Enhanced enrichment performance of nickel oxide nanoparticles via fabrication of a nanocomposite with a graphene template†
Batool Fatimaab,
Fahmida Jabeena,
Zahra Padashbarmchic and
Muhammad Najam-ul-Haq *a
*a
aDivision of Analytical Chemistry, Institute of Chemical Sciences, Bahauddin Zakariya University, Multan 60800, Pakistan. E-mail: najamulhaq@bzu.edu.pk; Tel: +92 306 7552653
bAustralian Institute for Bioengineering and Nanotechnology, The University of Queensland, Brisbane, QLD 4072, Australia
cDepartment of Environmental Sciences, Faculty of Natural Resources, University of Tehran, 31585-4314, Karaj, Iran
First published on 19th February 2015
Abstract
Metal oxide based nanocomposites are applied in phosphoproteomics for enrichment through the surface hydroxyl groups of metal oxides, though the role of the metal is rarely described. Using graphene as a template after modification with nickel oxide, a nanocomposite with an increased surface area is fabricated and applied to phosphopeptides. Characterisation shows a narrow size distribution of 15–20 nm, BET surface area of 179.70 m2 g−1 and a pore volume of 0.44 cm3 g−1. The graphene possesses well distributed NiO nanoparticles showing selectivity up to 1000 folds of complexity with a sensitivity as low as 1 femtomole. The G–NiO nanocomposite shows a higher selectivity towards phosphopeptides compared to TiO2, ZrO2 and NiO nanoparticles. The enrichment with the G–NiO nanocomposite is tested for biological samples like egg yolk, non-fat milk and human serum. Phosphopeptides having phosphorylations of up to 6 phosphate groups, derived from phosvitin and lipovitellin, are enriched in the egg yolk digest. Phosphopeptides characteristic of casein variants are enriched in the non-fat milk digest with a recovery of αS1 21.9%, αS2 30% and β-casein 20%. Phosphorylated proteins are identified in human serum through the enrichment of phosphopeptides.
Introduction
Phosphoproteomics needs selective and sensitive enrichment strategies.1 Several analytical tools including mass spectrometric protocols have been introduced. Potential substitutes for the separation of phosphorylated species have been reported.2 Enrichment tools are based on immuno-affinity, binding through metal ions or ion-exchange interactions.3,4 The bi-dentate ligand chemistry offered by the hydroxyl groups on the metal oxides also helps their separation.Sample complexity is reduced by enrichment followed by MS detection. Low abundant phosphopeptides are suppressed in MS if detected without enrichment.5 Enrichment methods have thus been developed to achieve higher sensitivity and recovery.6 These two factors are limited by the nature of the molecules, materials, methods and tools of analysis. The loading and washing conditions enhance specificity at the cost of inferior sensitivity plus recovery.7 Nano-based enrichment methods address the specificity and sensitivity of peptides/proteins with PTMs (post translational modifications) like phosphorylation which is present in a low-abundance.8,9 On-going work focuses on the versatile nature of the phosphorylation/de-phosphorylation process. The development of enrichment methods is required to find phosphorylations at different complexity levels.10
In recent years, the phosphopeptide enrichment of nanocomposites and their identification with MS has led to biomarker identifications.11 Composites exhibit properties of each component when compared to individual metal oxides.12 Nanocomposites are synthesized by combining the materials through physical interactions or covalent bonding.13 They show higher surface to volume ratios resulting in a large number of binding sites.14,15 The high intensity signals of non-specifically bound entities restrain the ion signals of phosphopeptides.16 Some contain mono-/multi-phosphorylated species in which mono-phosphorylated peptides suppress the signal of multi-phosphorylated peptides.17,18
Both of these problems have been addressed by using nanocomposites which have a variety of chemistries and a short analysis time. They are created by chemical modification for phosphopeptide enrichment.19 Among carbon based materials, graphene is preferred as a support because of its characteristics20 and its nanocomposites with Fe3O4 and TiO2 have been previously used for phosphopeptide enrichment.21
The use of metal oxide based nanocomposites as an enrichment tool works on the principles of Metal Oxide Affinity Chromatography (MOAC). Surface hydroxyl groups interact with phosphate groups on peptides/proteins and enrichment is achieved. The metals in metal oxides affect the acidity of metal oxides as they are Lewis acids and may contribute to the difference in selectivity.22 Further explanation is required for the role of metals in MOAC-based enrichment strategies, as it may help to understand the difference in performance of metal oxides.
In this study, graphene nano-foam is derivatized to incorporate nickel oxide using Stober’s method and applied for phosphopeptide enrichment. A comparison of the synthesized nanocomposite with NiO and other metal oxides is made using β-casein digest. Selectivity and sensitivity measurements are also carried out. Biological fluids like egg yolk, non-fat milk and serum are analysed with MALDI-MS after enrichment with the graphene–nickel oxide nanocomposite.
Results and discussion
Nickel ion based affinity sorbents have been commercialized for the separation of His-tagged proteins whereas NiO NPs are reported for use in phosphopeptide enrichment.23 In G–NiO, there is a graphene support with NiO on its surface that has surface hydroxyl groups for binding with the phosphate groups of phosphorylated biomolecules. The type of metal and its coordination, a change in properties with pH shift and the distribution of particles on the support material also contribute towards the enrichment efficiency. At lower pH values, Ni2+ coordination occurs through the C-terminus and it can bind to acidic peptides through the carboxylate group from the glutamic acid or histidine anchoring binding site. Furthermore, at lower pH values, amide deprotonation occurs and coordination is unfavoured,24 thus histidine rich peptides/proteins are not enriched by NiO.Graphene has a negatively charged surface because of the presence of carboxylic, hydroxyl and epoxy groups which bind nickel oxide through electrostatic interactions. The morphology of nano-porous graphene–NiO nanocomposites is studied by transmission electron microscopy (TEM). Nano-porous graphene foam has a slightly creased wavy morphology which is observed in the TEM image before modification (Fig. 1a). After modification, the nano-porous graphene is homogenously decorated with nickel oxide (Fig. 1b). The graphene strongly interacts and acts as a dispersant as a two-dimensional growth template for NiO. Fig. 2a illustrates the X-ray diffraction (XRD) pattern of the graphene–NiO nanocomposite with three characteristic nickel oxide peaks. The nanocomposite contains graphene with nano-pores filled with nickel oxide obtained as a result of thermal treatment of the mixture. The average size of the nickel oxide nanoparticles on the graphene nanofoam is approximately 15–20 nm. The thermal stability is determined using thermogravimetric analysis (TGA). The nanocomposite is stable up to 400 °C with a drop in weight from 400–500 °C because of the burning of carbon (Fig. 2b).
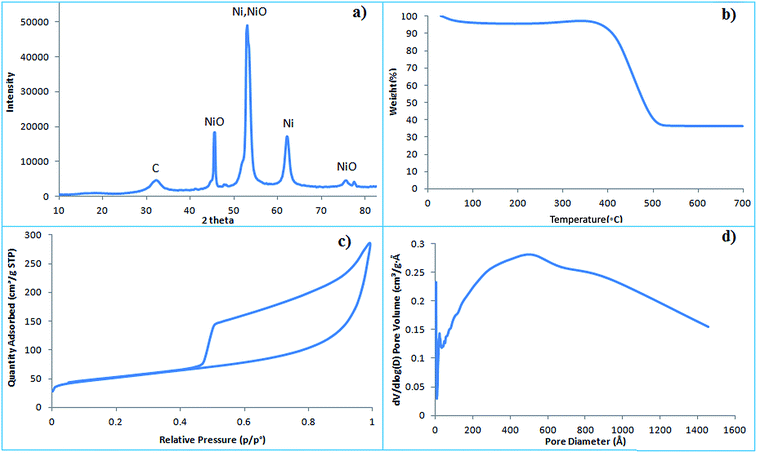 | ||
| Fig. 2 (a) X-ray diffraction (XRD) pattern; (b) thermogravimetric analysis (TGA); (c) BET surface area; (d) pore volume calculated for the graphene–NiO nanocomposite. | ||
Two factors contribute towards the change in surface area of the nanocomposite. The first is the overlapping of the graphene fibres and the second is the presence of nickel oxide nanoparticles. The silica spheres cause mesopores in the graphene foam, however after their removal, nanoporous graphene oxide is achieved. In nanoporous graphene foam without NiO nanoparticles (Fig. S1a†), the pore sizes are distributed around 50 nm which is close to the particle diameter of the silica sphere hard templates (Fig. S1b†). Nanoporous graphene foam without NiO nanoparticles thus shows a higher BET surface area of 401.01 m2 g−1 and a pore volume of 1.35 cm3 g−1. The pores inside the nanoporous graphene foam accommodate nickel nitrate and after the decomposition, NiO nanoparticles are placed within the pores. Therefore the surface area of the G–NiO composite is 179.70 m2 g−1 and the pore volume 0.44 cm3 g−1 which is less than the nanoporous graphene foam (Fig. 2c and d).
Application to phosphoproteomics – phosphopeptide enrichment (β-casein)
Having the metal oxide in nanocomposite form, the graphene–nickel oxide enriches phosphopeptides from a standard protein digest. The derivatization of the hydroxyl groups of graphene and its negatively charged surface is not suitable for the enrichment of phosphopeptides as the protein digest is applied to the graphene nanofoam and derivatized graphene without NiO (Fig. S2†). Having a negatively charged surface, no phosphopeptide is detected in the case of the graphene nanofoam whereas after derivatization with orthosilicate, four phosphopeptides are detected because of the presence of silica on the graphene nanofoam. It has been reported that NiO exhibits weak ferromagnetism or super-paramagnetism depending on the NP size. This may cause agglomeration of the nanoparticles. Therefore a graphene nanofoam is used as the support for an even distribution of the NiO NPs. G–NiO enriches 12 phosphopeptides from the β-casein digest. MS spectra were recorded for the β-casein digest before and after enrichment (Fig. 3). Phosphopeptides are suppressed in the presence of abundant non-phosphopeptides and only one mono-phosphopeptide is observed at m/z 2061 before enrichment. After applying G–NiO to the β-casein digest (20 μL mg−1), 8 phosphopeptides characteristic of β-casein were observed out of which four are mono-phosphorylated and four are multi-phosphorylated. The peaks at 1104 (KFQS*EEQQQT), 2061 (FQS*EEQQQTEDELQDK), 2556 (FQS*EEQQQTEDELQDKIHPF) and 3054 (KIEKFQS*EEQQQTEDELQDKIHPF) correspond to mono-phosphopeptides whereas 3122 (RELEELNVPGEIVES*LS*S*S*EESITRI), 3187 (RELEELNVPGEIVES*LS*S*S*EESIT), 3477 (RELEELNVPGEIVES*LS*S*S*EESITRINKK) and 3605 (RELEELNVPGEIVES*LS*S*S*EESITRINKKI) are multi-phosphorylated peptides. Four phosphopeptides are also detected from α-casein because of its contamination in commercial β-casein. The peaks at m/z 1253 (TVDMMES*TEVF), 1594 (TVDMES*TEVFTK) are mono-phosphorylated and 1329 (EQLS*TS*EENSK), 1927 (KDIGS*ES*TEDQAMEDIKQ) are di-phosphorylated peptides from α-casein.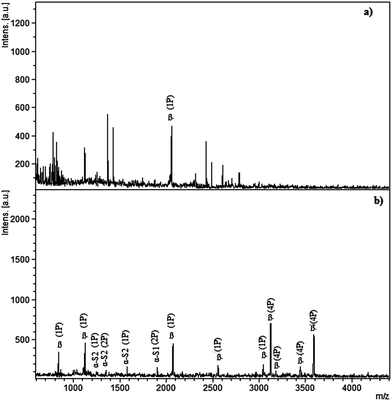 | ||
| Fig. 3 MALDI-MS spectra of tryptic β-casein digest: (a) direct analysis (b) analysis of eluted fraction after enrichment by the graphene–NiO nanocomposite. | ||
Comparison to metal oxide NPs
Although graphene–NiO has performed better than the reported NiO NPs, it is necessary to perform the enrichment process using other metal oxides. Commercial nanoparticles of titania, zirconia and nickel oxide were applied to the β-casein digest. Metal oxides can act as a Lewis acid or Lewis base depending on the buffer pH applied during activation and sample loading. Therefore the selectivity of the metal oxide towards the phosphate groups depends on the net charge of the surface, which is affected by nature of the metal present in the metal oxide. The isoelectric points of NiO (9.9–10.8), ZrO2 (4–11) and TiO2 (3.9–8.2) show that NiO resides as a M–OH2+ species for a range of pH values whereas for titania and zirconia, a M–O− species predominates above pH 4 and causes a loss of phosphopeptides during washing. Zirconia NPs show a loss of multi-phosphopeptides (Fig. S3a†) whereas mono-phosphopeptides are lost for titania NPs (Fig. S3b†). This is characteristic for these two metal oxides and there is no clear explanation for it. NiO has a wide pH range in which it binds negatively charged species. It enriches a number of mono- and multi-phosphopeptides derived from β-casein, however, G–NiO enriches an enhanced number of phosphopeptides in a higher mass range because of the higher surface area of the nanocomposite as compared to NiO NPs (Fig. S3c†).Estimation of selectivity (spiked β-casein digest in de-HeLa cell extract)
De-phosphorylated HeLa cell extract spiked with β-casein digest was used to obtain varying complexity. Five mixtures (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100, 1
100, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300, 1
300, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500, 1
500, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 and 1
700 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000) were tested to determine the selectivity levels of the G–NiO nanocomposite. The phosphorylated peptides from the β-casein digest present in the non-specific background of the de-phosphorylated HeLa cell extract were enriched by G–NiO. The number of enriched phosphopeptides decreased as the complexity levels increased which is because of the saturation of the binding sites with non-phosphopeptides which were present in abundance (Fig. 4). Although non-specifically bound species are washed away, their initial presence decreased the phosphopeptide binding. At the complexity level of 100, seven phosphopeptides were identified, however, at the 1
1000) were tested to determine the selectivity levels of the G–NiO nanocomposite. The phosphorylated peptides from the β-casein digest present in the non-specific background of the de-phosphorylated HeLa cell extract were enriched by G–NiO. The number of enriched phosphopeptides decreased as the complexity levels increased which is because of the saturation of the binding sites with non-phosphopeptides which were present in abundance (Fig. 4). Although non-specifically bound species are washed away, their initial presence decreased the phosphopeptide binding. At the complexity level of 100, seven phosphopeptides were identified, however, at the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000 level, the number decreases to two.
1000 level, the number decreases to two.
Sensitivity – femtomolar concentration of β-casein
A sensitivity study determines the ability of a nanocomposite to enrich the lowest concentrations. Three dilutions in femtomole concentrations of β-casein were made. At 5 femtomoles, three phosphopeptides were detected at the intensity level of 400. With an increase in dilution to 3 femtomoles, the number of phosphopeptides remained the same however the intensity dropped. At 1 femtomole, phosphopeptides could still be distinguished from the noise (Fig. 5). | ||
| Fig. 5 Sensitivity study at femtomolar concentration of β-casein: (a) 5 femtomole, (b) 3 femtomole and (c) 1 femtomole. | ||
Enrichment of phosvitin/lipovitellin from egg yolk
Phosvitin is the phosphoprotein in egg yolk which is highly phosphorylated because of successive serine sites. The egg yolk was lyophilized, solubilised in ammonium bicarbonate and digested to enrich the multi-phosphorylated peptides using the G–NiO nanocomposite. The MS spectrum was first recorded for the egg yolk digest without enrichment. Only one phosphopeptide from lipovitellin is observed (Fig. 6a). Graphene without nickel oxide was also tested. Because of the negatively charged surface, no phosphopeptide bound and non-specific species were detected (Fig. 6b). The G–NiO nanocomposite binds phosphopeptides with a multi-phosphorylation level up to 6P (Fig. 6c) and the amino acid sequence for detected phosphopeptides is listed in Table 1.| Derived protein from egg yolk | m/z of the peptide fragments | Peptide sequence | MS spectra for fractions | ||
|---|---|---|---|---|---|
| Raw | GNF | NiO–GNF | |||
| Phosvitin | 1084.600 | SSSSSSVLSKI | — | — | * |
| 1170.111 | AKTSSSSSSASSTATSSSSSSASSPN | — | — | * | |
| 1256.711 | DSSSSSSSSVLSK | — | — | * | |
| 1337.926 | KPMDEEENDQ | — | — | * | |
| 1419.677 | GTEPDAKTSSSSSSASSTATSSSSSSASSPN | — | — | * | |
| 1678.892 | SGHLEDDSSSSSSVL | — | — | * | |
| 1726.810 | LEDDSSSSSSVLSKI | — | — | * | |
| 1856.920 | EDDSSSSSSVLSKIWG | — | — | * | |
| Lipovitellin | 1575.567 | EVNPESEEE | — | — | * |
| 2073.567 | EVNPESEEEDESSPYEDI | * | — | * | |
Enrichment of casein-phosphopeptides from milk
Milk is a source of all casein variants in which α- and β-casein are phosphorylated at serine sites. α-Casein has two variants namely α-S1 and α-S2. One phosphopeptide is detected in the case of the digest directly analyzed by MALDI-MS (Fig. 7a). Graphene as such does not bind phosphopeptides however abundant non-phosphopeptides at m/z 1267 (Y![[L with combining low line]](https://www.rsc.org/images/entities/char_004c_0332.gif) GY
GY![[L with combining low line]](https://www.rsc.org/images/entities/char_004c_0332.gif) EQ
EQ![[L with combining low line]](https://www.rsc.org/images/entities/char_004c_0332.gif)
![[L with combining low line]](https://www.rsc.org/images/entities/char_004c_0332.gif) R 106–115 α-S1), 1758 (HQG
R 106–115 α-S1), 1758 (HQG![[L with combining low line]](https://www.rsc.org/images/entities/char_004c_0332.gif)
![[P with combining low line]](https://www.rsc.org/images/entities/char_0050_0332.gif) QE
QE![[V with combining low line]](https://www.rsc.org/images/entities/char_0056_0332.gif)
![[L with combining low line]](https://www.rsc.org/images/entities/char_004c_0332.gif) NEN
NEN![[L with combining low line]](https://www.rsc.org/images/entities/char_004c_0332.gif)
![[L with combining low line]](https://www.rsc.org/images/entities/char_004c_0332.gif) R, α-S1) and 2187 (DM
R, α-S1) and 2187 (DM![[P with combining low line]](https://www.rsc.org/images/entities/char_0050_0332.gif)
![[I with combining low line]](https://www.rsc.org/images/entities/char_0049_0332.gif) QAF
QAF![[L with combining low line]](https://www.rsc.org/images/entities/char_004c_0332.gif)
![[L with combining low line]](https://www.rsc.org/images/entities/char_004c_0332.gif) YQ
YQ![[E with combining low line]](https://www.rsc.org/images/entities/char_0045_0332.gif)
![[P with combining low line]](https://www.rsc.org/images/entities/char_0050_0332.gif)
![[V with combining low line]](https://www.rsc.org/images/entities/char_0056_0332.gif)
![[L with combining low line]](https://www.rsc.org/images/entities/char_004c_0332.gif) G
G![[P with combining low line]](https://www.rsc.org/images/entities/char_0050_0332.gif)
![[V with combining low line]](https://www.rsc.org/images/entities/char_0056_0332.gif) R, 184–202, β) are enriched (Fig. 7b). Upon enrichment with the G–NiO nanocomposite, 22 phosphopeptides were enriched which belong to all three casein variants (Fig. 7c). The phosphopeptides enriched are given in Table 2. The enrichment by the nanocomposite is attributed to the negative graphene surface finely holding positively charged NiO without causing agglomeration of the oxide particles.
R, 184–202, β) are enriched (Fig. 7b). Upon enrichment with the G–NiO nanocomposite, 22 phosphopeptides were enriched which belong to all three casein variants (Fig. 7c). The phosphopeptides enriched are given in Table 2. The enrichment by the nanocomposite is attributed to the negative graphene surface finely holding positively charged NiO without causing agglomeration of the oxide particles.
 | ||
| Fig. 7 MALDI-MS spectra of tryptic digest of non-fat milk: (a) without enrichment (b) after enrichment with graphene (c) after enrichment with the graphene–NiO nanocomposite. | ||
| Milk casein variants | Amino-acid position | Amino acid sequences | No. of phosphate groups | [M + H]+ |
|---|---|---|---|---|
| α-S2 | 153–162 | TVDMMES*TEVF | 1 | 1254.94 |
| 141–151 | EQLS*TSEENS*K | 2 | 1329.52 | |
| 153–164 | TVDMES*TEVFTKK | 2 | 1594.28 | |
| 17–36 | NTMEHVS*S*S*EES*IISQETYK | 1 | 2616.23 | |
| 61–85 | NANEEEYSIGS*S*S*EES*AEVATEEVK | 4 | 3006.05 | |
| α-S1 | 121–134 | VPQLEIVPNS*AEER | 1 | 1660.57 |
| 57–73 | KDIGES*ES*TEDQAMEDIK | 1 | 1847.62 | |
| 43–58 | DIGS*ES*TEDQAMEDIK | 2 | 1927.21 | |
| 118–137 | KYKVPQLEIVPNS*AEERLHS | 1 | 2202.02 | |
| 104–138 | KYKVPQLEIVPNS*AEERLHSM | 1 | 2289.11 | |
| 51–76 | KVNELSKDIGS*ES*TEDQAMEDIKQME | 2 | 2857.43 | |
| β Casein | 47–48 | KFQS*EEQQQ | 1 | 975.83 |
| 47–49 | KFQS*EEQQQT | 1 | 1104.10 | |
| 33–48 | FQSEEQQQTEDELQDK | 1 | 2061.02 | |
| 22–40 | NVPGEIVESLS*S*S*EES*ITR | 4 | 2352.61 | |
| 47–67 | FQS*EEQQQTEDELQDKIHPF | 1 | 2556.23 | |
| 44–67 | KIEKFQS*EEQQQTEDELQDKIHPF | 1 | 2779.95 | |
| 16–40 | RELEELNVPGEIVES*LS*S*S*EESITR | 4 | 2965.63 | |
| 29–52 | KIEK FQS*EEQQQTEDELQDKIHPF | 1 | 3054.42 | |
| 26–41 | RELEELNVPGEIVES*LS*S*S*EESITRI | 3 | 3122.63 | |
| 26–39 | RELEELNVPGEIVES*LS*S*S*EESIT | 4 | 3180.54 |
Bottom-up identification of serum phosphoproteins
Serum, being a source of information, is employed to identify the phosphoproteins. Serum digestion is carried out to study the phosphorylation sites. Digested serum was used as a sample to enrich phosphopeptides via G–NiO (Fig. S4†). Two phosphoproteins related to tumours were identified through a bottom-up approach using a mascot search engine. One is an apoptosis-associated speck-like protein containing a CARD, known for its apoptosis initiating ability in tumour cells. It is involved in inhibition of tumorigenesis in primary melanoma whereas in metastatic melanoma, this effect is diminished.25 Phosphopeptides with phosphorylation at serine and threonine were also identified. The second protein is tumour protein D52 (TPD52) which is overexpressed in prostate and breast cancer. It is interesting to study the phosphorylation of tumor protein D52 as the nanocomposite has enriched phosphopeptides related to this protein.26 Phosphopeptides enriched from the serum digest are listed in Table S1.†Conclusion
A graphene nanofoam is derivatized with NiO and used in phosphoproteomics. During the fabrication of the nanocomposite, the graphene strongly interacts with NiO without the agglomeration of oxide particles. This contributes to a high enrichment efficiency, selectivity and sensitivity. G–NiO NPs bind mono- and multi-phosphopeptides as compared to ZrO2 and TiO2. The performance of NiO NPs is enhanced in the form of the G–NiO nanocomposite because of the better surface area. Egg yolk, non-fat milk and serum digests were used as samples for the phosphopeptide enrichment. From the serum, two phosphoproteins related to tumours were identified using a bottom-up approach. It can be concluded that the performance of NiO is enhanced by using a graphene support in the form of a G–NiO nanocomposite.Experimental
Reagents and materials
α-Casein, β-casein, ammonia solution, nickel oxide (<50 nm particle size (TEM), 99.8% trace metals basis), zirconium(IV) oxide (<100 nm particle size (TEM)), titanium(IV) oxide (21 nm particle size (TEM), iodoacetamide, dithiothretol, ethanol, bovine serum albumin (BSA), tri-fluoro acetic acid (TFA), 2,5-dihydroxybenzoic acid, acetonitrile, ammonium bicarbonate (NH4HCO3), ammonium hydroxide solution, tetraethyl ortho-silicate, hydrochloric acid, surfactant pluronic F108, dimethyl disulfide and nickel nitrate were purchased from Sigma Aldrich. Trypsin was obtained from Promega and Milli Q water was used to prepare the solutions.Synthesis of graphene–nickel oxide
Silica spheres were synthesized by Stober’s method.27 A mixture of 2.3 mL of tetraethyl ortho silicate, NH4OH solution (29%, 2.59 mL in 410 mL distilled water), 1 mL of water and 60 mL of ethanol was stirred for 6 hours at 50 °C. The mixture (37.5 mL) was dialyzed for 48 hours (diluted to 75 mL with water). The modification was made by HCl (15 mL, 12 M), F108 (surfactant pluronic, 0.6 g) and dimethoxydimethylsilane (DMDMS, 0.875) for 48 hours and neutralized by 29% ammonium hydroxide solution. Graphene oxide (GO) sheets were fabricated as previously reported28 and a GO suspension (1 mg mL−1) was prepared by stirring 0.3 g of GO in 300 mL of water. After sonication (2 hours) silica spheres (55 mL) were added to the suspension, stirred for 12 hours and dried at 50 °C. This was followed by calcination at 900 °C for 5 hours in the presence of argon at 2 °C min−1. The material was washed with hydrofluoric acid (HF, 5%, 20 mL) and 0.0730 g NGF (nanoporous graphene foam) was mixed with 0.1460 g Ni(NO3)2·6H2O by an impregnation method in 30 mL ethanol and stirred at room temperature until the solvent evaporated. It was calcinated in N2 at 600 °C for 6 hours at 2 °C min−1 to get the graphene–NiO nanocomposite.Sample preparation
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 for 14 hours at 37 °C. 10 μL of 0.1% TFA was added to stop the digestion.
50 for 14 hours at 37 °C. 10 μL of 0.1% TFA was added to stop the digestion.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100, 1
100, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300, 1
300, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500, 1
500, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 and 1
700 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000 using β-casein digest in the de-HeLa cells. Sensitivity was measured with concentrations of 5, 3 and 1 fmol.
1000 using β-casein digest in the de-HeLa cells. Sensitivity was measured with concentrations of 5, 3 and 1 fmol.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 3 minutes and the supernatant was removed. Following similar steps the particles were conditioned with 100 μL of 0.1% TFA. Peptide samples, 1 mg mL−1 (tryptic digests of standard proteins and non-fat milk) acidified with 0.1% TFA were added to the nanocomposite material. The mixture was vortexed and incubated for half an hour at 37 °C with gentle shaking. Removal of non-phosphopeptides was carried out by 10 μL of 70% ACN in 0.1% TFA. Finally, phosphopeptide elution was conducted using 20 μL of 2% ammonia solution (pH 11). The eluted phosphopeptides were subjected to MALDI-MS analysis. Egg yolk digest and non-fat milk were also applied to the graphene nanofoam prior to the derivatization. Zirconia, titania and nickel oxide nanoparticles were applied to the β-casein digest using their reported protocols.
000 rpm for 3 minutes and the supernatant was removed. Following similar steps the particles were conditioned with 100 μL of 0.1% TFA. Peptide samples, 1 mg mL−1 (tryptic digests of standard proteins and non-fat milk) acidified with 0.1% TFA were added to the nanocomposite material. The mixture was vortexed and incubated for half an hour at 37 °C with gentle shaking. Removal of non-phosphopeptides was carried out by 10 μL of 70% ACN in 0.1% TFA. Finally, phosphopeptide elution was conducted using 20 μL of 2% ammonia solution (pH 11). The eluted phosphopeptides were subjected to MALDI-MS analysis. Egg yolk digest and non-fat milk were also applied to the graphene nanofoam prior to the derivatization. Zirconia, titania and nickel oxide nanoparticles were applied to the β-casein digest using their reported protocols.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50% acetonitrile (1
50% acetonitrile (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) spiked with 1% phosphoric acid. The fractions which were collected i.e. eluted phosphopeptides, prior to enrichment and non-phosphopeptides were mixed with the matrix solution in equal volumes and spotted on a MALDI target plate. Calibration standard I (Bruker) was used for calibration. The mass spectra were exported using Flex analysis software (Version 3.3) based on the BLAST algorithm. The parameters for the BLAST search were as follows: enzyme, trypsin; cystein modification, iodoacetamide; missed cleavages, one; fixed modification, C-carbamidomethyl; variable modifications, oxidation (M), phosphorylation (ST), phosphorylation (Y) and peptide tolerance, 3.0 Da. A mono-isotopic mass list was used in Mascot (http://www.matrixscience.com) to query the Swiss-Prot database which identifies the phosphorylated peptides for α- (αS1 and αS2) and β-casein (Swiss-Prot accession number P02662, P02663 and P02666 respectively). Generated data was converted to MS spectra files and exported to BioTools for identification using a peak picking method suppressed up to 700 Da. With access to the Swiss-Prot (http://web.expasy.org) database, the program was set up to provide information about phosphopeptides, such as m/z, amino acid sequence, hit score, protein accession number, missed cleavage, error in Da and error in ppm.
1) spiked with 1% phosphoric acid. The fractions which were collected i.e. eluted phosphopeptides, prior to enrichment and non-phosphopeptides were mixed with the matrix solution in equal volumes and spotted on a MALDI target plate. Calibration standard I (Bruker) was used for calibration. The mass spectra were exported using Flex analysis software (Version 3.3) based on the BLAST algorithm. The parameters for the BLAST search were as follows: enzyme, trypsin; cystein modification, iodoacetamide; missed cleavages, one; fixed modification, C-carbamidomethyl; variable modifications, oxidation (M), phosphorylation (ST), phosphorylation (Y) and peptide tolerance, 3.0 Da. A mono-isotopic mass list was used in Mascot (http://www.matrixscience.com) to query the Swiss-Prot database which identifies the phosphorylated peptides for α- (αS1 and αS2) and β-casein (Swiss-Prot accession number P02662, P02663 and P02666 respectively). Generated data was converted to MS spectra files and exported to BioTools for identification using a peak picking method suppressed up to 700 Da. With access to the Swiss-Prot (http://web.expasy.org) database, the program was set up to provide information about phosphopeptides, such as m/z, amino acid sequence, hit score, protein accession number, missed cleavage, error in Da and error in ppm.Acknowledgements
This work is supported by the Higher Education Commission (HEC) of Pakistan. Additionally, Chengzhong Micheal and Liang Zhou from the Australian Institute for Bioengineering and Nanotechnology, The University of Queensland, Brisbane, Australia are acknowledged for providing the laboratory facilities. Furthermore, the authors declare that they have no conflict of interest.References
- M. Gao, C. Deng, W. Yu, Y. Zhang, P. Yang and X. Zhang, Proteomics, 2008, 8, 939–947 CrossRef CAS PubMed
.
- S. Y. Chang, N. Y. Zheng, C. S. Chen, C. D. Chen, Y. Y. Cheng and C. R. C. Wang, J. Am. Soc. Mass Spectrom., 2007, 18, 910–918 CrossRef CAS PubMed
.
- G. E. Lienhard, Trends Biochem. Sci., 2008, 33, 351–352 CrossRef CAS PubMed
.
- B. Bodenmiller, L. N. Mueller, M. Mueller, B. Domon and R. Aebersold, Nat. Methods, 2007, 4, 231–237 CrossRef CAS PubMed
.
- H. Y. Lin, W. Y. Chen and Y. C. Chen, Anal. Bioanal. Chem., 2009, 394, 2129–2136 CrossRef CAS PubMed
.
- B. Eyrich, A. Sickmann and R. P. Zahedi, Proteomics, 2011, 11, 554–570 CrossRef CAS PubMed
.
- L. Wu and D. K. Han, Expert Rev. Proteomics, 2006, 3, 611–619 CrossRef CAS PubMed
.
- L. Hu, H. Zhou, Y. Li, S. Sun, L. Guo, M. Ye, X. Tian, J. Gu, S. Yang and H. Zou, Anal. Chem., 2009, 81, 94–104 CrossRef CAS PubMed
.
- H. Zhou, R. Tian, M. Ye, S. Xu, S. Feng, C. Pan, X. Jiang, X. Li and H. Zou, Electrophoresis, 2007, 28, 2201–2215 CrossRef CAS PubMed
.
- S. Feng, M. L. Ye, H. J. Zhou, X. G. Jiang, X. N. Jiang, H. F. Zou and B. L. Gong, Mol. Cell. Proteomics, 2007, 6, 1656–1665 CAS
.
- I. L. Batalha, C. R. Lowe and A. C. A. Roque, Trends Biotechnol., 2012, 30, 100–110 CrossRef CAS PubMed
.
- J. Lu, M. Wang, Y. Li and C. Deng, Nanoscale, 2012, 4, 1577–1580 RSC
.
- Y. Li, X. Xu, D. Qi, C. Deng, P. Yang and X. Zhang, J. Proteome Res., 2008, 7, 2526–2538 CrossRef CAS PubMed
.
- M.-Q. Guo and B. X. Huang, Curr. Anal. Chem., 2012, 8, 3–21 CrossRef CAS
.
- S. C. Mithoe, P. J. Boersema, L. Berke, B. Snel, A. J. R. Heck and F. L. H. Menke, J. Proteome Res., 2012, 11, 438–448 CrossRef CAS PubMed
.
- A. Leitner, M. Sturm and W. Lindner, Anal. Chim. Acta, 2011, 703, 19–30 CrossRef CAS PubMed
.
- C. A. Nelson, J. R. Szczech, J. Dooley, Q. Xu, M. J. Lawrence, H. Zhu, S. Jin and Y. Ge, Anal. Chem., 2010, 82, 7193–7201 CrossRef CAS PubMed
.
- H.-T. Wu, C.-C. Hsu, C.-F. Tsai, P.-C. Lin, C.-C. Lin and Y.-J. Chen, Proteomics, 2011, 11, 2639–2653 CrossRef CAS PubMed
.
- S. Kjellstrom and O. N. Jensen, Anal. Chem., 2004, 76, 5109–5117 CrossRef PubMed
.
- Z. Yan, L. L. Ma, Y. Zhu, I. Lahiri, M. G. Hahm, Z. Liu, S. B. Yang, C. S. Xiang, W. Lu, Z. W. Peng, Z. Z. Sun, C. Kittrell, J. Lou, W. Choi and P. M. Ajayan, ACS Nano, 2012, 7, 58–64 CrossRef PubMed
.
- J. Lu, C. Deng, X. Zhang and P. Yang, ACS Appl. Mater. Interfaces, 2013, 5, 7330–7334 CAS
.
- A. Leitner, TrAC, Trends Anal. Chem., 2010, 29, 177–185 CrossRef CAS PubMed
.
- N. Hasan and W. Hui-Fen, Anal. Bioanal. Chem., 2011, 400, 3451–3462 CrossRef CAS PubMed
.
- M. A. Zoroddu, M. Peana, R. Medici and S. Anedda, Dalton Trans., 2009, 5523–5534 RSC
.
- W. Liu, Y. Luo, J. H. Dunn, D. A. Norris, C. A. Dinarello and M. Fujita, J. Invest. Dermatol., 2013, 133, 518–527 CrossRef CAS PubMed
.
- R. Ummanni, S. Teller, H. Junker, U. Zimmermann, S. Venz, C. Scharf, J. Giebel and R. Walther, FEBS J., 2008, 275, 5703–5713 CrossRef CAS PubMed
.
- Y. Kobayashi, H. Katakami, E. Mine, D. Nagao, M. Konno and L. M. Liz-Marzán, J. Colloid Interface Sci., 2005, 283, 392–396 CrossRef CAS PubMed
.
- X. D. Huang, K. Qian, J. Yang, J. Zhang, L. Li, C. Z. Yu and D. Y. Zhao, Adv. Mater., 2012, 24, 4419–4423 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: Additional information regarding the preparation of the HeLa cell extract. See: Fig. S1 for phosphopeptides enrichment of β-casein, Fig. S2 for comparison to metal oxide NPs, Fig. S3 for bottom-up identification of serum phosphoproteins and Table S1 for enrichment from serum. See DOI: 10.1039/c4ra17299j |
| This journal is © The Royal Society of Chemistry 2015 |



