Three-way catalytic performance of a Pd/Ce0.67Zr0.33O2–Al2O3 catalyst for automotive emission control: the role of pretreatment gas
Siyu Lin,
Xue Yang,
Linyan Yang and
Renxian Zhou*
Institute of Catalysis, Zhejiang University, Hangzhou 310028, P. R China. E-mail: zhourenxian@zju.edu.cn; Fax: +86 571 88273283; Tel: +86 571 88273290
First published on 2nd April 2015
Abstract
The influence of oxidizing, reducing and reacting pretreatment gases on an automotive Pd/Ce0.67Zr0.33O2–Al2O3 catalyst is examined and characterized using catalytic performance tests, in situ DRIFTS, CO chemisorption, HRTEM, XPS, H2-TPR and OSC. The pretreatment gas impacts both the Pd particle size and chemical state, giving oxidized state and less growth of Pd in oxidizing gas. In contrast, Pd is mainly in the metallic state and exhibits more growth in reducing gas. In addition, reducing gas pretreatment also increases the concentration of oxygen vacancies. As a result, the catalyst pretreated with oxidizing gas shows good catalytic performance for HC oxidation. Pretreatment with reducing gas not only promotes the conversion of CO, NO and NO2, but also broadens the operation window. However, for the Pd/CZA-RG catalyst pretreated with reacting gas, based on the analysis of in situ DRIFTS, some active sites in the catalyst surface are blocked by strongly adsorbed HC species, hindering NO conversion.
Introduction
Three-way catalysts (TWCs) have been widely used to control and simultaneously diminish pollutant emissions of hydrocarbons (HC), carbon monoxide (CO) and nitrogen oxides (NOx) from gasoline automobiles.1–3 Pd is usually used as the main active metal in TWC systems due to its better resistance to thermal sintering, lower price, and higher activity for HC oxidation than Rh and Pt.4–6 However, the dispersion state, particularly the chemical state of the PdOx species on the support surface, has an important influence on the redox performance of catalysts.7,8 For example, Ciuparu et al.9 reported that the Pd0 species was better than oxidized Pd for NO decomposition at the interface of CeO2. Roth et al.10 reported that in the methane oxidation reaction, Pd/Al2O3 catalysts with a small Pd particle size (<5 nm) showed better oxidation activity because the Pd species could be oxidized more rapidly than big Pd particles (>15 nm). As is known to all, there are many factors influencing the nature of the PdOx species in the catalysts, such as the Pd precursors, pretreatment gas, etc. In our previous study,11 we discovered that a Pd/Ce0.67Zr0.33O2 catalyst prepared with Pd(NO3)2 or Pd(NH3)4(NO3)2 as a precursor had a higher dispersion of PdOx, which promoted CO oxidation, while the catalyst prepared using H2PdCl4 as a precursor exhibited higher catalytic activity for NO conversion, due to a larger particle size. Liu et al.12 studied the effects of the pretreatment atmosphere on the catalytic performance of a Pd/Al2O3 catalyst for benzene degradation and reasoned that the oxidation state of the Pd species decreased with H2 pretreatment, leading to an increase in the catalytic activity, while the oxidation state increased with air pretreatment, accompanied by a decrease in the degradation of benzene. For Pd/Al2O3 three-way catalysts, it is also reported that below the PdO decomposition temperature, PdO particle size under H2 pretreatment conditions shows more pronounced growth than that under O2 gas, which is detrimental for C3H6 oxidation.13 Graham et al.14 studied Pd automotive three-way catalysts prepared with Zr-rich ceria–zirconia supports. They discovered that the Ce 3d spectrum for the catalysts after oxidative treatment is characteristic of Ce4+, while that after reductive treatment shows a mixture of Ce4+ and Ce3+, and H2 reduction becomes increasingly facile with increasing zirconia content.Ceria–zirconia mixed oxides and alumina are widely applied as the main coating materials in TWC systems.15–17 γ-Al2O3 introduced into CeO2–ZrO2 could act as “a diffusion barrier”, resulting in a fine dispersion of precious metals with good thermal stability.18,19 The geometrical and electronic state of Pd affected by Al2O3 would contribute to a desirable performance for NO reduction.20 However, the detailed nature of the Pd species dispersed on the mixed support of ceria–zirconia and alumina is not very clear. In this study, we investigated the influence of the nature of the PdOx on the three-way performance of a Pd/Ce0.67Zr0.33O2–Al2O3 catalyst using three kinds of pretreatment conditions (oxidizing, reducing and reacting gas). The physical and chemical properties were characterized using CO chemisorption, XPS, HRTEM, H2-TPR and in situ DRIFTS, in order to determine the relationship between the catalytic performance and nature of the PdOx.
Experimental
Catalyst preparation
Ce0.67Zr0.33O2 (CZ, SBET = 111 m2 g−1) was prepared using a co-precipitation method.21 The Ce0.67Zr0.33O2–Al2O3 sample (designated as CZA, SBET = 163 m2 g−1) was prepared by mechanically mixing CZ and γ-Al2O3 (supplied by Rhodia Company), with a CZ/γ-Al2O3 mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Pd/Ce0.67Zr0.33O2–Al2O3 catalyst samples with a loading content of 1.0 wt% Pd were prepared with Pd(NO3)2 as the metal precursor, employing the incipient wetness impregnation method at 30 °C. The samples were dried at 110 °C for 4 h and then treated at 500 °C in air, 5% H2/Ar or reaction gas for 2 h. The samples are designated as Pd/CZA-Air, Pd/CZA-H2 and Pd/CZA-RG in turn. The catalysts were also calcined at 1000 °C for 4 h to obtain the aged catalysts, which are designated as Pd/CZA-Air-a, Pd/CZA-H2-a and Pd/CZA-RG-a, respectively.
1. Pd/Ce0.67Zr0.33O2–Al2O3 catalyst samples with a loading content of 1.0 wt% Pd were prepared with Pd(NO3)2 as the metal precursor, employing the incipient wetness impregnation method at 30 °C. The samples were dried at 110 °C for 4 h and then treated at 500 °C in air, 5% H2/Ar or reaction gas for 2 h. The samples are designated as Pd/CZA-Air, Pd/CZA-H2 and Pd/CZA-RG in turn. The catalysts were also calcined at 1000 °C for 4 h to obtain the aged catalysts, which are designated as Pd/CZA-Air-a, Pd/CZA-H2-a and Pd/CZA-RG-a, respectively.
Catalytic performance test
The three-way catalytic performance tests were carried out in a fixed-bed continuous flow reactor at atmospheric pressure. 0.2 ml of the catalyst (0.3–0.45 mm) was used. The reaction mixture, containing NO (0.121%)–NO2 (0.034%)–C3H6 (0.067%)–C3H8 (0.033%)–CO (0.748%)–O2 (0.745%) and balancing Ar, was fed into the reactor at a gas hourly space velocity (GHSV) of 43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h−1. The effluent gas was analyzed using an on-line Fourier transform infrared spectrophotometer (BRUKER EQ55) equipped with a multiple reflection transmission cell (Infrared Analysis Inc.; path length 10.0 m). All spectra were measured at a resolution of 2 cm−1 for 128 scans. The air/fuel ratio experiments were carried out at 400 °C. The concentration of O2 was adjusted in the air/fuel ratio tests from 6620 ppm to 8720 ppm. The λ value of the simulated exhaust, which represents the ratio between the available oxygen and the oxygen needed for full conversion to CO2, H2O and N2, is defined as λ = {2[O2] + [NO] + 2[NO2]}/{9[C3H6] + 10[C3H8] + [CO]}; λ = 1 at stoichiometry and the corresponding concentration of O2 was 7450 ppm.22
000 h−1. The effluent gas was analyzed using an on-line Fourier transform infrared spectrophotometer (BRUKER EQ55) equipped with a multiple reflection transmission cell (Infrared Analysis Inc.; path length 10.0 m). All spectra were measured at a resolution of 2 cm−1 for 128 scans. The air/fuel ratio experiments were carried out at 400 °C. The concentration of O2 was adjusted in the air/fuel ratio tests from 6620 ppm to 8720 ppm. The λ value of the simulated exhaust, which represents the ratio between the available oxygen and the oxygen needed for full conversion to CO2, H2O and N2, is defined as λ = {2[O2] + [NO] + 2[NO2]}/{9[C3H6] + 10[C3H8] + [CO]}; λ = 1 at stoichiometry and the corresponding concentration of O2 was 7450 ppm.22
Characterization techniques
The dispersion of Pd was measured by CO chemisorption at 30 °C, using a CHEMBET-3000 apparatus (Quantachrome Co.). The catalyst was first reduced under H2 and the dispersion was calculated as reported in the literature.23 X-ray photoelectron spectroscopy (XPS) analysis was performed on a Thermo ESCALAB 250 spectrometer with Al Kα radiation (1486.6 eV), operating at 150 W and with energy pass of 20 eV. High resolution transmission electron microscopy (HRTEM) analysis was carried out on a TECNAI G220 apparatus operated at 200 kV. The X-ray energy dispersive spectrometry (XEDS) analysis was used to record elemental maps to determine the chemical composition.Hydrogen temperature-programmed reduction (H2-TPR) experiments were carried out on a GC-1690 chromatograph. Each sample (50 mg) was pretreated under N2 (30 ml min−1) at 200 °C for 30 min and then cooled down to −50 °C in liquid nitrogen. A flow of 5% H2/Ar (40 ml min−1) was then directed into the system, and the temperature was raised to 900 °C at a rate of 10 °C min−1. The consumption of H2 was measured using a thermal conductivity detector (TCD), and the water formed during the program was absorbed with 5A molecular sieves. The oxygen storage capacity complete (OSCC) measurement was carried out using a CHEMBET-3000 apparatus (Quantachrome Co.). A sample (100 mg) was first reduced under H2 (10 ml min−1) at 550 °C for 60 min, and then cooled down to 400 °C and flushed with He (30 ml min−1) for 30 min. 0.15 ml of O2 was pulsed into the sample bed every 5 min until no consumption of oxygen could be detected.
Diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) studies were conducted on a Nicolet 6700 FTIR fitted with an mercury cadmium telluride (MCT) detector. The DRIFTS cell was fitted with CaF2 windows and a heating cartridge that allowed samples to be heated to 500 °C. Spectra were collected at a resolution of 4 cm−1 with 32 scans. DRIFTS spectra were obtained every 40 °C under reaction conditions from 30 to 400 °C. The CO chemisorption DRIFTS experiments were also performed following in situ pretreatment, the same as the Pd dispersion test, and the spectrum was recorded after CO adsorption at 30 °C.
Results and discussion
Catalytic performance test
The conversions of (A) HC, (B) CO, (C) NO and (D) NO2 over the fresh and aged catalysts as a function of reaction temperature with stoichiometric CO + NOx + HC + O2 are shown in Fig. 1. It can be found that for the fresh catalysts the catalytic performance of the Pd/CZA catalysts can be remarkably affected by the pretreatment gas, and the three kinds of catalyst show different behaviors for HC, CO, NO and NO2 conversions. According to the T90 (the temperature to obtain 90% conversion), Pd/CZA-H2 shows the best catalytic performance for CO, NO and NO2 conversions, and Pd/CZA-Air is favourable for HC oxidation. However, the Pd/CZA-RG catalyst exhibits poor catalytic performance for all the reactants. After the aging treatment, the catalytic performance of all the catalysts decreases due to deactivation, which is caused by metal particle growth and adverse metal–support interactions.24–26 In addition, the aged catalysts show less difference than the fresh catalysts in their catalytic performances. | ||
| Fig. 1 Light-off and full-conversion temperatures of HC (A), CO (B), NO (C) and NO2 (D) under the reaction conditions over different catalysts. | ||
Fig. 2 shows the results of the operation window experiments carried out under different air/fuel ratios (λ) at 400 °C. As shown in Fig. 2, the left side of the theoretical stoichiometric value (λ < 1.0) is low oxygen conditions and the right (λ > 1.0) is oxygen-rich conditions. For the fresh catalysts, the conversion of HC reaches 100% over all the catalysts in the test range from 0.90 to 1.15. All the catalysts show 100% CO conversion under oxygen-rich conditions, and under low oxygen conditions the CO conversion performance follows the order of Pd/CZA-H2 > Pd/CZA-Air > Pd/CZA-RG. The conversion for NO drops immediately with increasing O2 content when λ > 1.05 for the Pd/CZA-Air and Pd/CZA-RG catalysts, while the Pd/CZA-H2 catalyst still retains good catalytic performance. The conversion of NO2 in all the catalysts reaches almost 100% until λ > 1.10. On the whole, the Pd/CZA-H2 catalyst shows the best operation window, and the order of width W (λ value width, where all reactants reach 90% conversion) is Pd/CZA-H2 (0.18) > Pd/CZA-RG (0.16) > Pd/CZA-Air (0.14). After the aging treatment, the width of the operation window is obviously decreased, especially for the Pd/CZA-RG-a catalyst, and the order of W is Pd/CZA-Air-a (0.11) > Pd/CZA-H2-a (0.10) > Pd/CZA-RG-a (0.08). It should be pointed out that the operation window for NO and NO2 conversion is broadened compared with the fresh catalysts, which is probably due to the increased interaction between Ce0.67Zr0.33O2 and Al2O3 during the aging treatment, as reported in our previous study.19 In order to determine the immanent cause, a series of characterization techniques have been carried out, as detailed below.
 | ||
| Fig. 2 Conversion curves of HC (A), CO (B), NO (C) and NO2 (D) as a function of air/fuel ratio (λ) over fresh and aged catalysts at 400 °C. | ||
In situ DRIFTS studies
In situ DRIFTS was used to reveal the adsorption and desorption state of the various reactants on the surface of the catalysts during the reaction process. The spectra, obtained every 20 °C under the reaction conditions over the fresh Pd/CZA-Air, Pd/CZA-H2 and Pd/CZA-RG catalysts, are shown in Fig. 3 and 4. Bands in the region of 1700–2300 cm−1 can be assigned to NCO and carbonyl species adsorbed on the support and different PdOx sites.27–29 The presence of carbonyls adsorbed on Pd2+ (2160 cm−1) is observed for the Pd/CZA-Air and Pd/CZA-RG catalysts, and only carbonyls chemisorbed on Pd0 (2051 cm−1) are observed for the Pd/CZA-H2 catalyst. This means that the PdOx species mainly exist in the oxidized state in the Pd/CZA-Air and Pd/CZA-RG catalysts, but in the metallic state in the Pd/CZA-H2 catalyst. When the reaction temperature increases up to 110 °C, the adsorbed carbonyls on the Pd/CZA-H2 catalyst disappear due to oxidation and thermal desorption, occurring at a lower temperature than that of other catalysts, which is in agreement with its good low-temperature CO conversion performance. It is interesting that a band (NO–Pd0) at 1710 cm−1, ascribed to NO species adsorbed on metal Pd sites,30,31 is also observed for the Pd/CZA-H2 catalyst, immediately following the disappearance of adsorbed carbonyls, but a strong band at 2248 cm−1, attributed to NCO species absorbed on the alumina surface29 of the Pd/CZA-H2 catalyst, appears when the temperature increases up to 270 °C, indicating that NO may be readily converted via a NO dissociation process on metallic Pd sites and subsequent bonding with CO to form NCO. However, compared with the Pd/CZA-H2 catalyst, the NO–Pd0 band is very weak for the Pd/CZA-Air and Pd/CZA-RG catalysts, and the Al–NCO band doesn’t appear until the temperature increases up to 290 °C. The formation of NCO is an important path for NO reduction; thus, this band is usually regarded as a useful fingerprint for monitoring the NO dissociation process.27,29 This means that the Pd/CZA-H2 catalyst has better NO reduction activity, in agreement with the catalytic performance test results. | ||
| Fig. 3 DRIFTS spectra (1100–2300 cm−1) for fresh (A) Pd/CZA-Air, (B) Pd/CZA-H2 and (C) Pd/CZA-RG catalysts under stoichiometric CO + NOx + HC + O2 reaction conditions from 30 to 400 °C. | ||
 | ||
| Fig. 4 DRIFTS spectra (2550–3050 cm−1) for fresh catalysts under stoichiometric CO + NOx + HC + O2 reaction conditions from 30 to 400 °C. | ||
In order to track the conversion of HC as the temperature increased, the high frequency region of 2550–3050 cm−1 is shown in Fig. 4. The weak bands at 2929 and 2854 cm−1 are associated with –CH stretching vibrations of hydrocarbons adsorbed on the catalyst surface.32 Only in the Pd/CZA-RG catalyst can these bands be observed from room temperature. Considering that a weaker nitrite species band below 1200 cm−1 and a lack of nitrate bands between 1640–1200 cm−1 are observed for the Pd/CZA-RG catalyst at low temperature (Fig. 3C), this indicates that some active sites on the catalyst surface are blocked by HC species in the initial state, which leads to self-poisoning caused by the hydrocarbon33,34 and hence inhibits NO conversion. However, for the Pd/CZA-Air catalyst these –CH bands disappear at 290 °C, which is obviously lower than the temperatures for the Pd/CZA-H2 and Pd/CZA-RG catalysts. This means that the HC species adsorbed on Pd/CZA-Air are more easily oxidized, in agreement with the good oxidation activity of HC shown by the catalytic performance test.
Pd dispersion and chemical state
In order to obtain a visible observation of the dispersion state of Pd in the fresh and aged catalysts, an HRTEM study was carried out. According to Fig. 5A–C and the representative elemental mapping of the PdOx species in the Pd/CZA-Air catalyst, it can be seen that the PdOx particles are uniformly distributed on the surface of the support with a mean particle size smaller than 5 nm in the fresh catalysts. What should be pointed out is that the Pd particles in Pd/CZA-H2 seem a little bigger than those in the other catalysts. After the aging treatment, the PdOx particle size is obviously increased compared with the fresh catalysts as a result of sintering. | ||
| Fig. 5 HRTEM images of (A) Pd/CZA-Air (inset: elemental mapping of the PdOx species), (B) Pd/CZA-H2, (C) Pd/CZA-RG, (D) Pd/CZA-Air-a, (E) Pd/CZA-H2-a and (F) Pd/CZA-RG-a catalysts. | ||
The dispersion state of the PdOx species on the support surface is studied using CO pulse adsorption and in situ DRIFTS to obtain more information. Fig. 6 displays the in situ DRIFTS of the CO chemisorption experiments on pre-reduced catalysts at 30 °C, which is considered to be a useful technology to probe the catalyst surface.35,36 This is due to the fact that the CO adsorption band position on the metal Pd depends on the configuration of the metal sites, nature of exposed faces and particle size.35 The DRIFTS results show the adsorption of CO on metal Pd sites in two main regions. The first region of 2115–2025 cm−1 is assigned to carbonyl species chemisorbed on the steps and edges of the rows of metallic palladium in linear form (CO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pd = 1
Pd = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); the second (1990–1800 cm−1) is associated with a pillbox morphology of large Pd metal particles in bridging form (CO
1); the second (1990–1800 cm−1) is associated with a pillbox morphology of large Pd metal particles in bridging form (CO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pd = 1
Pd = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2).23,36,37 As can be seen from Fig. 6A, compared with the Pd/CZA-H2 and Pd/CZA-RG catalysts, the intensity of CO adsorption in the liner form is much stronger over Pd/CZA-Air, and this kind of Pd species may be helpful for HC conversion. The total area of the two CO adsorption bands is in the order of Pd/CZA-Air > Pd/CZA-RG > Pd/CZA-H2. In addition, relatively low intensity bands in the region of 1650–1200 cm−1 are observed, which can be ascribed to bicarbonate , bidentate and bridged carbonate species.38 After the aging treatment, the CO adsorption peaks decrease greatly in intensity and show a similar CO adsorption mode over the catalysts, due to the significant growth of the PdOx particles.
2).23,36,37 As can be seen from Fig. 6A, compared with the Pd/CZA-H2 and Pd/CZA-RG catalysts, the intensity of CO adsorption in the liner form is much stronger over Pd/CZA-Air, and this kind of Pd species may be helpful for HC conversion. The total area of the two CO adsorption bands is in the order of Pd/CZA-Air > Pd/CZA-RG > Pd/CZA-H2. In addition, relatively low intensity bands in the region of 1650–1200 cm−1 are observed, which can be ascribed to bicarbonate , bidentate and bridged carbonate species.38 After the aging treatment, the CO adsorption peaks decrease greatly in intensity and show a similar CO adsorption mode over the catalysts, due to the significant growth of the PdOx particles.
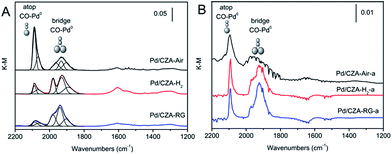 | ||
| Fig. 6 IR spectra of CO adsorbed on the fresh (A) and aged (B) Pd/CZA-Air, Pd/CZA-H2 and Pd/CZA-RG catalysts at 30 °C after the same pretreatment as the Pd dispersion test. | ||
According to the peak areas of each absorbed species in Fig. 6A, the average stoichiometries of CO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pd in the Pd/CZA-Air, Pd/CZA-H2 and Pd/CZA-RG catalysts are 1
Pd in the Pd/CZA-Air, Pd/CZA-H2 and Pd/CZA-RG catalysts are 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5, 1
1.5, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.7 and 1
1.7 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.8, respectively. The order of the Pd dispersion is Pd/CZA-RG (32.9%) > Pd/CZA-Air (29.6%) > Pd/CZA-H2 (24.6%), and the calculated PdOx particle sizes are 3.4, 3.7 and 4.5 nm in turn, in agreement with the HRTEM results. The lower Pd dispersion in the Pd/CZA-H2 catalyst indicates that reducing gas pretreatment may lead to slight agglomeration and more growth of the Pd particles. Considering the catalytic performance test results, it can be inferred that the bigger Pd particles in the Pd/CZA-H2 catalyst may promote NO conversion, because NO conversion is sensitive to the configuration of Pd, as several works have reported.27,29,39 In addition, the high Pd dispersion in the Pd/CZA-Air catalyst may promote good catalytic performance for HC conversion by providing more active sites.
1.8, respectively. The order of the Pd dispersion is Pd/CZA-RG (32.9%) > Pd/CZA-Air (29.6%) > Pd/CZA-H2 (24.6%), and the calculated PdOx particle sizes are 3.4, 3.7 and 4.5 nm in turn, in agreement with the HRTEM results. The lower Pd dispersion in the Pd/CZA-H2 catalyst indicates that reducing gas pretreatment may lead to slight agglomeration and more growth of the Pd particles. Considering the catalytic performance test results, it can be inferred that the bigger Pd particles in the Pd/CZA-H2 catalyst may promote NO conversion, because NO conversion is sensitive to the configuration of Pd, as several works have reported.27,29,39 In addition, the high Pd dispersion in the Pd/CZA-Air catalyst may promote good catalytic performance for HC conversion by providing more active sites.
In order to obtain information on the influence of different pretreatment gases on the chemical state of Pd, Pd 3d XPS studies were carried out. XPS-peak-differentiation analysis was used to avoid the disturbance of Zr 3p and Pd 3d overlap. As shown in Fig. 7, the binding energy of Pd 3d5/2 for Pd/CZA-Air is 337.3 eV, indicating that the PdOx species are in a higher oxidation state such as Pd2+, while the same for Pd/CZA-H2 is 336.4 eV, meaning that the PdOx species are mainly in the Pd0 state.39–42 This is in agreement with the in situ DRIFTS results above. The higher oxidation state of the PdOx species will be helpful for HC oxidation by the Pd/CZA-Air catalyst. On other hand, the higher Pd dispersion of the Pd/CZA-Air catalyst may promote Pd–support interactions, which is beneficial for the electron transfer from Pd to the support due to the lower redox potential of Pd2+/Pd0 than that of Ce4+/Ce3+.39,43 This may also lead to the higher Pd 3d5/2 binding energy for Pd/CZA-Air. The ratio of Pd0/(Pd0 + PdO) is also calculated based on Fig. 7. The percentage of Pd0 is about 81% for the Pd/CZA-H2 catalyst, much higher than those for the Pd/CZA-Air and Pd/CZA-RG catalysts (21% and 33%, respectively). Thus, the Pd/CZA-H2 catalyst shows a better catalytic performance for NO reduction because NO dissociation is most favored over the metallic Pd sites.9,29 The aged catalysts show a similar ratio value of Pd0/(Pd0 + PdO) of about 30%, indicating that a portion of the Pd0 species are re-oxidized in the Pd/CZA-H2 catalyst. Therefore, the aged catalysts show less difference in their catalytic performances.
Oxygen vacancies and OSC studies
The XPS spectra of Ce 3d and O 1s for fresh and aged catalysts, shown in Fig. 8, are used to understand how the nature of the support is influenced by different pretreatment gases. The complex spectrum of Ce 3d was decomposed into eight components, assigned to four pairs of spin–orbit doublets. The labeling and fitting of the peaks follow convention.44–46 Letters u’ and v’ represent the 3d104f1 initial electronic state ascribed to Ce3+. The relative percentage of Ce3+ in the total Ce species is obtained from Fig. 8. For the Pd/CZA-H2 catalyst, the percentage of Ce3+ (27%) is much higher than those in the Pd/CZA-Air and Pd/CZA-RG catalysts (19% and 21%, respectively). As reported in the literature,44,47 Ce3+ is associated with the formation of oxygen vacancies. Thus, this observation means that the reduction pretreatment seems to facilitate the formation of oxygen vacancies. In addition, the O 1s spectrum of ceria-based oxide can be fitted with two peak contributions: the peak (Oα) with a binding energy of 528.9–529.7 eV corresponding to lattice oxygen, and another peak (Oβ) with a binding energy of 531.0–532.0 eV attributed to chemisorbed oxygen.48,49 The ratio of Oβ/(Oβ + Oα) for the Pd/CZA-H2 catalyst (53%) is higher than those for the Pd/CZA-Air and Pd/CZA-RG catalysts (35% and 41%, respectively). This is caused by the higher concentration of oxygen vacancies in the Pd/CZA-H2 catalyst, which is in favor for the formation and migration of chemisorbed oxygen.49,50 The high concentration of oxygen vacancies would also promote the formation of nitrite/nitrate species and the conversion of NO on the catalyst surface, due to NO easily reacting with oxygen vacancies linked to Ce3+ cations,51,52 which promotes the catalytic activity for NO conversion and broadens its operation window. However, for the catalysts pretreated in oxidizing gas, the excess oxygen would enter the interstitial sites of the support, push the interstitial oxygen atoms back into the lattice and fill the oxygen vacancies. This leads to a decrease in oxygen vacancy concentration. For the aged catalysts, due to the sintering of the catalysts, the percentages of Ce3+ and chemisorbed oxygen decrease, and all the catalysts exhibit similar values of about 14% and 30%, respectively.One of the most important characteristics of three-way catalysts in real applications is the oxygen storage capacity (OSC), which makes catalyst able to store oxygen under low oxygen conditions and release it under oxygen-rich conditions. The OSC is determined by not only the nature of support but also the available active metal sites being in close contact with ceria.39,53 The order of the OSC is Pd/CZA-Air (394 μmol gcat−1) > Pd/CZA-RG (376 μmol gcat−1) > Pd/CZA-H2 (326 μmol gcat−1), and these OSC values are obviously higher than that of the CZA support (179 μmol gcat−1). This indicates that the strong Pd–support interaction would promote the back-spillover of oxygen, especially for the Pd/CZA-Air catalyst. The OSC value seems not to correspond with the operation window. In fact, the oxygen vacancies play a more important role in determining the operation window.27,39 For the aged catalysts, the sintering effect results in weakening of the Pd–support interaction, leading to a decrease in the OSC value, for which all catalysts show a similar value of between 221 and 246 μmol gcat−1.
H2-TPR studies
H2-TPR offers a convenient way to reveal information on the redox properties and Pd–support interactions.54 As shown in Fig. 9, two positive peaks and one negative peak can be observed. According to our previous studies,21,22,54 the positive reduction peaks can be assigned to two different species of Pd: the peak observed below 70 °C corresponds to PdOx species highly dispersed on the surface of the support, and another peak, more stable and reducible above 100 °C, can be attributed to the reduction of PdOx species having a strong interaction with the support. The negative peak at about 70 °C is caused by the decomposition of the β-hydride palladium phase, which is formed on hydrogen molecules adsorbed on metallic Pd at room temperature, and the formation is favored by the existence of large Pd particles.55,56 The Pd/CZA-H2 catalyst shows small H2 consumption peaks because the PdOx species are mainly in the metallic state and the catalyst is slightly oxidized at room temperature due to the high concentration of oxygen vacancies, which is beneficial for the formation and migration of chemisorbed oxygen. However, for the Pd/CZA-Air and Pd/CZA-RG catalysts, based on the calculations (using a standard 10 mg CuO sample test with a similar TPR procedure, the peak area corresponds to 125 μmol H2), the H2 consumptions are 327 and 272 μmol gcat−1 for the Pd/CZA-Air and Pd/CZA-RG catalysts, much higher than the PdO theoretical value of 94 μmol gcat−1. This is too large to be reasonably assigned to the reduction of PdO alone, and the reduction of interfacial Ce4+ at low temperature by the spillover of hydrogen from noble metal to support should be taken into account. Fan,44 Norman57 and He58 reported that the addition of a noble metal to the ceria zirconia materials increases the efficiency of the reduction process by the Ce in the support. Thus, the highest H2 consumption in Pd/CZA-Air implies that the Pd–support interaction is stronger than that in the other catalysts, which may be contributed to by the highest Pd dispersion. The negative peak in the Pd/CZA-H2 catalyst is very obvious, in agreement with the Pd dispersion results that the Pd mean particle size is relatively large in the Pd/CZA-H2 catalyst. The TPR for the aged catalysts is shown in Fig. 9B, in which the H2 consumptions of the three kinds of catalyst show a similar value between 184 and 213 μmol gcat−1. Compared with the fresh catalysts, the sintering effect weakens the Pd–support interaction, resulting in a decrease in H2 consumption by the Pd/CZA-Air and Pd/CZA-RG catalysts, while the increase for the Pd/CZA-H2 catalyst indicates that re-oxidation takes place during the aging treatment. Moreover, the growth of the Pd particles is also confirmed by the increased intensity of the negative peak.Conclusions
The effect of different pretreatment gases on the three-way catalytic performance and physicochemical properties of a Pd/Ce0.67Zr0.33O2–Al2O3 catalyst is investigated using catalytic performance tests, in situ DRIFTS, CO chemisorption, HRTEM, XPS, H2-TPR and OSC. The results show that the catalytic performance of the Pd/Ce0.67Zr0.33O2–Al2O3 catalyst for CO, HC and NOx elimination is greatly influenced by the pretreatment gas. The Pd/CZA-Air catalyst pretreated with oxidizing gas shows a good catalytic performance for HC oxidation due to the stronger Pd–support interaction, higher Pd dispersion and the oxidation state of the Pd. The Pd/CZA-H2 catalyst pretreated with reducing gas presents more initial Pd0 and Ce3+ species, and they act as electron donors during reaction process, promoting the NO decomposition. In addition, the higher concentration of oxygen vacancies produced by the reducing gas broadens the operation window. For the Pd/CZA-RG catalyst pretreated with reacting gas, some active sites on the catalyst surface are blocked by strongly adsorbed HC species, hindering NO conversion.Acknowledgements
We gratefully acknowledge the financial support from the Ministry of Science and Technology of China (no.: 2011AA03A406) and Zhejiang Leading Team of Science and Technology Innovation (no.: 2011R09020).Notes and references
- G. Li, B. Zhao, Q. Wang and R. Zhou, Appl. Catal., B, 2010, 97, 41–48 CrossRef CAS PubMed.
- B. Subramanian, S. Y. Christou, A. M. Efstathiou, V. Namboodiri and D. D. Dionysiou, J. Hazard. Mater., 2011, 186, 999–1006 CrossRef CAS PubMed.
- A. Kubacka, A. Martínez-Arias, M. Fernández-García and M. A. Newton, Catal. Today, 2009, 145, 288–293 CrossRef CAS PubMed.
- G. Li, Q. Wang, B. Zhao and R. Zhou, Fuel, 2012, 92, 360–368 CrossRef CAS PubMed.
- M. Cargnello, J. J. Delgado-Jaen, J. C. Hernandez-Garrido, K. Bakhmutsky, T. Montini, J. J. Calvino-Gamez, R. J. Gorte and P. Fornasiero, Science, 2012, 337, 713–717 CrossRef CAS PubMed.
- J. Wang, M. Shen, Y. An and J. Wang, Catal. Commun., 2008, 10, 103–107 CrossRef CAS PubMed.
- G. Wang, M. Meng, Y. Zha and T. Ding, Fuel, 2010, 89, 2244–2251 CrossRef CAS PubMed.
- C. Thomas, O. Gorce, F. Villain and G. Djéga-Mariadassou, J. Mol. Catal., A, 2006, 249, 71–79 CrossRef CAS PubMed.
- D. Ciuparu, A. Bensalem and L. Pfefferle, Appl. Catal., B, 2000, 26, 241–255 CrossRef CAS.
- D. Roth, P. Gélin, A. Kaddouri, E. Garbowski, M. Primet and E. Tena, Catal. Today, 2006, 112, 134–138 CrossRef CAS PubMed.
- S. Lin, L. Yang, X. Yang and R. Zhou, Chin. J. Catal., 2015, 36, 639–648 Search PubMed.
- J. Liu, H. Wang, Y. Chen, M. Yang and Y. Wu, Catal. Commun., 2014, 46, 11–16 CrossRef CAS PubMed.
- X. Chen, Y. Cheng, C. Y. Seo, J. W. Schwank and R. W. McCabe, Appl. Catal., B, 2015, 163, 499–509 CrossRef CAS PubMed.
- G. W. Graham, H. W. Jen, R. W. McCabe, A. M. Straccia and L. P. Haack, Catal. Lett., 2000, 67, 99–105 CrossRef CAS.
- A. Iglesias-Juez, A. B. Hungría, A. Martínez-Arias, J. A. Anderson and M. Fernández-García, Catal. Today, 2009, 143, 195–202 CrossRef CAS PubMed.
- M. Ozawa, M. Hattori and T. Yamaguchi, J. Alloys Compd., 2008, 451, 621–623 CrossRef CAS PubMed.
- J. R. Kim, W. J. Myeong and S. K. Ihm, J. Catal., 2009, 263, 123–133 CrossRef CAS PubMed.
- A. Morikawa, T. Suzuki, T. Kanazawa, K. Kikuta, A. Suda and H. Shinjo, Appl. Catal., B, 2008, 78, 210–221 CrossRef CAS PubMed.
- S. Lin, L. Yang, X. Yang and R. Zhou, Appl. Surf. Sci., 2014, 305, 642–649 CrossRef CAS PubMed.
- A. Martínez-Arias, A. B. Hungría, M. Fernández-García, A. Iglesias-Juez, J. A. Anderson and J. C. Conesa, J. Catal., 2004, 221, 85–92 CrossRef.
- B. Zhao, G. Li, C. Ge, Q. Wang and R. Zhou, Appl. Catal., B, 2010, 96, 338–349 CrossRef CAS PubMed.
- Q. Wang, B. Zhao, G. Li and R. Zhou, Environ. Sci. Technol., 2010, 44, 3870–3875 CrossRef CAS PubMed.
- S. Hinokuma, H. Fujii, Y. Katsuhara, K. Ikeue and M. Machida, Catal. Sci. Technol., 2014, 4, 2990–2996 CAS.
- S. B. Kang, H. J. Kwon, I.-S. Nam, Y. I. Song and S. H. Oh, Ind. Eng. Chem. Res., 2011, 50, 5499–5509 CrossRef CAS.
- H. Birgersson, M. Boutonnet, S. Järås and L. Eriksson, Top. Catal., 2004, 30–31, 1–4 Search PubMed.
- I. Heo, J. W. Choung, P. S. Kim, I.-S. Nam, Y. I. Song, C. B. In and G. K. Yeo, Appl. Catal., B, 2009, 92, 114–125 CrossRef CAS PubMed.
- M. Yang, M. Shen, J. Wang, J. Wen, M. Zhao, J. Wang and W. Wang, J. Phys. Chem. C, 2009, 113, 12778–12789 CAS.
- S. K. Matam, M. A. Newton, A. Weidenkaff and D. Ferri, Catal. Today, 2013, 205, 3–9 CrossRef CAS PubMed.
- M. Fernández-Garcíıa, A. Iglesias-Juez, A. Martíınez-Arias, A. B. Hungríıa, J. A. Anderson, J. C. Conesa and J. Soria, J. Catal., 2004, 221, 594–600 CrossRef PubMed.
- K. I. Hadjiivanov, Catal. Rev., 2000, 42, 71–144 CAS.
- P. S. Lambrou and A. M. Efstathiou, J. Catal., 2006, 40, 182–193 CrossRef PubMed.
- V. Matsouka, M. Konsolakis, I. V. Yentekakis, A. Papavasiliou, A. Tsetsekou and N. Boukos, Top. Catal., 2011, 54, 1124–1134 CrossRef CAS.
- A. Martínez-Arias, M. Fernández-García, A. B. Hungría, A. Iglesias-Juez, K. Duncan, R. Smith, J. A. Anderson, J. C. Conesa and J. Soria, J. Catal., 2001, 204, 238–248 CrossRef.
- A. B. Hungría, J. J. Calvino, J. A. Anderson and A. Martínez-Arias, Appl. Catal., B, 2006, 62, 359–368 CrossRef PubMed.
- I. Eswaramoorthi and A. K. Dalai, Int. J. Hydrogen Energy, 2009, 34, 2580–2590 CrossRef CAS PubMed.
- W. H. Cassinelli, S. Damyanova, N. V. Parizotto, D. Zanchet, J. M. C. Bueno and C. M. P. Marques, Appl. Catal., A, 2014, 475, 256–269 CrossRef CAS PubMed.
- S. Royer and D. Duprez, ChemCatChem, 2011, 3, 24–65 CrossRef CAS PubMed.
- Z. Wu, M. Li and S. H. Overbury, J. Catal., 2012, 285, 61–73 CrossRef CAS PubMed.
- M. Shen, M. Yang, J. Wang, J. Wen, M. Zhao and W. Wang, J. Phys. Chem. C, 2009, 113, 3212–3221 CAS.
- A. Aznárez, S. A. Korili and A. Gil, Appl. Catal., A, 2014, 474, 95–99 CrossRef PubMed.
- J. M. Padilla, G. Del Angel and J. Navarrete, Catal. Today, 2008, 133–135, 541–547 CrossRef CAS PubMed.
- S. Hinokuma, H. Fujii, M. Okamoto, K. Ikeue and M. Machida, Chem. Mater., 2010, 22, 6183–6190 CrossRef CAS.
- Y. Zhang, Y. Cai, Y. Guo, H. Wang, L. Wang, Y. Lou, Y. Guo, G. Lu and Y. Wang, Catal. Sci. Technol., 2014, 4, 3973–3980 Search PubMed.
- J. Fan, X. Wu, X. Wu, Q. Liang, R. Ran and D. Weng, Appl. Catal., B, 2008, 81, 38–48 CrossRef CAS PubMed.
- F. B. Noronha, E. C. Fendley, R. R. Soares, W. E. Alcarez and D. E. Resasco, Chem. Eng. J., 2001, 82, 21–31 CrossRef CAS.
- J. Fan, D. Weng, X. Wu, X. Wu and R. Ran, J. Catal., 2008, 258, 177–186 CrossRef CAS PubMed.
- C. Bozo, N. Guilhaume and J.-M. Herrmann, J. Catal., 2001, 203, 393–406 CrossRef CAS.
- G. Avgouropoulos and T. Ioannides, Appl. Catal., B, 2006, 67, 1–11 CrossRef CAS PubMed.
- K. M. Eblagon, P. H. Concepción, H. Silva and A. Mendes, Appl. Catal., B, 2014, 154–155, 316–328 CrossRef CAS PubMed.
- W. Cai, Q. Zhong, W. Zhao and Y. Bu, Appl. Catal., B, 2014, 158–159, 258–268 CrossRef CAS PubMed.
- S. C. Su, J. N. Carstens and A. T. Bell, J. Catal., 1998, 176, 125–135 CrossRef CAS.
- W. Lin, Y. Zhu, N. Wu, Y. Xie, I. Murwani and E. Kemnitz, Appl. Catal., B, 2004, 50, 59–66 CrossRef CAS PubMed.
- Q. Wang, G. Li, B. Zhao and R. Zhou, Appl. Catal., B, 2010, 100, 516–528 CrossRef CAS PubMed.
- G. Li, Q. Wang, B. Zhao, M. Shen and R. Zhou, J. Hazard. Mater., 2011, 186, 911–920 CrossRef CAS PubMed.
- J. Panpranot, O. Tangjitwattakorn, P. Praserthdam and J. G. Goodwin, Appl. Catal., A, 2005, 292, 322–327 CrossRef CAS PubMed.
- V. Ferrer, A. Moronta, J. Sánchez, R. Solano, S. Bernal and D. Finol, Catal. Today, 2005, 107–108, 487–492 CrossRef CAS PubMed.
- A. Norman, V. Perrichon, A. Bensaddik, S. Lemaux, H. Bitter and D. Koningsberger, Top. Catal., 2001, 16/17, 363–368 CrossRef CAS.
- H. He, H. X. Dai, L. H. Ng, K. W. Wong and C. T. Au, J. Catal., 2012, 206, 1–13 CrossRef.
| This journal is © The Royal Society of Chemistry 2015 |



