and b) show the FT-IR spectra of the neat PPC and the PPC/g-TTCP composites.
O bonds, and the broad peak at 3200–3500 cm−1 was related to the OH– from the PLA and the absorbed water. Fig. 2A(a and b) show the FT-IR spectra of the neat PPC and the PPC/g-TTCP composites.
The XRD patterns of electrospun PPC and PPC/g-TTCP hybrid scaffolds are shown in Fig. 2B. Two strong peaks are evident at 14.5° and 22.9° and are attributed to the crystalline peaks of the PPC copolymer scaffold. One strong peak at 14.5° was due to the g-TTCP/PPC scaffolds, but its intensity was slightly decreased. The peaks are known to broaden as the particle size decreases. The increases in the chemical interaction forces between the PPC molecules and g-TTCP resulted in strong bonding that reduced the crystal size of the composites.13
3.4. Thermal properties analysis and mechanical characterization
All electrospun PPC and PPC/g-TTCP hybrid scaffolds were subjected to DSC to characterize the thermal properties of all samples. The DSC traces of all specimens are shown in Fig. 2C, and the values of Tg are listed in Table 1. The midpoint of the inflection was considered to be the glass transition temperature (Tg), and the melting points were defined as the peak temperatures of the melting endotherms. For neat PPC and 2 wt% PPC/g-TTCP scaffolds, the Tg was observed at approximately 38.0 °C. When the g-TTCP content increased from 5 wt% to 15 wt%, the Tg decreased to approximately 36.5 °C. However, the melting peak was not evident in all samples. Tissue engineering scaffolds with a Tg of 37 °C (physiological temperature) or lower are desirable because scaffolds having a Tg higher than the physiological temperature tend to be brittle and can fracture when subjected to stress during use.31 Thus, the incorporation of g-TTCP particles improves the utility of these scaffolds.| PPC/g-TTCP (wt%) | Tg (°C) | Td,5% (°C) | Td,50% (°C ± SD) | Residual at 600 °C |
|---|---|---|---|---|
| a Glass transition temperature (Tg), and temperature at which 5% (Td, 5%) and 50% (Td, 50%) weight was loss of PPC/g-TTCP electrospun nanofibers containing 0, 2, 5, 10, and 15 wt% g-TTCP. | ||||
| 0 | 38.2 | 236.5 | 26.300 ± 4.680 | 0 |
| 2 | 37.7 | 231.5 | 31.030 ± 4.390 | 4.27 |
| 5 | 36.4 | 238.8 | 38.910 ± 6.973 | 7.16 |
| 10 | 36.9 | 238.8 | 47.870 ± 9.765 | 7.90 |
| 15 | 37.4 | 241.5 | 28.450 ± 5.384 | 12.74 |
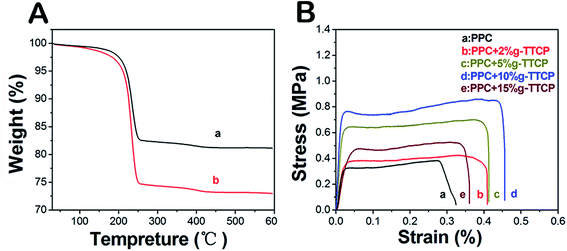
The TG curves of the neat PPC and PPC/g-TTCP are shown in Fig. 2D. The amount of the PPC was determined based on the weight loss percentage during heating, and the temperatures at which 5 and 50% weight loss were observed are listed in Table 1. The high residual obtained from PPC/g-TTCP scaffolds indicated that the g-TTCP particles were well dispersed. Considerable efforts have attempted to improve the composite interfacial bonding based on the layer of hydroxyl groups that covers the outermost surface of the calcium phosphate particles.19 Fig. 3A shows the TG curves of neat TTCP and g-TTCP. The PLA grafting percentage was about 23.3%. The surface functionalization of TTCP may play a significant role in producing well-dispersed calcium phosphate/polymer scaffolds. To the best of our knowledge, the use of TTCP in the surface modification of PPC composite scaffolds has not yet been reported. In this study, TTCP particles were surface-functionalized via a ring-opening polymerization to prepare novel PPC/g-TTCP scaffolds.
The mechanical behaviors of the electrospun PPC and PPC/g-TTCP scaffolds were assessed using a tensile test (Fig. 3B). Table 2 illustrates a comparison of the tensile properties of nanofibers with respect to the tensile stress strain and elastic modulus. Many researchers have reported that low contents of inorganic mineral powders such as nano-HA reinforce the polymer matrix.32,33 This phenomenon was also observed in our study. A preliminary study confirmed that g-TTCP dispersed well in solutions and enhanced the adhesion between the organic and inorganic TTCP phases.25 In our study, g-TTCP, especially low concentrations thereof (<10 wt%), increased the tensile strength of the PPC composite scaffolds due to the perfect distributions of g-TTCP particles in the fibers. However, the tensile strength decreased as the g-TTCP load increased (>15%), revealing that further increases in the g-TTCP weakened the interactions between the PPC matrix and g-TTCP crystal and even compromised the integrity of fibers.
| PPC/g-TTCP (wt%) | Maximum tensile strength (MPa ± SD) | Elongation at break (% ± SD) | Yong's modulus (MPa ± SD) |
|---|---|---|---|
| 0 | 0.323 ± 0.056 | 32.527 ± 5.238 | 26.300 ± 4.680 |
| 2 | 0.382 ± 0.067 | 43.949 ± 9.937 | 31.030 ± 4.390 |
| 5 | 0.632 ± 0.134 | 44.558 ± 3.322 | 38.910 ± 6.973 |
| 10 | 0.793 ± 0.192 | 46.381 ± 3.453 | 47.870 ± 9.765 |
| 15 | 0.476 ± 0.085 | 26.874 ± 2.373 | 28.450 ± 5.384 |
3.5. Cell viability and cytotoxicity test on scaffolds
One day after cell seeding, the majority of the cells on both scaffolds and the glass surface showed the same extension. However, differential cell proliferation was evident 3, 5 and 9 days after cell seeding; the cells rapidly proliferated on glass, but the cells on the PPC or PPC/g-TTCP surface did so to a greater extent. The initial adhesion of BMSC is especially critical for the long-term stability and differentiation of the cells. Our results indicate that the introduction of g-TTCP nanoparticles into the surface of the PPC could significantly promote the initial adhesion of BMSCs to the scaffolds. The cell proliferated more readily on the 15% PPC/g-TTCP scaffold than on PPC nanofibers on day 5 and day 9 (Fig. 4 and 5A(b)). This difference was related to the construction of the g-TTCP in scaffolds, which featured a high surface area and roughness to enhance cell attachment and potentially absorb more proteins and bioactive molecules in vivo to stimulate the cells and accelerate the synthesis of an ECM.14,34 Because many cell behaviors, such as adhesion, proliferation and differentiation, are regulated by the specific interaction of the ECM components and their integrin receptors,35,36 the interaction between the adhesive protein and the integrins on the surface of BMCS likely contributes to the good cellular proliferation compared to scaffolds devoid of any cell-binding structures, such as native PPC. | ||
| Fig. 4 The fluorescence morphology of BMSC grown on scaffolds: neat PPC nanofibers (A–F); 10 wt% PPC/g-TTCP composite nanofibers (G–L). Scale bars represent 50 μm. | ||
The results of the cytotoxicity test of the scaffolds are shown in Fig. 5A(a). None of the samples appeared to be cytotoxic to the BMSC for up to 5 days. The PPC/g-TTCP fibers and their negative control (glass) increased the cell viability throughout the culture.
3.6. Chondrogenesis of BMSC on the scaffolds and western blot analysis
In addition to attachment and proliferation, the ability to support cultured cell differentiation is another significant characteristic of scaffolds. The articular cartilage matrix contains special and unique components, including GAG and type II collagen. Because GAG is acidic, it is metachromatic to safranin o, and the cytoplasm takes on a red color after safranin o staining in the presence of GAG. At the chondrogenesis stage of differentiation, the high expression of the late cartilage differentiation marker type II collagen accompanied by the positive safranin o staining confirmed the maturation of the cartilage (Fig. 5B(a and b) and 6). A number of studies have demonstrated that TGF-β1 can induce BMSC to differentiate into chondrocytes, promote the proliferation and maturation of cartilage cells, and enhance the secretion of GAG and type II collagen.37,38 Therefore, BMSC were assumed to have differentiated into cartilage cells 14 days after induction. In accordance with previous results, the expression of GAG and type II collagen confirmed that the PPC/g-TTCP scaffolds encouraged cells to differentiate into cartilage-associated cells in vitro, which will ultimately be useful in cartilage tissue engineering.A Western blot analysis was used to verify the expression of type II collagen in induced BMSCs on scaffolds. The expression of collagen II by induced BMSCs was higher 7, 14 and 21 days after culture comparing with non-induced cells (Fig. 5B(c and d)). Furthermore, the expression of collagen II in induced BMSC continuously increased over time. Our results indicate that the BMSC seeded on scaffolds grew and proliferated well and that the in vitro construction of artificial cartilage was successful.
3.7. Histologic analysis of tissue response and in vivo chondrogenesis potential
To assess the ability PPC/g-TTCP fibrous scaffolds to elicit an in vivo host response, we performed a subcutaneous implant study in SD rats. Fig. 7 shows that the tissue/material interface did not show visible signs of inflammation or fibrosis 30 days after implantation and that the border between the tissue and the material was clear. A similar outcome was observed on the neat PPC scaffolds, which indicated that both of these composite scaffolds are biocompatible.To evaluate the capacity of the induced BMSC cultured on scaffolds to differentiate into cartilage-like cells in vivo, the implants containing induced cells were excised and stained with HE, safranin o or relevant antibodies. Inverted microscopy of the HE-stained sections revealed round and extensively distributed chondrocytes in the 10 wt% PPC/g-TTCP scaffolds with abundant ECM (Fig. 8G and J). In contrast, this phenomenon was not observed in the PPC scaffolds lacking g-TTCP particles (Fig. 8A and D). Additionally, the cells on PPC/g-TTCP fibrous scaffolds expressed more GAG and collagen II compared with those on the neat PPC groups based on safranin o and immunohistochemistry staining (Fig. 8B and F). This difference was due to the differentiation of progenitor cells along a chondrocyte lineage, which causes them to transition from a state of proliferation to a differentiated phenotype that is defined by matrix maturation.39 Therefore, the rate of cell proliferation is an important initial indicator of chondrocyte differentiation because the level of proliferation will begin to plateau as this phenotypic shift occurs. A previous study reported that TTCP can promote the collagen synthesis of the extracellular matrix.40 In other words, the presence of g-TTCP in composite scaffolds can effectively promote the chondrogenesis of BMSC. These findings indicate that cartilage cell formation, including cartilage-specific ECM formation, occurred in PPC/g-TTCP scaffolds.
In addition to the outstanding physicochemical properties and biocompatibility of these scaffolds, the scaffold-mediated differentiation of BMSCs leads to potent differentiation toward a chondrogenic lineage and accumulation of a cartilage ECM in vitro and in vivo.
4. Conclusion
Electrospun PPC fibrous scaffolds with different g-TTCP concentrations were successfully fabricated in the presented work. Our results suggest that the embedded g-TTCP particles changed the thermal stability and degree of crystallinity of the PPC scaffolds. Moreover, the g-TTCP particles further improved the mechanical properties of the PPC scaffolds. In vitro, BMSC culture confirmed that the PPC/g-TTCP composite scaffolds promoted cell adhesion and proliferation. These results show that the composite scaffold is a candidate scaffold for cartilage tissue engineering.Declaration of interest statement
The authors declare no conflicts of interest. The authors alone are responsible for the content and writing of the paper.Acknowledgements
This work was financially supported by National Natural Sciences Foundation of China (81372446), National S&T Major Project (2011ZX09102-001-10 and 2013ZX09301304-007). The authors deeply appreciate Wang Hui, Wen Jiqiu and Zhu Xiaohong (Analytical & Testing Center, Sichuan University) for their great help on SEM observation, XRD and FTIR measurement, respectively.References
- M. D. Crema, J. Cibere, E. C. Sayre, F. W. Roemer, H. Wong, A. Thorne, J. Singer, J. M. Esdaile, M. D. Marra, J. A. Kopec, S. Nicolaou and A. Guermazi, The relationship between subchondral sclerosis detected with MRI and cartilage loss in a cohort of subjects with knee pain: the knee osteoarthritis progression (KOAP) study, Osteoarthritis Cartilage, 2014, 22, 540 CrossRef CAS PubMed.
- P. Ducheyne, R. L. Mauck and D. H. Smith, Biomaterials in the repair of sports injuries, Nat. Mater., 2012, 11, 652 CrossRef CAS PubMed.
- J. R. Fuchs, B. A. Nasseri and J. P. Vacanti, Tissue engineering: a 21st century solution to surgical reconstruction, Ann. Thorac. Surg., 2001, 72, 577 CrossRef CAS.
- M. A. Moorman, D. W. Simonetti, S. Craig and D. R. Marshak, Multilineage potential of adult human mesenchymal stem cells, Science, 1999, 284, 143 CrossRef.
- H. Nejadnik, J. H. Hui, E. P. Feng Choong, B. C. Tai and E. H. Lee, Autologous bone marrow-derived mesenchymal stem cells versus autologous chondrocyte implantation: an observational cohort study, Am. J. Sports Med., 2010, 38, 1110 CrossRef PubMed.
- S. Wakitani, M. Nawata, K. Tensho, T. Okabe, H. Machida and H. Ohgushi, Repair of articular cartilage defects in the patello-femoral joint with autologous bone marrow mesenchymal cell transplantation: three case reports involving nine defects in five knees, J. Tissue Eng. Regener. Med., 2007, 1, 74 CrossRef PubMed.
- P. Sreejit and R. S. Verma, Enhanced cardiomyogenic lineage differentiation of adult bone-marrow-derived stem cells grown on cardiogel, Cell Tissue Res., 2013, 353, 443 CrossRef CAS PubMed.
- R. F. Canadas, J. M. Cavalheiro, J. D. Guerreiro, M. C. de Almeida, E. Pollet, C. L. da Silva, M. M. da Fonseca and F. C. Ferreira, Polyhydroxyalkanoates: waste glycerol upgrade into electrospun fibrous scaffolds for stem cells culture, Int. J. Biol. Macromol., 2014, 71, 131–140 CrossRef CAS PubMed.
- M. F. Pittenger, A. M. Mackay, S. C. Beck, R. K. Jaiswal, R. Douglas, J. D. Mosca, M. A. Moorman, D. W. Simonetti, S. Craig and D. R. Marshak, Multilineage potential of adult human mesenchymal stem cells, Science, 1999, 284, 143 CrossRef CAS.
- D. J. Huey, J. C. Hu and K. A. Athanasiou, Unlike bone, cartilage regeneration remains elusive, Science, 2012, 338, 917 CrossRef CAS PubMed.
- J. S. Park, M. S. Shim, S. H. Shim, H. N. Yang, S. Y. Jeon, D. G. Woo, D. R. Lee, T. K. Yoo and K. H. Park, Chondrogenic potential of stem cells derived from amniotic fluid, adipose tissue, or bone marrow encapsulated in fibrin gels containing TGF-β3, Biomaterials, 2011, 32, 8139 CrossRef CAS PubMed.
- G. Guo, S. Fu, L. Zhou, H. Liang, M. Fan, F. Luo, Z. Qian and Y. Wei, Preparation of curcumin loaded poly(ε-caprolactone)-poly(ethylene glycol)-poly(ε -caprolactone) nanofibers and their in vitro antitumor activity against Glioma 9L cells, Nanoscale, 2011, 3, 3825 RSC.
- P. Ghosh, A. P. Rameshbabu, N. Dogra and S. Dhara, 2, 5-Dimethoxy 2, 5-dihydrofuran crosslinked chitosan fibers enhance bone regeneration in rabbit femur defects, RSC Adv., 2014, 4, 19516–19524 RSC.
- X. He, B. Feng, C. Huang, H. Wang, Y. Ge, R. Hu, M. Yin, Z. Xu, W. Wang, W. Fu and J. Zheng, Electrospun gelatin/polycaprolactone nanofibrous membranes combined with a coculture of bone marrow stromal cells and chondrocytes for cartilage engineering, Int. J. Nanomed., 2015, 10, 2089 CAS.
- M. Xi, J. Jin and B. Y. Zhang, Surface modification of poly (propylene carbonate) by layer-by-layer assembly and its hemocompatibility, RSC Adv., 2014, 4, 38943–38950 RSC.
- J. Zhao, W. Han, H. Chen, M. Tu, S. Huan, G. Miao, R. Zeng, H. Wu, Z. Cha and C. Zhou, Fabrication and in vivo osteogenesis of biomimetic poly(propylene carbonate) scaffold with nanofibrous chitosan network in macropores for bone tissue engineering, J. Mater. Sci.: Mater. Med., 2012, 23, 517 CrossRef CAS PubMed.
- J. Frantzén, A. Pälli, E. Kotilainen, H. Heino, B. Mannerström, H. Huhtala, H. Kuokkanen, G. K. Sándor, K. Leino, M. Röyttä, R. Parkkola, R. Suuronen, S. Miettinen, H. T. Aro and S. Haimi, In Vivo and In Vitro Study of a Polylactide-Fiber-Reinforced β-Tricalcium Phosphate Composite Cage in an Ovine Anterior Cervical Intercorporal Fusion Model, Int. J. Biomater., 2011, 2011, 109638 Search PubMed.
- C. Moseke and U. Gbureck, Tetracalcium phosphate: Synthesis, properties and biomedical applications, Acta Biomater., 2010, 6, 3815 CrossRef CAS PubMed.
- P. Zhang, Z. Hong, T. Yu, X. Chen and X. Jing, In vivo mineralization and osteogenesis of nanocomposite scaffold of poly(lactide-co-glycolide) and hydroxyapatite surface-grafted with poly(L-lactide), Biomaterials, 2009, 30, 58–70 CrossRef CAS PubMed.
- R. Fan, X. Deng, L. Zhou, X. Gao, M. Fan, Y. Wang and G. Guo, Injectable thermosensitive hydrogel composite with surface-functionalized calcium phosphate as raw materials, Int. J. Nanomed., 2014, 9, 615 Search PubMed.
- H. J. Lee, H. W. Choi, K. J. Kim and C. L. Lee, Modification of Hydroxyapatite Nanosurfaces for Enhanced Colloidal Stability and Improved Interfacial Adhesion in Nanocomposites, Chem. Mater., 2006, 18, 5111 CrossRef CAS.
- D. J. Prockop, Marrow stromal cells as stem cells for nonhematopoietic tissues, Science, 1997, 276, 71 CrossRef CAS.
- L. Zhao, S. Jiang and B. M. Hantash, Transforming growth factor beta1 induces osteogenic differentiation of murine bone marrow stromal cells, Tissue Eng., Part A, 2010, 16, 725 CrossRef CAS PubMed.
- T. M. Farooque, C. H. Camp, C. K. Tison, G. Kumar, S. H. Parekh and C. G. Simon, Measuring stem cell dimensionality in tissue scaffolds, Biomaterials, 2014, 35, 2558 CrossRef CAS PubMed.
- R. Fan, L. Zhou, W. Song, X. Li, D. Zhang, R. Ye, Y. Zheng and G. Guo, Preparation and properties of g-TTCP/PBS nanocomposites and its in vitro biocompatibility assay, Int. J. Biol. Macromol., 2013, 59, 227 CrossRef CAS PubMed.
- J. Yang, W. Tian, Q. Li, Y. Li and A. Cao, Novel biodegradable aliphatic poly(butylene succinate-co-cyclic carbonate)s bearing functionalizable carbonate building blocks: II. Enzymatic biodegradation and in vitro biocompatibility assay, Biomacromolecules, 2004, 5, 2258 CrossRef CAS PubMed.
- T. J. Webster, R. W. Siegel and R. Bizios, Osteoblast adhesion on nanophase ceramics, Biomaterials, 1999, 20, 1221 CrossRef CAS.
- Z. Mazrouei-Sebdani, H. Hadadzadeh and M. Zarrebini, Synthesis and performance evaluation of the aerogel-filled PET nanofiber assemblies prepared by electro-spinning, RSC Adv., 2015, 5, 12830–12842 RSC.
- W. Song, X. Yu, D. C. Markel, T. Shi and W. Ren, Coaxial PCL/PVA electrospun nanofibers: osseointegration enhancer and controlled drug release device, Biofabrication, 2013, 5, 035006 CrossRef PubMed.
- A. B. Kutikov and J. Song, An amphiphilic degradable polymer/hydroxyapatite composite with enhanced handling characteristics promotes osteogenic gene expression in bone marrow stromal cells, Acta Biomater., 2013, 9, 8354–8364 CrossRef CAS PubMed.
- P. I. P. Park and S. B. Jonnalagadda, Predictors of glass transition in the biodegradable polylactide and poly-lactide-co-glycolide polymers, J. Appl. Polym. Sci., 2006, 100, 1983 CrossRef CAS PubMed.
- V. Thomas, D. R. Dean, M. V. Jose, B. Mathew, S. Chowdhury and Y. K. Vohra, Nanostructured biocomposite scaffolds based on collagen coelectrospun with nanohydroxyapatite, Biomacromolecules, 2007, 8, 631 CrossRef CAS PubMed.
- Y. Hu, X. Gu, Y. Yang, J. Huang, M. Hu, W. Chen, Z. Tong and C. Wang, Facile fabrication of poly(L-lactic acid)-grafted hydroxyapatite/poly(lactic-co-glycolic acid) scaffolds by pickering high internal phase emulsion templates, ACS Appl. Mater. Interfaces, 2014, 6, 17166 CAS.
- P. H. Chao Grace, H. Y. Hsu and H. Y. Tseng, Electrospun microcrimped fibers with nonlinear mechanical enhance ligament fibroblast phenotype, Biofabrication, 2014, 6, 035008 CrossRef PubMed.
- M. A. Schwartz, M. D. Schaller and M. H. Ginsberg, Integrins: emerging paradigms of signal transduction, Annu. Rev. Cell Dev. Biol., 1995, 11, 549 CrossRef CAS PubMed.
- C. K. Chan, C. C. Chen, C. A. Luppen, J. B. Kim, A. T. DeBoer, K. Wei, J. A. Helms, C. J. Kuo, D. L. Kraft and I. L. Weissman, Endochondral ossification is required for haematopoietic stem-cell niche formation, Nature, 2009, 457, 490 CrossRef CAS PubMed.
- L. A. Fortier, J. U. Barker, E. J. Strauss, T. M. McCarrel and B. J. Cole, The role of growth factors in cartilage repair, Clin. Orthop. Relat. Res., 2011, 469, 2706 CrossRef PubMed.
- P. W. Kopesky, E. J. Vanderploeg, J. D. Kisiday, D. D. Frisbie, J. D. Sandy and A. J. Grodzinsky, Controlled delivery of transforming growth factor β1 by self-assembling peptide hydrogels induces chondrogenesis of bone marrow stromal cells and modulates Smad2/3 signaling, Tissue Eng., Part A, 2011, 17, 83 CrossRef CAS PubMed.
- X. Xie, Y. Wang, C. Zhao, S. Guo, S. Liu, W. Jia, R. S. Tuan and C. Zhang, Comparative evaluation of MSCs from bone marrow and adipose tissue seeded in PRP-derived scaffold for cartilage regeneration, Biomaterials, 2012, 33, 7008 CrossRef CAS PubMed.
- A. Ehara, K. Ogata, S. Imazato, S. Ebisu, T. Nakano and Y. Umakoshi, Effects of alpha-TCP and TetCP on MC3T3-E1 proliferation, differentiation and mineralization, Biomaterials, 2003, 24, 831 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra04442a |
| This journal is © The Royal Society of Chemistry 2015 |