Biomimetic multi-layered hollow chitosan–tripolyphosphate rod with excellent mechanical performance
Abstract
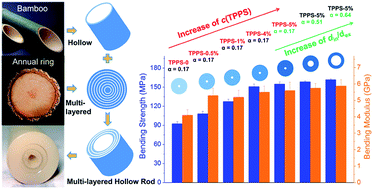
In this work, chitosan–tripolyphosphate hydrogel and dry rods were prepared, which possessed biomimetic features, i.e. multi-layered and hollow features. The ratio of internal to external diameter could be designed and controlled. The relationship between the biomimetic hierarchical structure and mechanical performance was also explored in this research. The resulting rods with three-dimensional, organized structure and excellent mechanical performance have a great potential application in bone tissue engineering.

 Please wait while we load your content...
Please wait while we load your content...