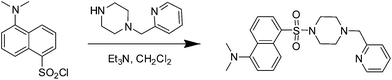
A dansyl-based fluorescent probe for selectively detecting Cu2+ and imaging in living cells†
Meijiao Cao,
Lina Jiang,
Fang Hu,
Yufeng Zhang,
Wen Chao Yang*,
Sheng Hua Liu* and
Jun Yin*
Key Laboratory of Pesticide and Chemical Biology, Ministry of Education, College of Chemistry, Central China Normal University, Wuhan 430079, PR China. E-mail: yinj@mail.ccnu.edu.cn; chshliu@mail.ccnu.edu.cn; tomyang@mail.ccnu.edu.cn; Fax: +86-27-67867725; Tel: +86-27-67867725
First published on 26th February 2015
Abstract
Copper is a crucial transition metal ion that plays an essential role in environmental, biological, and chemical systems. Therefore an efficient method for detection of copper ions is significant. In this work, a sulfonamide-based probe containing a dansyl fluorophore was synthesized in good yield. This probe exhibited good selectivity and sensitivity for copper ions in aqueous solution without any interference from other metal ions. And it displayed a very low detection limit (2.91 × 10−8 M) for Cu2+, which supported its further application in bioimaging. Its good water-solubility, bio-compatibility and cell permeability leads it to be employed as an efficient biomarker to monitor the level of Cu2+ in living cells. The binding model between fluorescent probe and Cu2+ was evaluated by density functional theory calculations.
Introduction
Copper ions, the third most abundant metal ions in the human body, play a vital role in numerous fundamental biological systems, such as enzyme function.1 An appropriate high level of Cu2+ in food supplements significantly increases the flexibility of body tissues and prevents hair bleaching and albinism. However, excess Cu2+ in the body can cause a series of diseases which influence normal physiological metabolism, leading to neurodegenerative diseases including Parkinson's disease, Wilson's disease and Alzheimer's disease, while its deficiency in the body may lead to hematological manifestations and a wide variety of neurological problems.2 Thus, simple and rapid sensing of copper ions in biological and environmental systems is very significant.Currently, some methods have been used to detect Cu2+.3 Among of them, the method based on fluorescent response is considered to be one of the most effective strategies due to its high sensitivity, simplicity and operability. Over the past decades, the fluorescence-dependent techniques and methods have been widely applied in many different fields such as luminescence material, fluorescent markers, fluorescent sensors, drug release and so on.4 Cu2+ as a paramagnetic ion, can strongly quench the fluorescence of fluorophores by electron or energy transfer. In recent years, a number of fluorescent probes have been used in the detection of Cu2+. However, many fluorescent probes usually suffered from some disadvantageous such as complicated synthesis of probes, poor water-solubility, low sensitivity, high detection limits, strong interference and so on. Therefore, the development of a simple and efficient fluorescent probe has potential value in practical applications.
The structure of dansyl-based fluorophore presents donor–acceptor system owing to it containing electron-donating and electron-withdrawing moieties. As a consequence, its derivatives show strong fluorescence with high emission quantum yields.5 More, sulfonyl chloride as a commercial available fluorophore has very high reactivity and can treat with a variety of amines to afford corresponding dansylsulfonamide derivatives, and numerous researches have shown that they can be employed as fluorescence probes to detect metal cations.6 Moreover, the affording metal complexes have further application ascribing to the fact that the metal complexes can be used as a tool to monitor some other biological species such as anions, amino acids and so on.7,8 Therefore, developing the dansyl-based fluorescent probes for metal ions contracts more attention. Herein, in the present work, we reported a dansyl-based fluorescence probe containing a piperazine-linking pyridine, as shown in Scheme 1, which was capable of detecting copper(II) and exhibited high selectivity and low interference due to co-existent metal ions in Scheme 1. Importantly, compared with other probes, our probe has the advantages of simple synthesis and low detection limits. These excellent properties support the use of this probe in living cells to monitor the level of copper ions (Scheme 2).
Experimental
Instruments and reagents
All reactions were taken place by using standard Schlenk techniques under an argon atmosphere, unless elaborated. All reagents and materials were purchased commercially and without further purification. Dansyl chloride (97%) and 1-pyridin-2-yl-methyl-piperazine (95%) are obtained by commercial approach from J&K Scientific Ltd. CH2Cl2 was dried with CaH2, and then distilled under nitrogen atmosphere. Column chromatography was performed over silica gel (200–300 mesh). NMR spectra were obtained on American Varian Mercury Plus 600 spectrometer (600 MHz) or 400 MHz and their chemical shifts are relative to TMS. Electrospray (EI) mass spectra were carried on Firmigan Trace. UV-Vis spectra were obtained on U-3310 UV Spectrophotometer. Fluorescence spectra were taken on a Fluoromax-P luminescence spectrometer (HORIBA JOBIN YVON INC.). All the density functional theory calculations were carried out at the B3LYP/6-31G* level using the Gaussian 09 suite of programs.Characterizations
A stock solution of probe 1 (1 mM) was prepared in DMSO, and a stock solution of metal ions (10 mM) were dissolved in distilled water. We selected a wide range of metal ions, such as Ca2+, Ni2+, Li+, Al3+, Hg2+, Cd2+, K+, Ag+, Zn2+, Pb2+, Mn2+, Cu2+, Fe3+, Na+. For selectivity, the work solution of 1 (10 μM) was prepared by mixing 1 mM of 1 and 0.1 mM of metal ions (10 mM) in 0.02 M HEPES buffer/DMSO (8/2, v/v). For titration, the work solution of 1 (10 μM) was obtained by mixing 1 mM of 1 in 0.02 M HEPES buffer/DMSO (8/2, v/v) with varying concentration of Cu2+ from 0 to 700 μM. For all fluorescence spectra were collected from 380 to 700 nm excitation with 360 nm, slit width at 5 nm.Calculation of dissociation constant [1]
The dissociation constant (Kd), was calculated by nonlinear fitting of eqn (1)
 | (1) |
F is the fluorescence intensity of [Cu2+]. Fmin and Fmax is the fluorescence intensity at minimal and maximal concentration of [Cu2+].
Cell culture and imaging
DMEM (Dulbecco's modified Eagle's medium) was used to dilute the concentration of probe 1. A stock solution of probe 1 (10 mM) was prepared in DMSO, and then using the DMEM to dilute the probe 1 from 10 mM to 100 μM. And then added it to HeLa cells incubated at 37 °C in humidified air and 5% CO2. After that DMEM was removed, washed with PBS (phosphate-buffered saline), and added PBS again. Experiments for Cu2+ ion were incubated for 30 min in the same medium.Synthesis of probe 1
5-(Dimethylamino) naphthalene-1-sulfonyl chloride was added dropwise at 0 °C to a solution of 1-benzylpiperazine in CH2Cl2 and Et3N (2 mL). The mixture was stirred for 12 h at room temperature. And then the solvent was removed in vacuum. The residue was purified by silica gel column chromatography to obtain the target compound as a yellow solid in 60% yield. 1H NMR (600 MHz, CDCl3): δ (ppm): 8.56 (d, 1H, Ph–H), 8.52 (d, 1H, Ph–H), 8.46 (d, J = 6 Hz, 1H, Ph–H), 8.19 (d, J = 6 Hz, 1H, Ph–H), 7.61 (t, J = 6 Hz, 1H, Ph–H), 7.54–7.50 (m, J = 6 Hz, 2H, Ph–H), 7.27 (s, 1H, Ph–H), 7.18 (d, J = 6 Hz, 1H, Ph–H), 7.15–7.13 (m, J = 6 Hz, 1H, Ph–H), 3.61 (s, 2H, CH2), 3.23 (t, 4H, CH2), 2.89 (s, 6H, CH3), 2.55 (t, 4H, CH2). 13C NMR (100 MHz, CDCl3): δ (ppm): 157.46, 151.48, 149.24, 136.30, 132.28, 130.42, 129.87, 127.78, 123.12, 122.98, 122.07, 119.64, 115.04, 63.91, 52.34, 45.49, 45.27. EI MS m/z = 410.18 [M]; calculated exact mass = 410.46.Results and discussion
UV-Vis spectrum
To investigate the sensitivity of 1 to different metal ions including Ca2+, Ni2+, Li+, Al3+, Hg2+, Cd2+, K+, Ag+, Zn2+, Pb2+, Mn2+, Cu2+, Fe3+, Na+, UV-Vis absorption was employed in DMSO/HEPES (2/8, v/v). From the instrument data, the absorption peak at 336 nm vanished dramatically upon addition the Cu2+, while no significant changes happened in other metal ions (Fig. 1S†). This result indicated that the probe has high selectivity towards Cu2+.Fluorescence spectrum
Subsequently, we further explore the fluorescence changes in the presence of metal ions. No drastically fluorescence changes were observed upon the addition of other metal ions, but there was almost 10 fold decrease in fluorescence intensity when the Cu2+ was added as shown in Fig. 1(b). It indicated 1 had a high selectivity towards Cu2+. And this phenomenon can be confirmed by the naked eye at the UV light resulting that the fluorescence of 1 was quenched by Cu2+ in Fig. 1(a).Additionally, an interference experiment of 1 toward Cu2+ in the presence of other metal ions was investigated. From Fig. 2, there were negligible fluorescence changes in the presence of other cations, which showed that there was no inference in detection of 1 toward Cu2+. The result demonstrated that this probe can discriminate Cu2+ from other metals ions.
 | ||
| Fig. 2 The fluorescence change of 1 with various metal ions. Black bars is 1 with other metal ions; red bars is 1 with Cu2+ in the present of other metal ions. | ||
In a titration experiment, there was a marked decrease from 0.2 to 5.0 equiv. at 564 nm following the addition of Cu2+, and no further decreases in fluorescence intensity were observed with further additions of Cu2+ (Fig. 3). When the concentration of Cu2+ was 5.0 equiv. total quenching occurred. When the concentration of 1 ranged from 2 μM to 20 μM, a good linear relationship between 1 and Cu2+ was observed. The linear regression equation was F = 1.41 × 106 to 3.91 × 106E (E is the equiv. of Cu2+) (Fig. 2S,† inset). From the linear equation of the titration plot, the limit of detection for 1 was calculated to be as low as 2.91 × 10−8 M, according to the equation DL = 3σ/m, where σ is the standard deviation of the blank solution and m is the slope of the calibration curve. The low detection limit made it possible to detect the level of Cu2+ in cells using this probe. The dissociation constant between 1 and Cu2+ was also calculated and was estimated to be 3.27 × 10−6 M.
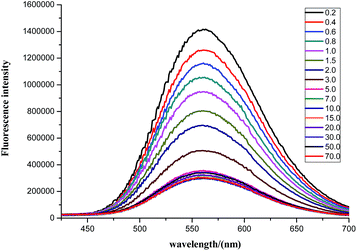 | ||
| Fig. 3 The fluorescence titration spectra of 1 with various concentration of Cu2+ in HEPES buffer (0.02 M, pH = 7.0)/DMSO (8/2, v/v) excited at 360 nm. | ||
Binding mode
To further investigate the binding mode of probe 1 and Cu2+, we have gone the ratio experiment which turned out to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 using the Job's plot in Fig. 3S.† The fluorescence intensity of probe 1 reached the minimum value at 560 nm when the concentration of Cu2+ was near to 5 μM, indicating the formation of a 1
1 using the Job's plot in Fig. 3S.† The fluorescence intensity of probe 1 reached the minimum value at 560 nm when the concentration of Cu2+ was near to 5 μM, indicating the formation of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex between probe 1 and Cu2+. The result indicated that introducing 1-pyridin-2-ylmethyl-piperazine group to dansyl fluorophore is useful to bind metal ions. So we could infer Cu2+ binding at the pyridyl and piperazine nitrogen atoms, which formed a tridentate coordination site as shown in Scheme 1.3a,9 Subsequently, the binding mode was further proved by density functional theory (DFT) calculations. From the frontier molecular orbitals, we could observe the electron density, as shown in Fig. 4. For probe 1, the electron density chiefly located in dansyl group in both HOMO and LUMO orbitals possible due to the donor–acceptor system of dansyl fluorophore. However, for 1–Cu2+ complex, the molecular orbitals contained both alpha and beta orbitals. The electron density distributed to the whole molecule, and beta orbital which played a major role to the quenching of fluorescence had the lower energy gap compared to alpha orbital. Furthermore, the binding also influenced the molecular donor–acceptor system, which resulted in the fluorescence quenching.
1 complex between probe 1 and Cu2+. The result indicated that introducing 1-pyridin-2-ylmethyl-piperazine group to dansyl fluorophore is useful to bind metal ions. So we could infer Cu2+ binding at the pyridyl and piperazine nitrogen atoms, which formed a tridentate coordination site as shown in Scheme 1.3a,9 Subsequently, the binding mode was further proved by density functional theory (DFT) calculations. From the frontier molecular orbitals, we could observe the electron density, as shown in Fig. 4. For probe 1, the electron density chiefly located in dansyl group in both HOMO and LUMO orbitals possible due to the donor–acceptor system of dansyl fluorophore. However, for 1–Cu2+ complex, the molecular orbitals contained both alpha and beta orbitals. The electron density distributed to the whole molecule, and beta orbital which played a major role to the quenching of fluorescence had the lower energy gap compared to alpha orbital. Furthermore, the binding also influenced the molecular donor–acceptor system, which resulted in the fluorescence quenching.
Cell imaging
As we know, high concentration of Cu2+ is harmful to biological system, leading various diseases.10 The excellent properties of probe such as good selectivity, high sensitivity and low detect limit made it is possible to apply to living cells. As HeLa cell was easy to incubate, we select HeLa cell to do the experiment. The ability of 1 to detect the level of Cu2+ ion in cell was demonstrated by fluorescence microscope images. To observe the cell permeability of 1, HeLa cells were incubated with 1 (100 μM) at humidified atmosphere of 5% CO2, 37 °C for 6 h. As displayed in Fig. 5(b), probe 1 displayed good adaptability in this environment, suggesting the low cytotoxicity and good cell permeability of probe 1. After removed the PBS, 1 mM CuCl2 with DMEM was added to the HeLa cells incubated in 5% CO2, 37 °C for 30 min, Fig. 5(c) indicated that the fluorescence of 1 was quenching by Cu2+ ion. These results showed that 1 could be used as a Cu2+-selective probe in living cells.Conclusions
In summary, we developed a sulfonamide-based probe containing a dansyl fluorophore, which exhibited high selectivity and sensitivity without interference from other metal ions, and a low detection limit for Cu2+. Significantly, its corresponding copper complex could be used as a fluorescence indicator to detect amino acids. According to DFT calculations, we explored the proposed quenching mechanism. Furthermore, the probe was successfully used in biological systems to visualize the level of copper ions. Further studies will focus on the development of novel efficient probes.Acknowledgements
We acknowledge financial support from National Natural Science Foundation of China (21272088, 21472059, 21402057), and the Program for Academic Leader in Wuhan Municipality (201271130441). The work was also supported by the Scientific Research Foundation for the Returned Overseas Chinese Scholars, Ministry of Education and Financially supported by self-determined research funds of CCNU from the colleges' basic research and operation of MOE (CCNU14A05009, CCNU14F01003).Notes and references
- (a) P. Xi, L. Huang, G. Xie, F. Chen, Z. Xu, D. Bai and Z. Zeng, Dalton Trans., 2011, 40, 6382 RSC; (b) Y. Zhao, X. Zhang, Z. Han, L. Qiao, C. Li and L. Jian, Anal. Chem., 2009, 81, 7022 CrossRef CAS PubMed; (c) D. G. Barceloux and J. Copper, J. Toxicol., Clin. Toxicol., 1999, 37, 217 CrossRef CAS; (d) R. Uauy, M. Olivares and M. Gonzalez, Am. J. Clin. Nutr., 1998, 67, 952 Search PubMed.
- G. Muthaup, A. Schlicksupp, L. Hess, D. Beher, T. Ruppert, C. L. Masters and K. Beyreuther, Science, 1996, 271, 1406 Search PubMed.
- (a) Y. H. Lee, N. Park, Y. B. Park, Y. J. Hwang, C. Kang and J. S. Kim, Chem. Commun., 2014, 50, 3197 RSC; (b) S. H. Kim, J. S. Kim, S. M. Park and S. K. Chang, Org. Lett., 2006, 8, 371 CrossRef CAS PubMed; (c) Y. Peng, Y. Dong, M. Dong and Y. Wang, J. Org. Chem., 2012, 77, 9072 CrossRef CAS PubMed; (d) W. Lin, L. Yuan, W. Tan, J. Feng and L. Long, Chem.–Eur. J., 2009, 15, 1030 CrossRef CAS PubMed; (e) N. Shao, Y. Zhang, S. M. Cheung, R. Yang, W. Chan and T. Mo, Anal. Chem., 2005, 77, 7294 CrossRef CAS PubMed; (f) Y. Luo, Y. Li, B. Lv, Z. Zhou, D. Xiao and M. M. F. Choi, Microchim. Acta, 2009, 164, 411 CrossRef CAS.
- (a) Q. Xu, P. Pu, J. Zhao, C. Dong, C. Gao, Y. Chen, J. Chen, Y. Liu and H. Zhou, J. Mater. Chem. A, 2015, 3, 542 RSC; (b) C. Dong, R. Eldawud, L. M. Sargent, M. L. Kashon, D. Lowry, Y. Rojanasakul and C. Z. Dinu, Environ. Sci.: Nano, 2014, 1, 595 RSC; (c) K. Joo, Y. Fang, Y. Liu, L. Xiao, Z. Gu, A. Tai, C. L. Lee, Y. Tang and P. Wang, ACS Nano, 2011, 5, 3523 CrossRef CAS PubMed; (d) Z. Yu, R. M. Schmaltz, T. C. Bozeman, R. Paul, M. J. Rishel, K. S. Tsosie and S. M. Hecht, J. Am. Chem. Soc., 2013, 135, 2883 CrossRef CAS PubMed; (e) M. M. Madathil, C. Bhattacharya, Z. Yu, R. Paul, M. J. Rishel and S. M. Hecht, Biochemistry, 2014, 53, 6800 CrossRef CAS PubMed; (f) K. Tonanka, T. Kabashima, T. Shibata, C. Tang, Z. Yu and M. Kai, Anal. Sci., 2008, 24, 471 CrossRef; (g) T. Kabashima, Z. Yu, C. Tang, Y. Nakagawa, K. Okumura, T. Shibata, J. Lu and M. Kai, Peptides, 2008, 29, 356 CrossRef CAS PubMed; (h) Z. Yu, T. Kabashima, C. Tang, T. Shibata, K. Kitazato, N. Kobayashi, M. K. Lee and M. Kai, Anal. Biochem., 2010, 397, 197 CrossRef CAS PubMed; (i) J. A. Lines, Z. Yu, L. M. Dedkova and S. Chen, Biochem. Biophys. Res. Commun., 2014, 443, 308 CrossRef CAS PubMed; (j) B. R. Schroeder, I. M. Ghare, C. Bhattacharya, R. Paul, Z. Yu, P. A. Zaleski, T. C. Bozeman, M. J. Rishel and S. M. Hecht, J. Am. Chem. Soc., 2014, 136, 13641 CrossRef CAS PubMed; (k) C. Bhattacharya, Z. Yu, M. J. Rishel and S. M. Hecht, Biochemistry, 2014, 53, 3264 CrossRef CAS PubMed; (l) Z. Yu, T. Kabashima, C. Tang, T. Shibata, K. Kitazato, N. Kobayashi, M. K. Lee and M. Kai, Anal. Biochem., 2010, 397, 197 CrossRef CAS PubMed; (m) J. Yin, Y. Kwon, D. Kim, D. Lee, G. Kim, Y. Hu, J. Ryu and J. Yoon, J. Am. Chem. Soc., 2014, 136, 5351 CrossRef CAS PubMed; (n) K. J. Siegrist, S. H. Reynolds, M. L. Kashon, D. T. Lowry, C. Dong, A. F. Hubbs, S. Young, J. L. Salisbury, D. W. Porter, S. A. Benkovic, M. McCawley, M. J. Keane, J. T. Mastovich, K. L. Bunker, L. G. Cena, M. C. Sparrow, J. L. Sturgeon, C. Z. Dinu and L. M. Sargent, Part. Fibre Toxicol., 2014, 11, DOI:10.1186/1743-8977-11-6.
- A. J. Parola, J. C. Lima, F. Pina, J. S. Pina, J. de Melo, C. Soriano, E. García-España, R. Aucejo and J. Alarcón, Inorg. Chim. Acta, 2007, 360, 1200 CrossRef CAS.
- (a) R. Metivier, I. Leray, B. Lebeau and B. Valeur, J. Mater. Chem., 2005, 15, 2965 RSC; (b) L. Ding, X. Cui, Y. Han, F. Lu and Y. Fang, J. Photochem. Photobiol., A, 2007, 186, 143 CrossRef CAS; (c) S. Liu, Y. He, G. Qing, K. Xu and H. Qin, Tetrahedron: Asymmetry, 2005, 16, 1527 CrossRef CAS; (d) P. Ceroni, V. Vicinelli, M. Maestri, V. Balzani, S. K. Lee, J. van Heyst, M. Gorka and F. Vogtle, J. Organomet. Chem., 2004, 689, 4375 CrossRef CAS; (e) S. K. Mohanty, S. Baskaran and A. K. Mishra, Eur. Polym. J., 2006, 42, 1893 CrossRef CAS.
- (a) D. Quang and J. S. Kim, Chem. Rev., 2010, 110, 6280 CrossRef CAS PubMed; (b) A. Bencini and V. Lippolis, Coord. Chem. Rev., 2012, 256, 149 CrossRef CAS; (c) Y. Zhou and J. Y. Yoon, Chem. Soc. Rev., 2012, 41, 52 RSC; (d) S. Zhang, C. Ong and H. Shen, Cancer lett., 2004, 208, 143 CrossRef CAS PubMed; (e) T. P. Dalton, H. G. Shertzer and A. Puga, Annu. Rev. Pharmacol. Toxicol., 1999, 39, 67 CrossRef CAS PubMed; (f) W. A. Kleinman and J. P. Richie, Biochem. Pharmacol., 2000, 60, 19 CrossRef CAS PubMed.
- (a) Y. Ruan, A. Li, J. Zhao, J. Shen and Y. Jiang, Chem. Commun., 2010, 46, 4938 RSC; (b) N. Shao, J. Jin, S. M. Cheung, R. Yang, W. Chan and T. Mo, Angew. Chem., Int. Ed., 2006, 45, 4944 CrossRef CAS PubMed; (c) Y. Yang, S. Shim and J. Tae, Chem. Commun., 2010, 46, 7766 RSC.
- (a) B. Zhao, J. Miao, W. Liu, H. Li and H. Lv, Spectrochim. Acta, Part A, 2012, 95, 658 CrossRef PubMed; (b) W. Zeng, J. Huang, M. Tang, M. Liu, M. Zhou, Z. Liu, Y. Cao, M. Zhu and S. Liu, Dyes Pigm., 2014, 107, 1 CrossRef.
- E. Lane, A. J. Holden and R. A. Coward, Analyst, 1999, 124, 245 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: See DOI: 10.1039/c5ra00740b |
| This journal is © The Royal Society of Chemistry 2015 |