The influence of electrode structure on the performance of an SnS anode in Li-ion batteries: effect of the electrode particle, conductive support shape and additive†
Alok M. Tripathi and
Sagar Mitra*
Electrochemical Energy Laboratory, Indian Institute of Technology Bombay, Mumbai, India-400076. E-mail: sagar.mitra@iitb.ac.in; Fax: +91-22-2576-4890; Tel: +91-22-2576-7890
First published on 19th February 2015
Abstract
The electrochemical performance of tin sulfide (SnS) nanorods (NRs) as an anode for lithium-ion batteries has been evaluated, after adding a carbon coating and reduced graphene oxide (r-GO) as a conductive support. Raman spectroscopic analysis of such types of carbon-coated SnS nanorod clearly shows the influence of the size and shape of the conductive graphite crystallites on their electrochemical performance against Li/Li+. The incorporation of an r-GO support for the SnS nanorods enhances the electrochemical performance. The SnS NRs/r-GO composite with both reduced graphene oxide and a carboxymethyl cellulose binder shows better performance than the carbon-coated sample. The ex situ transmission electron microscopy studies show that the carbon coating of the SnS nanorod is ruptured during the lithiation process and the particle size is not maintained uniformly, while the r-GO composite is able to maintain its shape and size during the continuous charge–discharge process. The discharge capacity of the SnS NRs/r-GO composite was 602 mA h g−1 at a current rate of 160 mA h g−1 after fifty continuous charge–discharge cycles. The current observations suggest that the practical energy density need not be sacrificed for power density, provided that the carbon coatings are optimized by the careful selection of additives.
1. Introduction
Alloy-based materials have been found to be suitable candidates for use in lithium ion batteries and have been successfully commercialized by Sony in NEXLION technology.1 The only problem faced by these alloy-based electrodes is their rate capability. Their poor rate capability has been attributed to low electronic conductivity and/or the slow diffusion of lithium ions across the two/three phase boundaries.2 To address these issues, researchers have optimized synthesis techniques to minimize particle size without compromising the electrode material purity, or have incorporated conductive additives to increase the electronic conductivity. The latter approach can involve coating the electrode particles with carbon by incorporating an organic or polymeric component with the precursors before final calcinations,3–6 adding metal particles to the mixture,4,7,8 or adding carbon nanotubes or graphene to make composites.9–13 All of these efforts have met with success in improving performance, however, it has recently been noted that a smaller particle size and the addition of excessive carbon additive could remarkably affect the particle tap density.14–16 On the other hand, any amount of carbon additive could intensify this effect and result in a low practical energy density. Therefore, it is imperative to optimize the carbon coating in any composite electrode material to achieve efficient performance. As a conductive material, carbon has been widely used in both cathode and anode fabrication as a conductive material. The use of carbon material in the cathode is a safer strategy because of its higher potential; very few side reactions occur in presence of the electrolyte. However, in the anode, conductive carbon can engage in several side reactions that lead to the generation of various organic gases and salts, because the side reactions occur at a lower Li intercalation potential. When the battery is operating at a high rate, these side reactions happen more quickly, which can lead to a fire or explosion in the lithium-ion battery.17 Thus, from a safety point of view, the use of pure carbon for such technology is likely to shift to materials like titanate-based insertion materials, or alloying/conversion-based anode materials.As a conversion- and alloying-based anode, tin mono-sulfide (SnS) is known to exhibit less irreversible loss than other tin-based compounds, such as its oxide and phosphide etc.18 The only problem with such a material is the large amount of lithium accumulation, which leads to a large volume expansion and creates mechanical stress in the electrode. These chemical and physical changes result in the loss of electrical connectivity between the electro-active material and current collector. These alloy-based anode materials mostly lose their initial shape during charge–discharge cycling and finally end up with a spherical shape.18 The shape and size of the final particles and their distribution ultimately decide the future electrochemical performance of the electrode, as observed in our previous work.18,19 As observed, the elastomeric binders also play a crucial role in cell performance and cellulose binders like CMC are known to be better binders for alloying- and conversion-based electrodes, in comparison to the regular PVDF binder. The CMC binder is better because it can achieve the proper maintenance of the particle size of the electro-active material during cycling.18,20,21 Another factor that affects the stability of alloying-based anode materials is the kind of conducting carbon present in the electrode.
In this report, we have initiated a study to discover the effect of the structure of residual electrode materials after cycling on the electrochemical performance, when two different forms of carbon are used. Herein, we prepared SnS nanorods (NRs) solvothermally to provide better cycling stability, and two surface modification processes for SnS NRs were adopted. One process involved the 3-D carbon-coating of the SnS NRs surface and another involved supporting the NRs on a 2-D graphene sheet. In brief, the SnS NRs were coated with 4–5 nm thick graphitized carbon without any phase change of the SnS. The same SnS NRs were used to prepare a composite with a 2-D carbon support of reduced graphene oxide (r-GO), produced by the solvothermal reduction of graphene oxide prepared by Hummer’s process.22 The electrochemical performance of both SnS NRs samples was studied on the addition of r-GO, a carbon coating and binders (PVDF, CMC) using the charge–discharge test against Li/Li+ and the morphological changes were studied using ex situ TEM analysis.
2. Experimental section
2.1 Synthesis of SnS nanorods
The synthesis of SnS NRs was done using our previously reported method.18 The hydrated salt of tin(II) chloride (2 g) was used as a tin source and sodium sulfide (Na2S) fused flakes (1.64 g) were used as a sulfur source for the reaction. Both salts were taken in a (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) molar ratio and dissolved in 50 mL of N,N-dimethyl formamide (DMF) solvent. The mixture was kept in a Teflon-lined steel-coated solvothermal vessel at 180 °C for 48 h. After the completion of the reaction, the sample was washed with Milli-Q water and dried in a vacuum oven at 80 °C for 5 h.
2) molar ratio and dissolved in 50 mL of N,N-dimethyl formamide (DMF) solvent. The mixture was kept in a Teflon-lined steel-coated solvothermal vessel at 180 °C for 48 h. After the completion of the reaction, the sample was washed with Milli-Q water and dried in a vacuum oven at 80 °C for 5 h.
2.2 Carbon-coating of the SnS nanorods (C-SnS)
The carbon-coating of the SnS NRs was performed using the thermal carbonization process with a suitable carbon precursor. Polyvinyl alcohol (PVA) was used as a carbon source and dimethyl sulphoxide (DMSO) as a solvent for the carbon-coating process. Initially, a 3 wt% solution of PVA was prepared by stirring for 12 h in DMSO. Then, 1 wt% of SnS NRs was dispersed in a DMSO–PVA solution and sonicated for 5 min, followed by stirring for 2 h. The viscous slurry was transferred to a tubular furnace and calcined at 150 °C for 1 h, and then at 500 °C for 5 h in an Ar/H2 (90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) environment to get a uniform carbon coating.
10) environment to get a uniform carbon coating.
2.3 Synthesis of graphene oxide (GO)
Initially, the graphene oxide (GO) was synthesized by Hummer’s method22 and dispersed in water. In brief, 2 g of graphite flakes was dispersed in ice cold H2SO4 and then 1 g of NaNO3 was added with constant stirring. The oxidizing agent KMnO4 (5 g) was added slowly to the mixture and the temperature was maintained at 20 °C for 30 min, before being increased to 35 °C with constant stirring. After 35 minutes of mixing, 200 mL of water was added to the reaction mixture with vigorous stirring. In the final stage, 500 mL of a 3% solution of H2O2 was added. The whole suspension was filtered and the solid cake was dispersed in water and sonicated for 2 h. The filtrate of the sonicated sample was collected and used as a graphene oxide–water (GO–H2O) colloid.2.4 Synthesis of the SnS NRs/r-GO composite
The composite of SnS nanorods with the reduced graphene oxide (r-GO) was prepared by solvothermal reduction in DMF. GO was separated from 40 mL of GO–H2O colloid, with a concentration of 2.4 mg mL−1, by the addition of 5 mL of ethanol (C2H5OH) and 5 mL of HCl followed by centrifugation at 8000 rpm for 15 min. The separated GO was pressed between cellulose filter paper to remove extra solvent. The separated GO was finally dispersed in 20 mL N,N-dimethyl formamide (DMF). Tin sulfide (SnS) nanorods were also dispersed in 10 mL of DMF by sonication for 5 min. Both dispersions were mixed and volume was made up to 50 mL by adding pure DMF. To facilitate GO reduction, 0.1 mg of thiourea was added. This dispersion of GO and SnS nanorods in DMF was kept in a steel-body, thin Teflon-lined solvothermal bomb at 160 °C for 8 h. The black precipitate was obtained after the completion of the reaction, and was washed with water and isopropyl alcohol (IPA) and dried in a vacuum oven at 60 °C for 5 h.2.5 Material characterization
Powder X-ray diffraction (PXRD) of the samples was performed using the Rigaku Smart Lab run on 40 kW power with a Cu-target and a wavelength (λ) of 1.54 nm in the scan range of 10–70° (2θ scale). The Raman spectra were recorded using a Ramnor HG-2S Spectrometer (Horiba Jobin Yvon, France) with an attached Quanta LASER system with a wavelength of 514.4 nm. The power of the LASER was adjusted to 5 mW and a 20× objective lens was used to focus. To record the FT-IR spectra, the sample was mixed with KBr and pressed to form pellets, which were analyzed using a Magna 550, Nicolet Instrument (USA). Morphological analysis of the SnS/r-GO composite, carbon-coated SnS NRs and their ex situ morphological changes after cycling were studied using field emission gun transmission electron microscopy (FEG-TEM), performed on a JEOL JEM-2100F accelerated with 200 kV. The electrochemical tests of all the materials were done with a Biologic VMP-3 electrochemical work station (France) and Arbin (USA) battery testing unit at a constant temperature of 20 °C against Li/Li+.2.6 Cell preparation for electrochemical testing
The electrochemical performance of the carbon-coated SnS NRs and SnS NRs/r-GO composite was evaluated in lab scale Swagelok and coin type cells (CR2032) in a half cell mode. The slurry of electro-active material was prepared by mixing 90 wt% composite with 10 wt% of binder CMC/PVDF in 10 mL of N-methyl pyrrolidone (NMP)/water without the further addition of any external carbon. All of the slurries were cast on a thin copper foil using the doctor blade technique. All films were dried in a vacuum oven at 120 °C for 12 h prior to any electrochemical tests. Dried films were cut into circular discs and used as anodes in a half cell assembly against a thin metallic lithium foil, which acted as the reference and counter electrode. Both electrodes were separated by a Whatman GF/D borosilicate glass fiber soaked in LP-30 (Merck, Germany) electrolyte.2.7 Sample preparation for ex situ TEM analysis
For ex situ TEM analysis, the cells were fabricated in a Swagelok type cell assembly. After the constant current charge–discharge cycling test, the used cells were opened and anode portion of cell was removed inside an argon-filled glove box. The anode film was scratched with the tip of a micropipette of acetone solvent. The debris of the electrode was dropped on a holey carbon grid. The grids were dried under an IR lamp and further analyzed using FEG-TEM for morphological changes after cycling.3. Results and discussion
Several forms of conductive carbon are used in battery technology, such as highly ordered pyrolytic graphite (HOPG) carbon, carbon nanotubes (CNTs), carbon coatings on particles, and reduced graphene oxide (r-GO).3–13,23 HOPG carbon has d-spacing superior to that of natural graphite for Li intercalation, and CNTs are a form of carbon with relatively high conductivity and a tubular structure that can participate in Li intercalation. On the other hand, regular carbon is simply a three dimensional (3-D) structure resulting from the graphitization of a carbon source on electro-active materials and graphene is a 2-D conducting single sheet of carbon used for the support of electro-active species. All of these types of carbon have differences in their density, due to the differing arrangement of the carbon atoms, which influences the conductivity that arises solely through their sp2 hybridization.24,25In our previous study, it was established that these SnS NRs have stable electrochemical performance with bare carbon and CMC binder against Li/Li+ in the potential window of 0.01–1.2 V.18 This combination of SnS NRs with bare carbon and CMC binder is not stable in wide potential window of 0.01–2.5 V. To enhance the cycling performance of the SnS NRs using a wider potential window (0.01–2.5 V), two forms of carbon modifications were introduced, as explained above. SnS NRs were carbon-coated by the thermal carbonization of polyvinyl alcohol (PVA) in dimethyl sulfoxide (DMSO) solution. PVA polymer expands its chain (swelling of polymer) in DMSO solvent when it is stirred for a long period of time and forms a viscous transparent solution. The thermal carbonization (removal of oxygenic functional groups and the restoration of double bonds) of PVA occurs in two steps in an inert atmosphere, at ∼350 °C and ∼450 °C, and finally generates polyenes or substituted olefins.26,27 The SnS NRs are dispersed into the solution of PVA–DMSO, which is adsorbed onto the surface of the SnS nanorods, and during calcinations in an Ar/H2 environment the PVA molecules become carbonized and form a uniform nano layer of carbon on the SnS NRs surface. Another modification to SnS NRs was achieved by the formation of a composite with graphene through the solvothermal reduction of graphene oxide (GO) in the presence of thiourea and DMF. The temperature and thiourea play an important role in the reduction of the oxygen functional groups of GO and convert it into r-GO.28
In both modification processes, the precursors have an oxygenic functional group and tin is more prone to oxidation, which makes the probability of oxidation very high during the attachment of carbon to the SnS NRs. The primary criterion for composite formation is that both components of the mixture should not change their phase and morphology. To gather information about the phase of the composite, a powder X-ray diffraction (PXRD) study was performed on the powder sample of the carbon-coated SnS NRs, SnS NRs/r-GO composite and pristine SnS NRs, as shown in Fig. 1. The PXRD patterns of all three samples of the pure NRs, carbon-coated NRs and the SnS NRs/r-GO composite showed a good crystalline peak for SnS having the space group Pnma, as reported previously.18
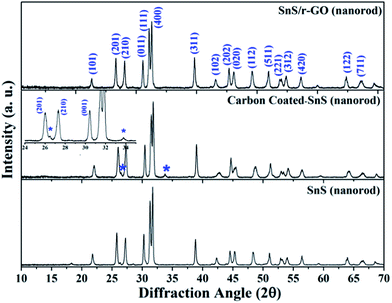 | ||
| Fig. 1 Powder X-ray diffraction (PXRD) pattern of pure SnS nanorods (NRs), carbon-coated NRs and the SnS/r-GO composite. | ||
From the XRD analysis, it can be concluded that the phase of the material has not changed during the formation of the SnS NRs composite. The only small peaks indicating impurities were observed in the carbon-coated SnS NRs sample, and were positioned at the 2θ values of 26.5° and 34°, corresponding to tin oxide (SnO2) formation (JCPDS card no. 01-077-0451).
The formation of a small amount of impurities may happen during the initiation of the carbonization process with an oxygenic carbon precursor. The carbon peaks of modified SnS NRs are not very conspicuous because their 2θ positions are merged with the SnS peak positions. Thus, to confirm the presence of carbon and its physical nature, Raman spectroscopy was performed on the powder samples.
The Raman spectra (Fig. 2) reveal the internal conditions of the carbon i.e. their graphitic nature and degree of disorder. The Raman spectra shown in Fig. 2 correspond to pure SnS NRs, carbon-coated SnS NRs, GO, r-GO and the SnS NRs composite with r-GO, respectively. The pure SnS sample has peaks at 160 cm−1, 220 cm−1 and 312 cm−1, corresponding to the B3g and B1u vibration modes of SnS and the A1g mode of SnS2, respectively.29,30 The samples containing carbon material have shown the characteristic D-band (E2g) mode and G-band (A1g) mode at ∼1350 cm−1 and ∼1580 cm−1, respectively, in GO, r-GO and the SnS NRs/r-GO composite.31 The SnS/r-GO composite also has the B3g and B1u peaks, corresponding to SnS, and a small hump for the A1g mode of SnS2. Here, we can observe the difference in the metallic peak intensities with the various modifications, which may happen due to the orientation dependence of Raman spectra.29 The Raman spectra reveal that the reduction of the GO to r-GO has increased the disorderness in the carbon, which is a normal phenomenon in chemically synthesized GO. The ID/IG ratio has also changed from 1.03 to 1.44 with the reduction of GO to r-GO, and 1.03 to 1.61 in the case where GO is reduced to form the SnS NRs/r-GO composite. This increase in the ID/IG ratio strongly suggests that the reduction process is quite good and that the nanorods were distributed on the surface of the GO, which helps to increase the disorderness of r-GO in the composite and results in an overall increase in the ID/IG ratio.
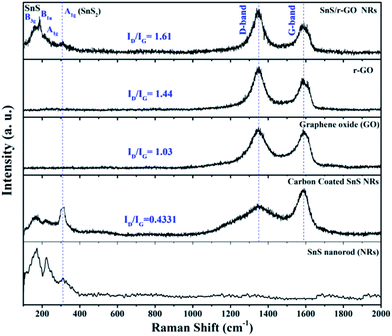 | ||
| Fig. 2 Dispersive Raman spectra of pure SnS nanorods (NRs), carbon-coated SnS NRs, GO, r-GO and SnS NRs/r-GO composite samples. | ||
In this section, the differences in the chemical functionality of the morphologically different forms of carbon attached to the SnS NRs will be assessed. The FT-IR spectra (Fig. 3) have shown that both forms of carbon, whether in the form of a carbon coating or reduced graphene oxide (r-GO), have similar carbon natures, regarding the type of surface functional group present after carbonization and reduction. With regard to the FT-IR spectra, the peak position at 3450 cm−1 corresponds to the (–O–H) stretch of the hydroxyl group of various functional groups, such as aldehydes, alcohols, acids, etc. The FT-IR peak at 2920 cm−1 corresponds to CH3 stretching, that at 2854 cm−1 corresponds to the C–H stretching of an aldehyde and those from 1400 cm−1 to 1800 cm−1 correspond to the carbonyl group (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) stretching of various functional groups. The aromatic basal plane of the graphene layers of the graphitized carbon coating and r-GO sheet appears at 1644 cm−1 for the C
O) stretching of various functional groups. The aromatic basal plane of the graphene layers of the graphitized carbon coating and r-GO sheet appears at 1644 cm−1 for the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond stretch. Some of the terminal bonds with an sp3 carbon are represented in the CH3 bend at 1390 cm−1. Other oxygen functionalities are shown at 1110 cm−1 for the C–O stretching of alcohol and 1034 cm−1 for the epoxide bond of the –C–O– group.32 The peak at ∼460 cm−1 corresponds to Sn–S bond stretching. Thus, FT-IR analysis has confirmed that during the reduction process most of the oxygen functionalities have been removed and only a few of the inherent oxygen functionalities remain in the carbon.
C bond stretch. Some of the terminal bonds with an sp3 carbon are represented in the CH3 bend at 1390 cm−1. Other oxygen functionalities are shown at 1110 cm−1 for the C–O stretching of alcohol and 1034 cm−1 for the epoxide bond of the –C–O– group.32 The peak at ∼460 cm−1 corresponds to Sn–S bond stretching. Thus, FT-IR analysis has confirmed that during the reduction process most of the oxygen functionalities have been removed and only a few of the inherent oxygen functionalities remain in the carbon.
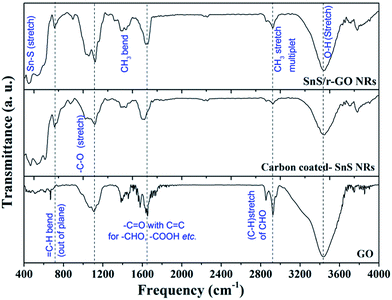 | ||
| Fig. 3 Fourier transform infrared (FT-IR) spectra of samples containing GO, carbon-coated SnS nanorods (NRs) and the SnS NRs/r-GO composite. | ||
3.1 Microscopic analysis (FEG-TEM)
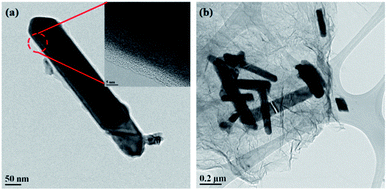
As mentioned previously, the composite should not change in phase and morphology during composite formation and this is crucial for electrochemical performance. Our XRD, Raman and FT-IR spectroscopy experiments have shown that the phase of the SnS NRs did not change during the composite formation processes. The retention of the nanorods’ morphologies was then confirmed by the high resolution field emission gun transmission electron microscopy (HR FEG-TEM) imaging of carbon-coated and graphene composite SnS NRs samples.The FEG-TEM images (Fig. 4a and b) shows that the morphology of the SnS nanorods remains intact after surface modification by carbon-coating and reduced graphene oxide (r-GO) wrapping. The carbonization of PVA at 500 °C and 400 °C generated a uniform carbon coating (Fig. 4a and S1†) on the surface of the SnS NRs. The thickness of these carbon coatings was found to be ∼4–5 nm (Fig. 4a, inset). The graphene composite of the SnS NRs was also prepared with chemically obtained GO and solvothermally synthesized SnS by the solvothermal reduction process (Fig. 4b). The TEM image shows that the SnS NRs are supported on the r-GO surface after the solvothermal reduction, keeping the morphology intact as before. Thus, FEG-TEM analysis shows that both forms of carbon supported the SnS NRs as desired.
3.2 Electrochemical performance
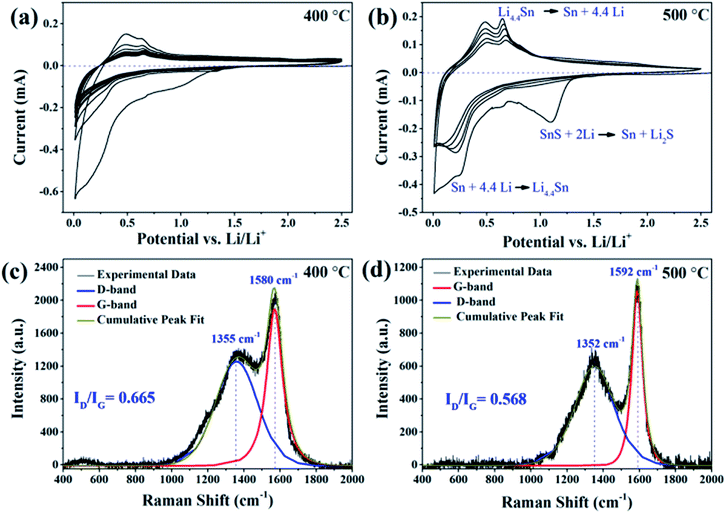
The surface modification of the SnS NRs was done with two kinds of carbon to enhance the electrochemical performance of the SnS NRs as a lithium battery anode in applications requiring a wider potential window (0.01–2.5 V). It has already been shown in our previous work that bare SnS material remains electrochemically stable in the narrow potential window of 0.01–1.2 V with external carbon and a CMC binder.18 The electrochemical tests with modified SnS NRs were first started by checking the compatibility of the SnS NRs with Li by performing cyclic voltammetry (CV) experiments against Li/Li+ in a half cell assembly.Again, the carbon-coating of the SnS NRs sample was performed at two different carbonization temperatures, 400 °C and 500 °C. A carbon coating was formed on the SnS NRs under both conditions, the only difference observed was in their electrochemical performances (Fig. 5a and b) against Li/Li+. The sample carbonized at 400 °C showed poor peak currents in the conversion (∼1.1 V region) and alloying (0.65–0.30 V) regions in their first reduction cycle. A similar finding was obtained in their first oxidation scan. From the second cycle onwards, the peak current was reduced and there was no redox behavior corresponding to alloying and de-alloying, but instead the redox activity of pure carbon was shown.33,34 The FEG-TEM image of the carbon-coated SnS NRs sample (shown as ESI, Fig. S1†) shows a uniform carbon coating with a thickness of 4–5 nm. From these observations, it is expected that in the initial cycle some poorly coated SnS NRs reacted with Li but afterwards the uniformly coated sample did not allow Li to penetrate and react with the active SnS NRs material.
To improve the performance of the carbon-coated SnS NRs, the carbonization temperature was increased from 400 °C to 500 °C. The SnS NRs carbonized and calcined at 500 °C have a thickness similar to that of the previous ones i.e. 4–5 nm, as observed from TEM analysis. The corresponding CV results (Fig. 5b) also show much improved peaks at 1.11 V and 0.22 V in the first reduction scan, and 0.47 V and 0.66 V in first oxidation scan. The CV peaks at 1.11 V and 0.22 V in first reduction scan correspond to the conversion of SnS to Sn + S and the alloying of tin with lithium, respectively. In the oxidation scan, the CV peaks at 0.47 V and 0.22 V correspond to the two steps in the de-alloying of Sn and lithium, respectively. One peculiar observation is noticed here, which is that there is no peak for the partial conversion of Sn to SnO2 or SnS with the carbon-coated sample, which is a regular observation in the literature.35 Thus, the CV experiments indicate that increasing the carbonization temperature of the SnS NRs sample results in good electrochemical behavior against Li/Li+, which may be attributed to the improved conductive behavior of the carbon coating due to the higher temperature carbonization process. A more detailed explanation for this is given in the latter part of the discussion.
To establish the reason for such different electrochemical behavior emerging with the use of the same active material, Raman spectroscopy was performed on both samples. As mentioned previously, the nature of carbon can be characterized by its D-band (E2g mode), which corresponds to the disorderness of the system, and G-band (A1g mode), which corresponds to the extent of the sp2 carbon network.36 The characteristic D-bands are observed at 1355 cm−1 and 1352 cm−1 in both cases, for samples heated at 400 °C and 500 °C, respectively. The characteristic G-band situated at 1380 cm−1 in the sample carbonized at 400 °C has shifted to 1592 cm−1 on performing the carbonization at 500 °C. Here, another peculiarity is that the D-band and G-band overlap in the sample carbonized at 400 °C and are separated in the sample carbonized at 500 °C, with an increase in the sharpness of the G-band and reduction in the intensity of the D-band. After the Gaussian–Lorentzian mixed fitting of the Raman spectra of the carbon-coated SnS NRs, the ID/IG ratios were found to be 0.665 and 0.568 for the samples carbonized at 400 °C and 500 °C, respectively. This indicates that, on increasing the carbonization temperature, the sp2 hybridized basal plane of the carbon chain increased, with a simultaneous reduction in the defect level of the graphitized carbon coating.
Further, Tuinstra and Koenig’s (TK) formula is used to approximate the crystallite size (La) or inter-defect distance of the sp2 hybridized graphite layer, as shown in eqn (1). The crystallite size was calculated by using a constant for the LASER wavelength (Cλ) ≈ 4.4 nm and the intensities of the Raman D-band (ID) and G-Band (IG).36,37
| La = Cλ[ID/IG]−1 | (1) |
The crystallite size of the graphitic plane in the sample carbonized at 400 °C was found to be ∼6.60 nm, while that of the sample carbonized at 500 °C was found to be ∼7.73 nm. This difference in the crystallite size may be attributed to the addition of more sp2 hybridized carbon to the graphitized basal plane in comparison to the sample carbonized at 400 °C, which further results in the reduction of the defect level. Similar shifts were also observed by others for the calcination of pure carbon.38
It is a known fact that the D-band comes from the edges of the graphite plane and these graphite edges are more reactive towards the LiPF6 of the electrolyte salt. The electrolyte salt breaks down to LiF more on the edges than on the basal planes of the graphitized surface.39 Therefore, it is expected that the smaller crystallite size of graphitic carbon in the carbon coating will have more free bonds, which results in more surface reactions and the easier breakdown of the LiPF6 salt, which blocks the Li-diffusion path in the SnS NRs and hence stops the electrochemical lithiation. On the other hand, the sample carbonized at 500 °C increases the sp2 carbon content of its basal plane and reduces the free terminal bonds, resulting in more favourable Li diffusion and the proper lithiation of the SnS NRs.
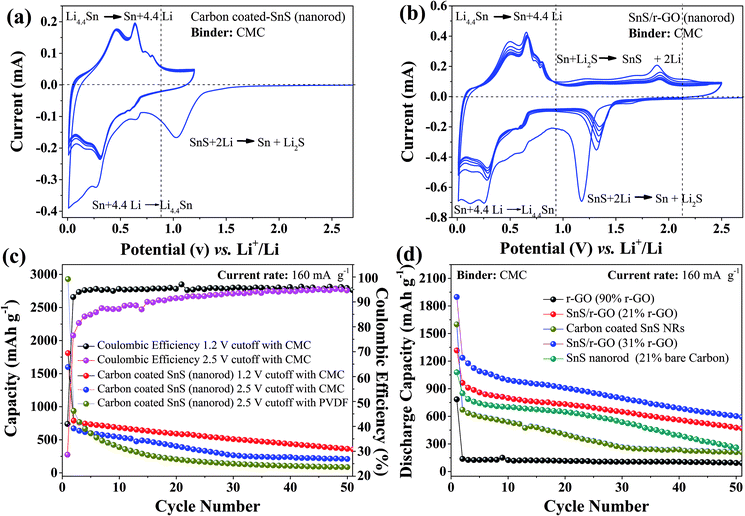
Although the carbon-coated SnS NRs sample became more reactive towards lithium after being carbonized at 500 °C, it lacked in cycling stability (Fig. 5b) in the potential window of 0.01–2.5 V. However, the same carbon-coated SnS NRs show stable peak currents for alloying and de-alloying reactions when the charge–discharge potential window is narrowed down to 0.01–1.2 V (Fig. 6a), as it was with the external carbon reported previously.18 To observe the effect of the binder on the electrochemical performance, the charge–discharge profiles of the carbon-coated SnS NRs carbonized at 500 °C were obtained in both potential windows with CMC and PVDF binders against Li/Li+. The carbon content of the carbon-coated SnS NRs was found to be 18.5% by CHN analysis. The first discharge capacities of the SnS NRs with a CMC binder (Fig. 6c and S3†) were found to be 1810.4 mA h g−1 and 1599.43 mA h g−1 with coulombic efficiencies of 40% and 44% in the two different potential windows of 0.01–1.2 V and 0.01–2.5 V, respectively. This difference in first discharge capacity is due to the variation in the loading of electro-active material on the electrodes and the extra reactions that occurred in the wider potential window. The second discharge capacities of the SnS NRs were found to be 790 and 672 mA h g−1 with coulombic efficiencies of 91% and 89% for the potential windows of 0.01–1.2 V and 0.01–2.5 V, respectively. After the 50th cycle of discharge, the capacities of the carbon-coated SnS NRs were found to be 363 mA h g−1 and 210 mA h g−1 in the potential windows of 0.01–1.2 V and 0.01–2.5 V, respectively. This observation shows that the widening of the operating potential window results in a greater loss in the capacity value. However, the same carbon-coated SnS NRs were tested with a PVDF binder in the potential window of 0.01–2.5 V and poor cycling stability was observed compared to the CMC binder and, after the 50th cycle of discharge, the capacity was found to be 86 mA h g−1.
Therefore, it is worth mentioning here that the carbon-coated SnS NRs show a higher degree of stability in electrochemical performance when a CMC binder is used and the charge–discharge potential window is limited to 0.01–1.2 V. Therefore, a carbon coating, in place of the external use of conductive carbon, is not very effective in an alloy-based anode electrode material. It is also clear that the loss in capacity is raised by 15% just by increasing the potential window. To find out the reason behind the instability of the carbon-coated sample, it was further studied using ex situ TEM, as discussed in section 3.3.
To further enhance their electrochemical properties, composites of the SnS NRs with r-GO were prepared using the solvothermal reduction method. Herein, two compositions were prepared, one having a carbon percentage of 21% and another having 31% carbon as r-GO, as estimated by CHN analysis. The CV of SnS NRs/r-GO composite (with 31% carbon) with a CMC binder in the wider potential window of 0.01–2.5 V shows the first reduction peaks at 1.17 V, 0.62 V and 0.25 V in the reduction scan, for the conversion of SnS to Sn and sulfur and the alloying of Sn with lithium in two steps, respectively. In the following oxidation scan, the profile shows peak currents at 0.48 V and 0.66 V, and minor ones at 0.74 V and 0.79 V for delithiation from the Sn22Li5 that is formed in first reduction step. Similarly, the oxidation scan peak at 1.89 V corresponds to the partial conversion of Sn to SnS. In the second discharge scan, all of the reduction peak positions showed a minor shift in potential and appeared at 1.32 V, 0.61 V and 0.29 V. This is due to the formation of a SEI layer in the first reduction cycle. In the initial two cycles, the peak in the CV profile shows a reduction in the peak current but from the 3rd cycle onwards the peak current of the alloying–de-alloying region became stabilized. The signature for the conversion reaction remained visible after the first cycle and appeared at 1.89 V (oxidation scan) and 1.32 V (reduction scan). These peaks correspond to the partial conversion of Sn to SnS in the oxidation scan and SnS to Sn conversion in the reduction scan.18,35,40 It is noticeable that the peak current corresponding to these conversion reactions diminishes with an increase in cycle number and shows clearly that the alloying reaction predominates over the conversion reaction after the complete conversion of SnS to Sn.
To study the roles of the 2D graphene (21%, 31% carbon) sheets, the use of external carbon (21% carbon), the 3D coating of sp2 carbon (18.5% carbon) and pure r-GO (90% carbon) in influencing the electrochemical properties of the SnS NRs, all of the cells were prepared with a CMC binder and tested at a constant current density of 160 mA g−1 in the wider potential window of 0.01–2.5 V, as shown in Fig. 6d. It was noticed that the electrochemical charge–discharge behavior was more stable for the sample with 21% r-GO, in comparison to that of the samples with a similar amount of carbon in the form of the carbon coating (18.5%) or externally used carbon (21%). The first, second and 50th cycle discharge capacities of the SnS NRs/r-GO composite (21% carbon) were found to be 1317 mA h g−1, 964 mA h and 482 mA h g−1, respectively. The percentage of retention of the discharge capacity from the 2nd cycle to the 50th cycle in the wider potential window (0.01–2.5 V) was found to be 50% and that is above the graphite theoretical limit (372 mA h g−1). The graphene supported SnS NRs showed better capacity retention than those in the recent work by Cai et al. on SnS nanorods, even at a current rate that was three times higher.41 For comparison, an electrode comprising SnS NRs with 21% external carbon and a CMC binder was tested and showed first, second and 50th cycle discharge capacities of 1079 mA h g−1, 850 mA h g−1 and 263 mA h g−1, respectively. SnS NRs with an external carbon electrode initially showed a trend similar to that of the r-GO containing electrode, however after the 20th cycle the capacity loss became more severe and at the end of 50th cycle it showed 46% less capacity than the r-GO supported electrode.
Similarly, when the electrode with pure graphene (having 90% carbon and 10% CMC binder) was tested, the discharge capacities were observed to be 785 mA h g−1, 138 mA h g−1 and 94 mA h g−1 after the 1st, 2nd and 50th cycle, respectively, under the same electrochemical conditions. The current data show that the attachment of carbon to the SnS NRs is better in the case of reduced graphene oxide (r-GO) than bare carbon, coated carbon and pure graphene (r-GO).
As per the above discussion, it is clear that r-GO alone or SnS with bare carbon or coated carbon could show a lower capacity (lower than the graphite theoretical limit) at a moderately high current rate. On the other hand, combining SnS NRs with r-GO in the form of a composite could result in an enhancement in the capacity and cycling stability. The 2-D r-GO support was able to provide better electrochemical kinetics in the composite, which facilitates the better reaction of lithium with SnS and minimizes the losses due to secondary reactions, in comparison to the use of bare carbon and the carbon coating.
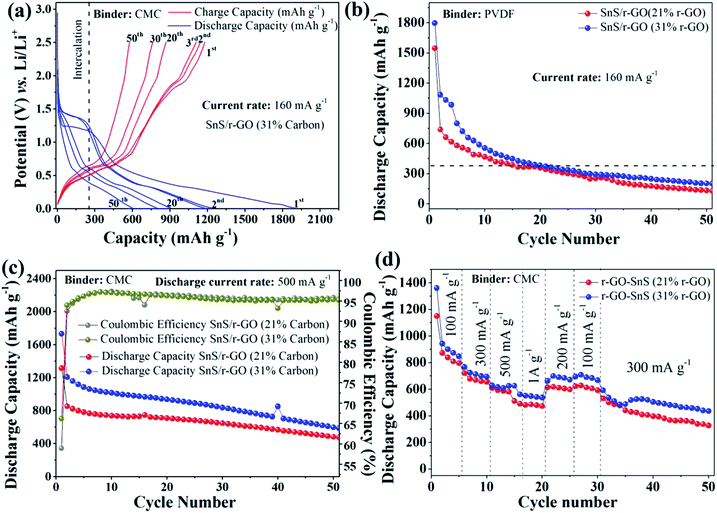
On increasing the carbon content in the SnS NRs to 31% in the form of r-GO, the performance of the SnS NRs cell improved relative to that of the r-GO electrode containing 21% carbon. The above electrode showed discharge capacities of 1897 mA h g−1, 1237 mA h g−1 and 602 mA h g−1 after the 1st, 2nd and 50th cycle, respectively, and these are rather higher than those of the sample containing 21% carbon. The enhancement in the first discharge capacity in the case with a greater amount of r-GO may be attributed to more irreversible reactions with remaining oxygen functionalities of the r-GO sheet taking place. Here, it can be concluded that the r-GO support plays a crucial role in improving the cycling stability of SnS NRs in comparison to the externally used carbon and carbon in the form of a graphitized (in situ) coating.
As we previously discovered, the binder significantly influences the cycling stability and power performance, particularly in alloying- and conversion-based electrodes.18,42 Here again, we confirm the role of the binder in the cycling stability of the cells containing the r-GO composite samples with 21% carbon and 31% carbon. Both samples were tested along with PVDF and CMC binders at the same current rate of 160 mA g−1 and in the potential window of 0.01–2.5 V against Li/Li+. Here, it was observed (Fig. 7b) that, just by changing the binder from CMC to PVDF, the electrode performance deteriorated drastically. The 21% and 31% r-GO containing SnS NRs electrodes showed first discharge capacities of ∼1547 mA h g−1 and 1796 mA h g−1, second discharge capacities of ∼737 mA h g−1 and 1082 mA h g−1 and 50th cycle discharge capacities of 132 mA h g−1 and 201 mA h g−1, respectively, at a current density of 160 mA g−1. Therefore, here it is clear that the presence of r-GO and the interactive binder together makes it possible to maintain the electrochemical stability of the SnS NRs. In this case, the combination of r-GO and CMC was found to be better than the combination of r-GO and PVDF.
To further confirm the stability of the SnS NRs/r-GO composite with the CMC binder, it was tested at high rates, at a current rate of around 500 mA g−1, and the results are shown in Fig. 7c. The first, second and fiftieth cycle discharge capacities (Fig. 7c) of the SnS NRs/r-GO composite (21% carbon) were found to be 1314 mA h g−1, 852 mA h g−1 and 476 mA h g−1, which are similar to those obtained in the test performed at a current rate of 160 mA g−1. However, this should not mask the fact that the electrode coulombic efficiency is better for this electrode. For the first cycle it was 58%, which increased to 92% in second cycle and eventually reached 95%. At the same current rate of 500 mA g−1, the SnS NRs/r-GO composite electrode with 31% carbon could show very similar trends in discharge capacity in the first, second and 50th cycles (1731 mA h g−1, 1206 mA h g−1 and 598 mA h g−1) with coulombic efficiencies of 66%, 93% and 95% in the rest of the cycles, respectively.
As a further potential “figure of merit”, the rate capability as a function of cycle number of our r-GO supported electrodes was determined. For that, a rate capability test (i.e., power rate) was carried out for the composites and both r-GO electrodes with variable current densities of 100 mA g−1, 300 mA g−1, 500 mA g−1, 1000 mA g−1 and reversing current rate to 200 mA g−1, 100 mA g−1 for 5 cycles at each current rate and then 20 cycles at a current rate of 300 mA h g−1. The plot of the power performance (Fig. 7d) of SnS/r-GO (21% carbon) shows a discharge capacity of 846 mA h g−1 at the end of the 5 initial cycles with a current rate of 100 mA g−1, and 694 mA h g−1 at the end of next 5 cycles, i.e. the 10th discharge cycle with a current rate of 300 mA g−1. A capacity of 625 mA h g−1 was then obtained after 5 subsequent cycles, i.e. the 15th discharge cycle at a current rate of 500 mA g−1, and 536 mA h g−1 at the end of the next 5 cycles i.e. after the 20th discharge cycle at a current rate of 1 A g−1. This shows that at a high current of 1 A g−1 the r-GO composite is capable of providing a charge storage capacity above the graphite theoretical capacity level. Again, when the current was lowered to 200 mA g−1 from 1000 mA g−1 in the previous cycle, the composite electrode started gaining capacity and, at the end of next 5 cycles at 200 mA g−1, provided a discharge capacity of 671 mA h g−1. On further lowering the current to 100 mA g−1 for next 5 cycles, the capacity was found to be 667 mA h g−1 after the 30th discharge cycle. After the completion of the 30th cycle with various low and high current densities, the cell was again tested at a current rate of 300 mA g−1 for the next 20 cycles and the discharge capacity after the 50th cycle was found to be 434 mA h g−1, which is higher than the graphite theoretical capacity (372 mA h g−1).
The power performance plot of SnS/r-GO (31% carbon) with a CMC binder shows very similar performance and is shown in the same figure. After the cell was operated at several high and low current rates, the cell was again charge–discharged at a current rate of 300 mA g−1 until the end of 50th cycle, and it is notable that the capacity observed at the end of the 50th cycle was 326 mA h g−1. Therefore, the variable rate performance of the electrode again concludes that the SnS NRs with 31% r-GO and a CMC binder is better in electrochemical stability than that of the 21% r-GO containing electrode. The cause of such variation in the electrochemical performance could be further analyzed using ex situ TEM analysis and the effects of the morphological changes and conductive support on the overall cell performance could be established.
3.3 Ex situ morphological analysis before and after the charge–discharge process
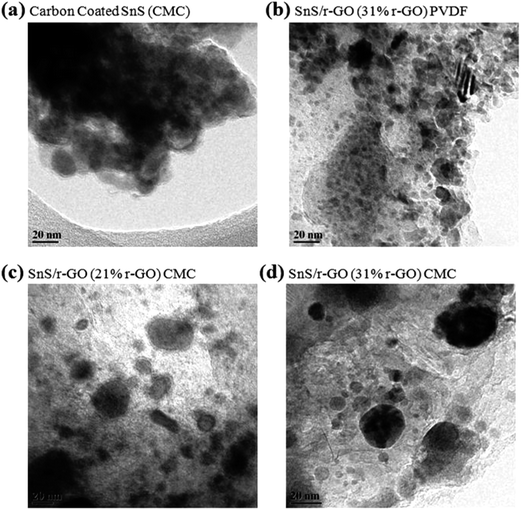
To investigate the origin of the capacity fading in samples containing a PVDF binder and carbon coating, we first examined the sample morphology after cycling, as shown in Fig. 8. Each sample, in its discharged state after 50 cycles, was collected and analyzed using an ex situ TEM instrument. The cell underwent the charge–discharge process at a current density of 160 mA g−1 for 50 cycles, in the potential window of 0.01–1.2 V for the carbon-coated SnS NRs electrode and 0.01–2.5 V for the SnS NRs/r-GO composite with PVDF and CMC binders.The ex situ TEM analysis of the carbon-coated SnS NRs with a CMC binder was done for a cell cycled in the potential window of 0.01–1.2 V, because it was only stable in this potential window, unlike the cells cycled in the potential window of 0.01–2.5 V. The ex situ TEM analysis (Fig. 8a) has shown that the carbon-coated SnS NRs electrode is not able to retain the basic morphology of the NRs. With the lithiation and delithiation process, the carbon-coated SnS NRs become converted to non-uniform and irregularly shaped particles in an agglomerated state. The selected area electron diffraction (SAED) pattern of the carbon-coated sample (Fig. S4a†) shows a dotted pattern with poor intensity. The SAED pattern indicates the formation of the agglomerated particles state after the 50th discharge for the carbon-coated sample. Therefore, it is expected that in the initial cycles of lithiation, the carbon coating is damaged due to the excessive volume expansion of Sn on lithiation in the discharge cycles and the carbon coating is not able to provide proper support to the electro-active material. With the addition of graphene to the SnS NRs system, the stability was increased (Fig. 8b–d) and the particle size retention and distribution were distinguishably better in comparison to the carbon-coated sample. The ex situ TEM analysis of the SnS NRs/r-GO composite (31% carbon) with a PVDF binder shows that the electrode will have a better particle size distribution in comparison to the electrode with carbon-coated SnS NRs and a CMC binder. In the SAED pattern of the SnS NRs/r-GO electrode with a PVDF binder, a diffuse ring is shown, which is a characteristic of nanoparticle formation, as shown in Fig. S4b.† Here, again, it is clear that the r-GO acts as a better support to maintain the electro-active particle size during lithiation and de-lithiation during electrochemical cycling against Li/Li+. The ex situ TEM image of the electrode with SnS NRs/r-GO and a CMC binder (Fig. 8c and d) shows even better particle size retention than the electrodes with SnS NRs/r-GO and a PVDF binder or carbon-coated SnS NRs and a CMC binder. The particle size distribution in the r-GO electrodes with 21% and 31% carbon have shown better particle distribution than the carbon-coated one. The SAED patterns of Fig. S4c and d† show the dotted ring patterns, which could be attributed to the presence of nanoparticles with an even distribution on the r-GO support after the 50th charge–discharge. Therefore, we can conclude here that the difference in the capacity fading has occurred due to the improper support of the SnS NRs in the 21% r-GO containing samples in comparison to the 31% r-GO containing sample. The ex situ TEM analysis of the electrodes with SnS NRs/r-GO and a CMC binder and SnS NRs/r-GO and a PVDF binder with 31% carbon as reduced graphene oxide (r-GO) has also established the fact that the binder has a synergistic effect with r-GO in the morphological retention during cycling, which clearly reflects the electrochemical performances against Li/Li+ in the wider potential window.
4. Conclusions
The real Achilles heel of employing alloying-based reactions in practical applications is the severe volume change over the course of the lithium insertion–extraction process. These changes induce cracks in the electrode structure, which rapidly leads to the end of the cycle life. It has been shown by various authors that this problem may be controlled by properly modifying the electrode configuration, e.g., by decreasing the particle size and selecting an optimized binder. Here, we have used a strategy to improve the electrochemical performance of SnS alloy-based nanoparticles along with their cycling stability by incorporating a 3D carbon coating and/or (2D) r-GO support with SnS. The electrochemical performance of electrodes fabricated using both conductive coating processes were compared in the presence of a new generation of less expensive binders, such as CMC. It was noticed during the analysis that the carbon coating became ruptured and was not able to provide proper support to the electrode’s active materials, which retain the electrochemical performance of the SnS NRs electrode. The further enhancement in the electrochemical performance of the SnS NRs by using an r-GO support and a CMC binder may be due to the synergistic effect of the r-GO and CMC binder, which was established with electrochemical tests and ex situ TEM analysis. The capacity fading mechanism of the carbon-coated SnS NRs electrode under high current battery operation was investigated by combined electrochemical and TEM morphological analysis. Our results indicate that the morphological breakdown occurred mainly on the electrode surface, due to the large volume change in the reaction with lithium, which was somewhat restricted with the use of the r-GO support and CMC binder. However, the dependency of the electrochemical activity on the cutoff voltage could be due to several reasons, such as secondary reactions, particle whiskering, etc., and needs further in-depth data collection. In brief, in the future, the process of carbon-coating and composite formation can be used on other sulfide based nanostructured materials to improve their electrochemical performance as lithium and sodium ion battery anodes.Acknowledgements
This manuscript is based upon work supported in part under the US–India Partnership to Advance Clean Energy-Research (PACER) for the Solar Energy Research Institute for India and the United States (SERIIUS), funded jointly by the U.S. Department of Energy (Office of Science, Office of Basic Energy Sciences, and Energy Efficiency and Renewable Energy, Solar Energy Technology Program, under Subcontract DE-AC36-08GO28308 to the National Renewable Energy Laboratory, Golden, Colorado) and the Government of India, through the Department of Science and Technology under Subcontract IUSSTF/JCERDC-SERIIUS/2012 dated 22nd Nov. 2012. The authors are indebted to SAIF, IIT-B for their assistance in Raman, HR-TEM and FEG-SEM analysis.References
- C. M. Park, J. H. Kim, H. Kim and H. J. Sohn, Chem. Soc. Rev., 2010, 39, 3115–3141 RSC.
- W. J. Zhang, J. Power Sources, 2011, 196, 13–24 CrossRef CAS PubMed.
- Z. Li, G. Wu, D. Liu, W. Wu, B. Jiang, J. Zheng, Y. Li, J. Li and M. Wu, J. Mater. Chem. A, 2014, 2, 7471–7477 CAS.
- W. Wei and J. M. Lee, J. Mater. Chem. A, 2014, 2, 1589–1626 Search PubMed.
- M. M. Doeff, J. D. Wilcox, R. Kostecki and G. Lau, J. Power Sources, 2006, 163, 180–184 CrossRef CAS PubMed.
- P. Gao, J. Fu, J. Yang, R. Lv, J. Wang, Y. Nauli and X. Tang, Z. Phys. Chem., 2009, 11, 11101–11105 CAS.
- N. R. Srinivasan, S. Mitra and R. Bandyopadhyaya, Phys. Chem. Chem. Phys., 2014, 16, 6630–6640 RSC.
- W. M. Zhang, X. L. Wu, J. S. Hu, Y. G. Guo and L. J. Wan, Adv. Funct. Mater., 2008, 18, 3941–3946 CrossRef CAS.
- L. Zhuo, Y. Wu, J. Ming, L. Wang, Y. Yu, X. Zhang and F. Zhao, J. Mater. Chem. A, 2013, 1, 1141–1147 CAS.
- Q. Xiao, Y. Fan, X. Wang, R. A. Susantyoko and Q. Zhang, Energy Environ. Sci., 2014, 7, 655–661 CAS.
- X. Huang, J. Chen, H. Yu, S. Peng, R. Cai, Q. Yan and H. H. Hng, RSC Adv., 2013, 3, 5310–5313 RSC.
- G. Zhou, F. Li and H. M. Cheng, Energy Environ. Sci., 2014, 7, 1307–1338 CAS.
- N. Li, H. Song, H. Cui, G. Yang and C. Wang, J. Mater. Chem. A, 2014, 2, 2526–2537 CAS.
- Z. Chen and J. R. Dahn, J. Electrochem. Soc., 2002, 149, A1184–A1189 CrossRef CAS PubMed.
- W. Liu, P. Gao, Y. Mi, J. Chen, H. Zhou and X. Zhang, J. Mater. Chem. A, 2013, 1, 2411–2417 CAS.
- B. Zhang, Y. Liu, Z. Huang, S. Oh, Y. Yu, Y. W. Mai and J. K. Kim, J. Mater. Chem., 2012, 22, 12133–12140 RSC.
- J. Vetter, P. Novak, M. R. Wagner, C. Veit, K. C. Moller, J. O. Besenhard, M. Winter, M. W. Mehrens, C. Vogler and A. Hammouche, J. Power Sources, 2005, 147, 269–281 CrossRef CAS PubMed.
- A. M. Tripathi and S. Mitra, RSC Adv., 2014, 4, 10358–10366 RSC.
- A. M. Tripathi and S. Mitra, ChemElectroChem, 2014, 1, 1327–1337 CrossRef CAS.
- J. Li, R. B. Lewis and J. R. Dahn, Electrochem. Solid-State Lett., 2007, 10, A17–A20 CrossRef CAS PubMed.
- I. Kovalenko, B. Zdyrko, A. Magasinski, B. Hertzberg, Z. Milicev, R. Burtovyy, I. Luzinov and G. Yushin, Science, 2011, 334, 75–79 CrossRef CAS PubMed.
- W. S. Hummers and R. E. Offeman, J. Am. Chem. Soc., 1958, 80, 1339 CrossRef CAS.
- Q. Zhang, Z. Yu, P. Du and C. Su, Recent Pat. Nanotechnol., 2010, 4, 100–110 CrossRef CAS.
- L. Dai, D. W. Chang, J. B. Baek and W. Lu, Small, 2012, 8, 1130–1166 CrossRef CAS PubMed.
- E. Frackowiak and F. Beguin, Carbon, 2001, 39, 937–950 CrossRef CAS.
- P. S. Thomas, J. P. Guerbois, G. F. Russell and B. J. Briscoe, J. Therm. Anal. Calorim., 2001, 64, 501–508 CrossRef CAS.
- B. J. Holland and J. N. Hay, Polymer, 2001, 42, 6775–6783 CrossRef CAS.
- Y. Liu, Y. Li, Y. Yang, Y. Wen and M. Wang, J. Nanosci. Nanotechnol., 2011, 11, 10082–10086 CrossRef CAS PubMed.
- H. R. Chandrasekhar, R. G. Humphreys, U. Zwick and M. Cardona, Phys. Rev. B: Condens. Matter Mater. Phys., 1977, 15, 2177–2183 CrossRef CAS.
- D. G. Mead and J. C. Irwin, Solid State Commun., 1976, 20, 885–887 CrossRef CAS.
- A. C. Ferrari, J. C. Meyer, V. Scardaci, C. Casiraghi, M. Lazzeri, F. Mauri, S. Piscanec, D. Jiang, K. S. Novoselov, S. Roth and A. K. Geim, Phys. Rev. Lett., 2006, 97, 187401–187405 CrossRef CAS.
- M. Acik, G. Lee, C. Mattevi, A. Pirkle, R. M. Wallace, M. Chhowalla, K. Cho and Y. Chabal, J. Phys. Chem. C, 2011, 115, 19761–19781 CAS.
- L. Fransson, T. Eriksson, K. Edstrom, T. gustafsson and J. O. Thomas, J. Power Sources, 2001, 101, 1–9 CrossRef CAS.
- D. Zhang, B. S. Haran, A. Durairajan, R. E. White, Y. Podrazhansky and B. N. Popov, J. Power Sources, 2000, 91, 122–129 CrossRef CAS.
- M. Mohamedi, S. J. Lee, D. Takahashi, M. Nishizawa, T. Itoh and I. Uchida, Electrochim. Acta, 2001, 46, 1161–1168 CrossRef CAS.
- A. C. Ferrari and J. Robertson, Phys. Rev. B: Condens. Matter Mater. Phys., 2000, 61, 14095–14107 CrossRef CAS.
- F. Tuinstra and J. L. Koenig, J. Chem. Phys., 1970, 53, 1126–1130 CrossRef CAS PubMed.
- F. Li and J. S. Lannin, Appl. Phys. Lett., 1992, 61, 2116–2118 CrossRef CAS PubMed.
- E. Peled, D. Golodnitsky, A. Ulus and V. Yufit, Electrochim. Acta, 2004, 50, 391–395 CrossRef CAS PubMed.
- K. J. Rhodes, R. Meisner, M. Kirkham, N. Dudney and C. Daniel, J. Electrochem. Soc., 2012, 159, A294–A299 CrossRef CAS PubMed.
- J. Cai, Z. Li and P. K. Shen, ACS Appl. Mater. Interfaces, 2012, 4, 4093–4098 CAS.
- P. S. Veluri and S. Mitra, RSC Adv., 2013, 3, 15132–15138 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: TEM image of carbon-coated SnS NRs prepared at carbonization temperature of 400 °C, the potential vs. capacity plot of a SnS NRs/r-GO (21% carbon) electrode with a CMC binder in the potential window of 0.01–2.5 V at a current rate of 160 mA g−1 against Li/Li+ and the potential vs. capacity plot of a carbon-coated SnS NRs (18.5% carbon) electrode with a CMC binder in the potential window of 0.01–1.2 V at a current rate of 160 mA g−1 against Li/Li+. See DOI: 10.1039/c5ra00226e |
| This journal is © The Royal Society of Chemistry 2015 |