Diastereoselective synthesis of functionalised carbazoles via a sequential Diels–Alder/ene reaction strategy†
Joseph Cowell,
Matokah Abualnaja
,
Stephanie Morton‡,
Ruth Linder,
Faye Buckingham,
Paul G. Waddell,
Michael R. Probert and
Michael J. Hall*
School of Chemistry, Newcastle University, Newcastle upon Tyne, NE1 7RU, UK. E-mail: michael.hall@newcastle.ac.uk
First published on 20th January 2015
Abstract
An operationally simple one-pot, three-component, diastereoselective synthesis of saturated carbazoles and related pyridazino[3,4-b]indoles, based on two sequential intermolecular pericyclic reactions, is described. The reaction sequence involves an intermolecular Diels–Alder (D–A) reaction of a 3-vinyl-1H-indole, containing an electron withdrawing N-protecting group, with a suitable dienophile. Due to the electron withdrawing nature of the N-protecting group the resultant D–A cycloadducts are sufficiently stabilised to allow for a subsequent in situ diastereospecific intermolecular ene reaction to take place with an added enophile, generating functionalised carbazoles with relative stereocontrol of up to four stereocentres.
Introduction
Carbazole scaffolds are common in many bioactive compounds (e.g. staurosporine)1,2 with the indolocarbazole scaffold in particular being found in a number of molecules with potential therapeutic application, several of which have entered clinical trials for the treatment of cancer (midostaurin (PKC412), lestaurtinib (CEP-701), CEP-751, CEP-1347, edotecarin and becatecarin).3,4 Therefore there is a growing interest in the development of new synthetic routes to functionalised carbozoles.5–9 Herein we describe our progress in the development of a diastereoselective one-pot, three-component approach for the synthesis of functionalised, partially saturated carbazoles and pyridazino[3,4-b]indoles.Results and discussion
The vinyl-indole synthesis of carbazoles, originally developed by Nolan, Pindur, Porter and others, involves the D–A cycloaddition of a 2- or 3-vinyl-1H-indole (typically either unprotected or containing an electron donating N-protecting group) with a dienophile.10–22 The resulting D–A cycloadducts are often unstable and are therefore typically oxidised or undergo an in situ 1,3-H shift to rearomatise the indole. We postulated that if the intermediate D–A cycloadduct could instead be intercepted via an alternative intermolecular reaction this would provide a new multi-component route to functionalised carbazoles. Following on from our recent work on the D–A reactions of vinyl-imidazoles,23,24 we decided to investigate if the D–A cycloadducts of 3-vinyl-1H-indoles could be reacted in situ with enophiles to give a new stereoselective three-component, intermolecular D–A/intermolecular ene approach to the carbazole or pyridazino[3,4-b]indole scaffold (Fig. 1).25–29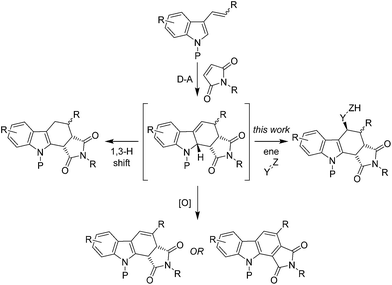 | ||
| Fig. 1 Typical products of the D–A reaction between 3-vinyl-1H-indoles and maleimides verses our proposed trapping of the D–A cycloadduct via an intermolecular ene reaction. | ||
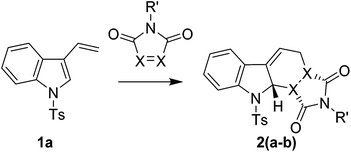
We decided to focus our investigation on the D–A reactions of 3-vinyl-1H-indoles containing an electron withdrawing N-protecting group as we postulated that this would stabilise the desired D–A cycloadducts sufficiently to allow either isolation or further in situ chemistry.19,20 Despite the extensive body of work that has been published on the vinyl-indole synthesis of carbazoles,11–18 the incorporation of electron withdrawing N-protecting groups has been less well studied with the phenylsulfonyl group being the most common.25,30–33 We therefore decide to focus our initial investigation on tosyl protected systems and embarked on the synthesis of two N-tosyl protected 3-alkenyl-indoles through reaction of 1H-indole-3-carbaldehyde with tosyl chloride, followed by a Wittig reaction with methylenetriphenyl-λ5-phosphane or ethyl 4-(triphenyl-λ5-phosphanylidene)butanoate to give 1a and 1b respectively (Scheme 1).
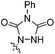
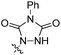
When we reacted 1-tosyl-3-vinyl-1H-indole 1a with 1-methyl-1H-pyrrole-2,5-dione in DCM at reflux for 48 hours, we were pleased to isolate, in a 74% yield, the N-tosyl protected endo-cycloadduct 2a, which showed little propensity towards spontaneous rearomatisation or oxidation. Whilst 4-phenyl-1,2,4-triazole-3,5-dione (PTAD) reacted rapidly with 1a at −78 °C in DCM to give the stable D–A cycloadduct 2b in 88% yield (Table 1).
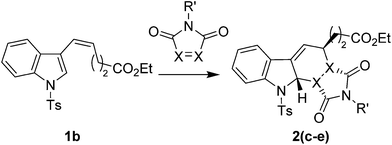
Attempts to react the more sterically demanding ethyl (Z)-5-(1-tosyl-1H-indol-3-yl)pent-4-enoate 1b with 1H-pyrrole-2,5-diones under thermal conditions proved unsuccessful with no D–A reaction being observed after prolonged heating in toluene, DCM or iso-propanol. The addition of 20 mol% of 1,3-bis(3,5-bis(trifluoromethyl)phenyl)thiourea34 also showed no improvement in the D–A reaction, whilst the addition of one equivalent of TiCl4 at −78 °C for 10 minutes resulted in an efficient D–A reaction but was accompanied by the unwanted rearomatisation of the indole. Addition of one equivalent of AlCl3 or Me2AlCl in DCM at r.t., followed by heating to reflux gave low yields of the desired product 2c along with recovered starting material. Further optimisation resulted in a final protocol whereby 2 equivalents of Me2AlCl were added to a DCM solution of 1b and the requisite 1H-pyrrole-2,5-dione at −78 °C, followed by warming to reflux in DCM for 48 hours to give N-tosyl protected D–A cycloadducts 2(c–e) in good yields (Table 2). The structures of 2(a), 2(b) and 2(d) were confirmed by single crystal X-ray analysis and are consistent with an endo-selective D–A reaction (see ESI†).
| Starting material | Dienophile | Reaction conditions | R′ | X | Yielda | Product | |
|---|---|---|---|---|---|---|---|
| a Isolated yields.b Structure confirmed by single crystal X-ray analysis. | |||||||
| 1 | 1b |  |
2 eq. Me2AlCl, −78 °C 30 min, then 40 °C 48 h | Me | CH | 85% | 2c |
| 2 | 1b |  |
2 eq. Me2AlCl, −78 °C 30 min, then 40 °C 48 h | H | CH | 64% | 2db |
| 3 | 1b | 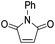 |
2 eq. Me2AlCl, −78 °C 30 min, then 40 °C 48 h | Ph | CH | 71% | 2e |
Next we examined the reactivity of N-tosyl protected endo-cycloadducts 2(a–e) towards enophiles. Reaction of 2a with nitrosobenzene proceeded well at r.t. in 18 hours to give the ene adduct 3a in 68% isolated yield. We therefore reacted D–A adducts 2(a–e) with nitrosobenzene and 1-methyl-2-nitrosobenzene at r.t. in DCM, giving high yields of the corresponding ene adducts 3(a, b and e–h). The ene reactions of 2(a–c) also proceeded smoothly with PTAD at 0 °C to give 3(c, i and j). The reaction of 2a with 2,3,4,5,6-pentafluorobenzaldehyde under thermal conditions was unsuccessful. However addition of one equivalent of Me2AlCl at −78 °C to a mixture of 2a and 2,3,4,5,6-pentafluorobenzaldehyde resulted in formation of the ene cycloadduct 3d as a 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of diastereomers, epimeric at the exo-cyclic hydroxyl position35 (Table 3).
1 mixture of diastereomers, epimeric at the exo-cyclic hydroxyl position35 (Table 3).
| Starting material | R | R′ | X | Enophile | Reaction conditions | R′′ | Yielda | Product | |
|---|---|---|---|---|---|---|---|---|---|
a Isolated yields.b Isolated as a 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of diastereomers. 1 mixture of diastereomers. |
|||||||||
| 1 | 2a | H | Me | CH |  |
r.t., 18 h | N(Ph)OH | 68% | 3a |
| 2 | 2a | H | Me | CH | 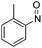 |
r.t., 18 h | N(o-Tol)OH | 72% | 3b |
| 3 | 2a | H | Me | CH |  |
0 °C, 2.5 h |  |
73% | 3c |
| 4 | 2a | H | Me | CH |  |
1 eq. Me2AlCl, −78 °C, 15 min r.t., 18 h | CH(C6F5)OH | 82% | 3db |
| 5 | 2b | H | Ph | N |  |
r.t., 18 h | N(Ph)OH | 74% | 3e |
| 6 | 2b | H | Ph | N |  |
r.t., 18 h | N(o-Tol)OH | 72% | 3f |
| 7 | 2c | (CH2)2CO2Et | Me | CH |  |
r.t., 24 h | N(o-Tol)OH | 59% | 3g |
| 8 | 2e | (CH2)2CO2Et | Ph | CH | 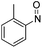 |
r.t., 24 h | N(o-Tol)OH | 58% | 3h |
| 9 | 2c | (CH2)2CO2Et | Me | CH | 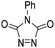 |
0 °C, 6 h | 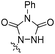 |
56% | 3i |
| 10 | 2d | (CH2)2CO2Et | H | CH |  |
0 °C, 6 h |  |
56% | 3j |
Since both the D–A and ene reactions are performed in DCM we then decided to examine the potential for reaction telescoping by attempting a D–A/ene reaction sequence under “domino” conditions.36 1-Tosyl-3-vinyl-1H-indole 1a, 1-methyl-1H-pyrrole-2,5-dione and nitrosobenzene were stirred together for 5 days at r.t. in DCM, until 1a had been consumed by TLC. Examination of the crude reaction mixture showed the formation of a number of by-products (including a rearomatised isomer of D–A cycloadduct 2a) but the desired domino D–A/ene product 3a could be isolated as a single diastereomer in a 40% yield.
To improve both the yield and reaction flexibility whilst maintaining operational simplicity we next examined a one-pot, sequential addition approach. 1-Tosyl-3-vinyl-1H-indole 1a and 5-methoxy-1-tosyl-3-vinyl-1H-indole 1c (synthesised as previously via tosyl protection of 5-methoxy-1H-indole-3-carbaldehyde followed by a Wittig reaction with methylenetriphenyl-λ5-phosphane) were reacted with 1-methyl-1H-pyrrole-2,5-dione or 1H-pyrrole-2,5-dione in refluxing DCM for 48 hours to give the corresponding D–A cycloadducts. Nitrosobenzene, 1-methyl-2-nitrosobenzene, 2,3,4,5,6-pentafluorobenzaldehyde with one equivalent of Me2AlCl, or PTAD, were then added directly to the reaction vessels containing the D–A cycloadducts and the ene reactions conducted were under the previously optimised conditions, depending on the enophile. This one-pot three-component approach gave the corresponding D–A/ene products 3(a–d and k–q) in excellent (70–89%) yields with no purification or work-up of the intermediate D–A cycloadducts required (Table 4).
We therefore continued with this approach, reacting 1a and 1c with PTAD as the dienophile followed by in situ addition of enophiles (nitrosobenzene, 1-methyl-2-nitrosobenzene, or PTAD) again giving the D–A/ene products 3(e, f, r and s) cleanly and in good yields (Table 4).
| Starting Material | P | R′′′ | R′ | X | Enophile | R′′ | Reaction conditions | Product | Yielda | |
|---|---|---|---|---|---|---|---|---|---|---|
a Isolated yields.b 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 endo 1 endo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exo.c Structure confirmed by single crystal X-ray analysis.d 25 exo.c Structure confirmed by single crystal X-ray analysis.d 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 endo 1 endo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exo.e 10 exo.e 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 endo 1 endo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exo.f Only one diastereomer observed. exo.f Only one diastereomer observed. |
||||||||||
| 1 | 1a | Tos | H | Me | CH |  |
–N(Ph)OH | (i) 40 °C, 48 h, (ii) r.t., 18 h | 3a | 71% |
| 2 | 1a | Tos | H | Me | CH | 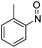 |
–N(o-Tol)OH | (i) 40 °C, 48 h, (ii) r.t., 18 h | 3b | 71% |
| 3 | 1a | Tos | H | Me | CH |  |
 |
(i) 40 °C, 48 h, (ii) 0 °C, 4 h | 3c | 76% |
| 4 | 1a | Tos | H | Me | CH |  |
–CH(C6F5)OH | (i) 40 °C, 48 h, (ii) Me2AlCl, −78 °C to r.t., 18 h | 3d | 72%b |
| 5 | 1a | Tos | H | Ph | N |  |
–N(Ph)OH | (i) −78 °C, 4 h, (ii) r.t., 24 h | 3e | 66% |
| 6 | 1a | Tos | H | Ph | N |  |
–N(o-Tol)OH | (i) −78 °C, 4 h, (ii) r.t., 24 h | 3f | 66% |
| 7 | 1a | Tos | H | H | CH | 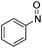 |
–N(Ph)OH | (i) 40 °C, 48 h, (ii) r.t., 4 h | 3k | 89% |
| 8 | 1a | Tos | H | H | CH | 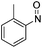 |
–N(o-Tol)OH | (i) 40 °C, 48 h, (ii) r.t., 4 h | 3l | 82%c |
| 9 | 1a | Tos | H | H | CH |  |
–CH(C6F5)OH | (i) 40 °C, 48 h, (ii) Me2AlCl, 0 °C to r.t., 18 h | 3m | 71%d |
| 10 | 1a | Tos | H | H | CH |  |
 |
(i) 40 °C, 48 h, (ii) 0 °C, 4 h | 3n | 75% |
| 11 | 1c | Tos | OMe | H | CH |  |
–N(o-Tol)OH | (i) 40 °C, 48 h, (ii) r.t., 24 h | 3o | 76% |
| 12 | 1c | Tos | OMe | Me | CH |  |
–CH(C6F5)OH | (i) 40 °C, 48 h, (ii) Me2AlCl, −78 °C, 15 min then r.t., 18 h | 3p | 70%e |
| 13 | 1c | Tos | OMe | H | CH |  |
 |
(i) 40 °C, 48 h, (ii) 0 °C, 4 h | 3q | 72% |
| 14 | 1a | Tos | H | Ph | N |  |
 |
(i) −78 °C, 4 h, (ii) 0 °C, 4 h | 3r | 65%c |
| 15 | 1c | Tos | OMe | Ph | N |  |
–N(o-Tol)OH | (i) −78 °C then r.t. 23 h, (ii) r.t., 23 h | 3s | 72% |
| 16 | 1d | DMAS | H | Me | CH |  |
–N(o-Tol)OH | (i) 40 °C, 48 h, (ii) r.t., 3 h | 3t | 74% |
| 17 | 1d | DMAS | H | Ph | CH | 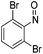 |
–N(2,4-(Br)2C6H4)OH | (i) 40 °C, 48 h, (ii) r.t., 18 h | 3u | 69%c |
| 18 | 1d | DMAS | H | Me | CH |  |
–CH(C6F5)OH | (i) 40 °C, 48 h, (ii) Me2AlCl, −78 °C, 1 h | 3v | 77%c,f |
| 19 | 1d | DMAS | H | Ph | N | 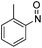 |
–N(o-Tol)OH | (i) −78 °C, 1 h, (ii) r.t., 4 h | 3w | 76% |
| 20 | 1d | DMAS | H | H | CH |  |
–N(o-Tol)OH | (i) 40 °C, 48 h, (ii) r.t., 3 h | 3x | 77% |
| 21 | 1e | Cbz | H | Me | CH |  |
–N(Ph)OH | (i) 40 °C, 24 h, (ii) r.t., 18 h | 3y | 74% |
| 22 | 1e | Cbz | H | Me | CH |  |
–N(o-Tol)OH | (i) 40 °C, 24 h, (ii) r.t., 18 h | 3z | 78% |
| 23 | 1e | Cbz | H | Me | CH |  |
 |
(i) 40 °C, 24 h, (ii) 0 °C, 1 h then r.t., 18 h | 3aa | 54% |
| 24 | 1e | Cbz | H | H | CH |  |
–N(Ph)OH | (i) 40 °C, 24 h, (ii) r.t., 18 h | 3bb | 70% |
| 25 | 1e | Cbz | H | H | CH |  |
–N(o-Tol)OH | (i) 40 °C, 24 h, (ii) r.t., 4 h | 3cc | 83% |
| 26 | 1e | Cbz | H | H | CH |  |
 |
(i) 40 °C, 24 h, (ii) 0 °C, 1 h | 3dd | 58% |
| 27 | 1e | Cbz | H | Ph | N |  |
–N(Ph)OH | (i) −78 °C, 5 h, (ii) r.t., 3 h | 3ee | 72% |
| 28 | 1e | Cbz | H | Ph | N | 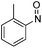 |
–N(o-Tol)OH | (i) −78 °C, 5 h, (ii) r.t., 18 h | 3ff | 68% |
| 29 | 1f | Cbz | OMe | Me | CH |  |
–N(Ph)OH | (i) 40 °C, 18 h, (ii) r.t., 1.5 h | 3gg | 73% |
| 30 | 1f | Cbz | OMe | Me | CH |  |
–N(o-Tol)OH | (i) 40 °C, 18 h, (ii) r.t., 3 h | 3hh | 74% |
| 31 | 1f | Cbz | OMe | H | CH | 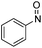 |
–N(Ph)OH | (i) 40 °C, 18 h, (ii) r.t., 2.5 h | 3ii | 79% |
| 32 | 1f | Cbz | OMe | H | CH | 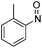 |
–N(o-Tol)OH | (i) 40 °C, 18 h, (ii) r.t., 3.5 h | 3jj | 76% |
| 33 | 1f | Cbz | OMe | Ph | N |  |
–N(Ph)OH | (i) −78 °C, 1.5 h, (ii) r.t., 20 h | 3kk | 78% |
| 34 | 1f | Cbz | OMe | Ph | N | 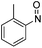 |
–N(o-Tol)OH | (i) −78 °C, 1.5 h, (ii) r.t., 24 h | 3ll | 82% |
We then decided to examine the range of electron withdrawing N-protecting groups tolerated in our one-pot D–A/ene reaction sequence with a view towards flexibility in the deprotection of the products. Boc protection of 1H-indole-3-carbaldehyde proceeded in high yield (triethylamine, Boc anhydride, DCM, 18 h, r.t.), however attempts to synthesise tert-butyl 3-vinyl-1H-indole-1-carboxylate, by reaction of tert-butyl 3-formyl-1H-indole-1-carboxylate with methylenetriphenyl-λ5-phosphane, gave only a 13% yield of the desired product with the major products arising from loss of the Boc group. We therefore focused our efforts on use of the DMAS (dimethylaminosulfonyl) and Cbz protecting groups, and synthesised N,N-dimethyl-3-vinyl-1H-indole-1-sulfonamide 1d (DMAS), benzyl-3-vinyl-1H-indole-1-carboxylate 1e and benzyl-5-methoxy-3-vinyl-1H-indole-1-carboxylate 1f (Cbz) via appropriate protection of 1H-indole-3-carbaldehyde followed by reaction with methylenetriphenyl-λ5-phosphane as previously.
N,N-Dimethyl-3-vinyl-1H-indole-1-sulfonamide 1d was reacted in DCM with 1H-pyrrole-2,5-dione, 1-methyl-1H-pyrrole-2,5-dione, 1-phenyl-1H-pyrrole-2,5-dione and PTAD. After 48 hours at 40 °C for the maleimides, or 1 hour at −78 °C for PTAD, the D–A reactions were complete and in situ ene reactions with nitrosobenzene, 1-methyl-2-nitrosobenzene, 1,3-dibromo-2-nitrosobenzene23 or 2,3,4,5,6-pentafluorobenzaldehyde (catalysed by one equivalent of Me2AlCl) were carried out to give 69–77% isolated yields of the desired three-component D–A/ene products 3(t–x) (Table 4). Cbz protected 3-vinyl-1H-indoles 1(e and f) also underwent D–A reactions with 1H-pyrrole-2,5-dione, 1-methyl-1H-pyrrole-2,5-dione or PTAD followed by in situ ene reactions with nitrosobenzene, 1-methyl-2-nitrosobenzene or PTAD to give 54–82% yields of 3(y–ll) (Table 4). The Cbz protected products 3(y–ll) were isolable by silica gel chromatography but proved less stable than their DMAS or Tos protected counterparts. NMR investigations of these compounds showed evidence of decomposition in solution at r.t., however they could be stored as solids under an atmosphere of nitrogen at −20 °C for months at a time. Interestingly the ene reactions of 2,3,4,5,6-pentafluorobenzaldehyde with D–A cycloadducts 3(d, m, p and v) gave a mixture of diastereomers at the exo-cyclic hydroxyl group, with ratios from 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to >25
1 to >25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The relative stereochemistry of 3v was confirmed through the solution of a single crystal X-ray structure (see ESI†) and is consistent with an endo-selective ene reaction, providing some support for an ene mechanism in this reaction rather than a nucleophilic attack of the D–A cycloadducts to the carbonyl carbon of the aldehyde in the manner of an vinylogous enamine.37
1. The relative stereochemistry of 3v was confirmed through the solution of a single crystal X-ray structure (see ESI†) and is consistent with an endo-selective ene reaction, providing some support for an ene mechanism in this reaction rather than a nucleophilic attack of the D–A cycloadducts to the carbonyl carbon of the aldehyde in the manner of an vinylogous enamine.37
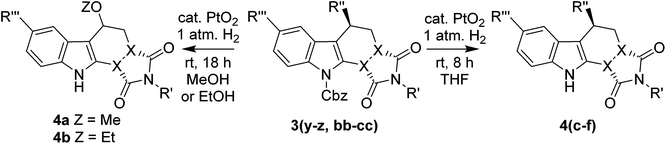
Finally we investigated the deprotection of our D–A/ene reaction products. Tosyl and DMAS protected compounds 3(a–x) proved intransient to a range of basic (NaOH, KOH or KOEt in EtOH, MeOH or H2O with Bu4NBr) and reducing (Mg, Mg/Hg or Na/Hg) deprotection conditions. We therefore focused on the deprotection of Cbz protected compounds 3(y–ll). Initial attempts at Cbz removal with H2 and Pd/C proved unsuccessful. Atmospheric pressure hydrogenation with Adam's catalyst in either MeOH and EtOH resulted in the removal of the Cbz group from 3z, however unexpected nucleophilic substitutions of the hydroxy(aryl)amino group by MeOH or EtOH also occurred to give 4a and 4b respectively. This gave the first indication that the removal of the electronically stabilising N-protecting group perhaps unsurprisingly lowers the activation energy barrier towards substitution chemistry at the indolylic position.38–40 Replacement of the alcoholic solvent with THF resulted in a cleaner deprotection of Cbz protected indoles 3(y–ll) to give 4(c–p) in 38–91% yields. However the products 4(c–p) showed some evidence of decomposition in CDCl3 after a few hours at r.t., with the appearance of new peaks in the 1H NMR spectra. Therefore NMR analysis of 4(c–p) was carried out in either d2-DCM or d6-DMSO (depending on solubility) in which decomposition was slowed, although the appearance of minor peaks in the 1H NMR could still be observed over time. The deprotection of indoles 3(y–ll) with H2 and Adam's catalyst in THF has allowed us to successfully demonstrate a one-pot, three-component approach to our target library of deprotected partially saturated carbazoles and pyridazino[3,4-b]indoles, which will be the focus of future investigations (Table 5).
| Starting material | R′ | X | R′′ | R′′ | Solvent | Product | Yielda | |
|---|---|---|---|---|---|---|---|---|
| a Isolated yields. | ||||||||
| 1 | 3z | Me | CH | –N(o-Tol)OH | H | MeOH | 4a | 60% |
| 2 | 3z | Me | CH | –N(o-Tol)OH | H | EtOH | 4b | 24% |
| 3 | 3y | Me | CH | –N(Ph)OH | H | THF | 4c | 75% |
| 4 | 3z | Me | CH | –N(o-Tol)OH | H | THF | 4d | 87% |
| 5 | 3aa | Me | CH | 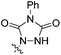 |
H | THF | 4e | 91% |
| 6 | 3bb | H | CH | –N(Ph)OH | H | THF | 4f | 41% |
| 7 | 3cc | H | CH | –N(o-Tol)OH | H | THF | 4g | 70% |
| 8 | 3dd | H | CH | 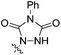 |
H | THF | 4h | 64% |
| 9 | 3ee | Ph | N | –N(Ph)OH | H | THF | 4i | 65% |
| 10 | 3ff | Ph | N | –N(o-Tol)OH | H | THF | 4j | 44% |
| 11 | 3gg | Me | CH | –N(Ph)OH | OMe | THF | 4k | 70% |
| 12 | 3hh | Me | CH | –N(o-Tol)OH | OMe | THF | 4l | 70% |
| 13 | 3ii | H | CH | –N(Ph)OH | OMe | THF | 4m | 60% |
| 14 | 3jj | H | CH | –N(o-Tol)OH | OMe | THF | 4n | 85% |
| 15 | 3kk | Ph | N | –N(Ph)OH | OMe | THF | 4o | 38% |
| 16 | 3ll | Ph | N | –N(o-Tol)OH | OMe | THF | 4p | 61% |
Conclusions
In conclusion, we have developed a practically simple three-component approach to Tos, DMAS and Cbz protected partially saturated carbazoles and pyridazino[3,4-b]indoles, based on a one-pot D–A/ene reaction, including the examination of a deprotection strategy of the Cbz group. Current work is looking into controlling the reactivity and biological activity of the final products as well as investigating enantioselective D–A/ene approaches for molecules of this type.Experimental
3-Vinyl-1H-indole
In a Schlenk flask, methyltriphenylphosphonium iodide (2.14 g, 5.3 mmol) was dissolved in dry THF (13 mL). The solution was cooled to −78 °C and nbutyllithium (2.9 mL, 4.6 mmol) was added over 10 minutes. The yellow solution was warmed to 0 °C and was left to stir for 1 hour before being cooled to −78 °C. In a separate Schlenk flask, 1H-indole-3-carboxylate (0.67 g, 4.6 mmol) was dissolved in THF (7 mL) and to the solution sodium bis(trimethylsilyl)amide (2.3 mL, 4.6 mmol) was added. This solution was transferred into the first Schlenk flask and the red solution was allowed to stir at room temperature for 1 hour. The reaction was poured into water (30 mL) and extracted with ethyl acetate (2 × 20 mL). The combined organic layers were dried over MgSO4, filtered and the solvent removed under pressure to leave the crude product as yellow oil. The product was purified using column chromatography (petrol (40/60)–diethyl ether 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, column diameter = 4 cm, silica = 20 cm) to give 3-vinyl-1H-indole (0.636 g, 4.4 mmol, 95%) as a yellow powder.
3, column diameter = 4 cm, silica = 20 cm) to give 3-vinyl-1H-indole (0.636 g, 4.4 mmol, 95%) as a yellow powder.
Mp: 78.4–80.7 °C; Rf: 0.76 (Pet(40/60)–EA, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (300 MHz, CDCl3): δH 8.02 (1H, br s), 7.85–7.80 (1H, m), 7.32–7.28 (1H, m), 7.18 (1H, s), 7.18–7.09 (2H, m), 6.83 (1H, ddd, J = 17.7, 11.2, 0.5 Hz), 5.65 (1H, dd, J = 17.7, 1.5 Hz), 5.11 (1H, dd, J = 11.2, 1.5 Hz); 13C NMR (101 MHz, CDCl3): δ 136.8, 129.5, 125.7, 123.6, 122.6, 120.4, 120.2, 115.9, 111.4, 110.9; IR (neat): νmax/cm−1 3660, 2981.
1); 1H NMR (300 MHz, CDCl3): δH 8.02 (1H, br s), 7.85–7.80 (1H, m), 7.32–7.28 (1H, m), 7.18 (1H, s), 7.18–7.09 (2H, m), 6.83 (1H, ddd, J = 17.7, 11.2, 0.5 Hz), 5.65 (1H, dd, J = 17.7, 1.5 Hz), 5.11 (1H, dd, J = 11.2, 1.5 Hz); 13C NMR (101 MHz, CDCl3): δ 136.8, 129.5, 125.7, 123.6, 122.6, 120.4, 120.2, 115.9, 111.4, 110.9; IR (neat): νmax/cm−1 3660, 2981.
1a – 1-tosyl-3-vinyl-1H-indole
Into a Schlenk flask, was placed 1H-indole-3-carbaldehyde (5.0 g, 34.5 mmol) and DCM (100 mL). The resulting stirred solution was cooled to 0 °C before triethylamine (12 mL, 86.2 mmol) was added dropwise via syringe. To the stirred solution, p-toluenesulfonyl chloride (7.23 g, 37.9 mmol) in DCM was added dropwise over a period of 20 minutes. The solution was stirred at 0 °C for a further one hour before warming to room temperature over 18 hours. The solution was washed into a separating funnel with DCM (20 mL) and washed with water (2 × 100 mL) and brine (100 mL). The organic extracts were dried over MgSO4, filtered and the solvent removed under reduced pressure to give the crude product as a pale orange oil. The product was purified by recrystallisation from hot ethyl acetate (150 mL) to give 1-tosyl-1H-indole-3-carbaldehyde (8.38 g, 28 mmol, 82%) as orange crystals.Mp: 145.3–148.8 °C; Rf: 0.83 (Pet(40/60)–EA 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4); 1H NMR (300 MHz, CDCl3): δH 10.11 (1H, s), 8.29–8.26 (1H, m), 8.19–8.16 (1H, m), 8.16 (1H, s), 7.89–7.85 (1H, m), 7.78 (2H, d, J = 8.4 Hz), 7.36–7.25 (2H, m), 7.22 (2H, d, J = 8.4 Hz), 2.29 (3H, s); 13C NMR (101 MHz, CDCl3): δ 185.3, 146.1, 136.2, 135.4, 134.1, 130.3, 130.2, 127.2, 127.1, 126.2, 124.9, 122.5, 122.2, 113.1, 21.5; IR (neat): νmax/cm−1 3140, 1663; anal. calcd for C16H13NO3S: C, 64.20; H, 4.38; N, 4.68. Found: C, 63.97; H, 4.52; N, 4.72.
4); 1H NMR (300 MHz, CDCl3): δH 10.11 (1H, s), 8.29–8.26 (1H, m), 8.19–8.16 (1H, m), 8.16 (1H, s), 7.89–7.85 (1H, m), 7.78 (2H, d, J = 8.4 Hz), 7.36–7.25 (2H, m), 7.22 (2H, d, J = 8.4 Hz), 2.29 (3H, s); 13C NMR (101 MHz, CDCl3): δ 185.3, 146.1, 136.2, 135.4, 134.1, 130.3, 130.2, 127.2, 127.1, 126.2, 124.9, 122.5, 122.2, 113.1, 21.5; IR (neat): νmax/cm−1 3140, 1663; anal. calcd for C16H13NO3S: C, 64.20; H, 4.38; N, 4.68. Found: C, 63.97; H, 4.52; N, 4.72.
A Schlenk flask was charged with methyltriphenylphosphonium iodide (1.29 g, 3.2 mmol) dissolved in dry THF (25 mL) under a nitrogen atmosphere. The solution was cooled to −78 °C and nbutyllithium (1.81 mL, 2.97 mmol) was added dropwise via syringe over 10 minutes. The solution was warmed to 0 °C and left to stir for 2 hours. In a separate Schlenk flask, 1-tosylindoline-3-carbaldehyde (0.8 g, 2.7 mmol) was dissolved in THF (5 mL). The indole solution was transferred via cannula to the Schlenk flask containing the solution of methyltriphenylphosphonium iodide and the solution was stirred for 18 hours. The reaction poured into water (50 mL) and extracted with ether (3 × 40 mL). The organic layers were washed with brine (20 mL), dried over MgSO4, filtered and the solvent was removed under reduced pressure to leave the crude product as an orange oil. The crude product was purified by column chromatography (petrol (40/60)–ethyl acetate; 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2 cm diameter column) to give 1-tosyl-3-vinyl-1H-indole (0.56 g, 3.9 mmol, 70%) as a pale yellow powder.
1, 2 cm diameter column) to give 1-tosyl-3-vinyl-1H-indole (0.56 g, 3.9 mmol, 70%) as a pale yellow powder.
Mp: 90.3–94.6 °C; Rf: 0.86 (Pet(40/60)–EA, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (300 MHz, CDCl3): δH 7.93–7.90 (1H, m), 7.69 (2H, d, J = 8.4 Hz), 7.70–7.65 (1H, m), 7.53 (1H, s), 7.29–7.19 (2H, m), 7.29–7.17 (2H, m), 7.15 (2H, d, J = 8.4 Hz), 6.70 (1H, app ddd, J = 17.9, 11.3, 0.7 Hz), 5.72 (dd, 1H, J = 17.9, 1.2 Hz), 5.28 (1H, dd, J = 11.3, 1.2 Hz), 2.26 (3H, s); 13C NMR (101 MHz, CDCl3): δC 145.1, 135.6, 135.2, 130.0, 129.1, 127.6, 126.9, 125.0, 124.2, 123.6, 121.0, 120.5, 115.4, 113.8, 21.7; IR (neat): νmax/cm−1 3119, 3072; anal. calcd for C17H15NO2S: C, 68.66; H, 5.08; N, 4.71. Found: C, 68.70; H, 5.21; N, 4.61.
1); 1H NMR (300 MHz, CDCl3): δH 7.93–7.90 (1H, m), 7.69 (2H, d, J = 8.4 Hz), 7.70–7.65 (1H, m), 7.53 (1H, s), 7.29–7.19 (2H, m), 7.29–7.17 (2H, m), 7.15 (2H, d, J = 8.4 Hz), 6.70 (1H, app ddd, J = 17.9, 11.3, 0.7 Hz), 5.72 (dd, 1H, J = 17.9, 1.2 Hz), 5.28 (1H, dd, J = 11.3, 1.2 Hz), 2.26 (3H, s); 13C NMR (101 MHz, CDCl3): δC 145.1, 135.6, 135.2, 130.0, 129.1, 127.6, 126.9, 125.0, 124.2, 123.6, 121.0, 120.5, 115.4, 113.8, 21.7; IR (neat): νmax/cm−1 3119, 3072; anal. calcd for C17H15NO2S: C, 68.66; H, 5.08; N, 4.71. Found: C, 68.70; H, 5.21; N, 4.61.
1b – ethyl (Z)-5-(1-tosyl-1H-indol-3-yl)pent-4-enoate
In a Schlenk flask, (4-ethoxy-4-oxobutyl) triphenylphosphonium bromide (3.32 g, 7.26 mmol) was dissolved in dry THF (20 mL). The solution was cooled to −78 °C and sodium bis (trimethylsilyl) amide (1.00 M in THF, 8.58 mL, 8.58 mmol) was added dropwise over 10 min. The mixture was warmed to 0 °C and left to stir. After 2 hours, 1-(toluene-4-sulfonyl)-1H-indol-3-carboxaldehyde (2.00 g, 6.60 mmol) was dissolved in dry THF (10 mL) in a separate round bottomed flask, and transferred via cannula into the reaction solution. The reaction mixture was stirred at room temperature and for 48 hours, quenched with saturated NH4Cl(aq.) (30 mL), extracted with EtOAc (2 × 200 mL), and the combined organic layers washed with brine, dried over MgSO4 and filtered. The solvent was removed under reduced pressure to give a crude product as orange oil. The product was purified by column chromatography (petrol (40/60)–ethyl acetate 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give two fractions the first containing ethyl (Z)-5-(1-tosyl-1H-indol-3-yl)pent-4-enoate (1.568 g, 3.94 mmol, 60%) as a colourless oil, and a second fraction containing a 20
1) to give two fractions the first containing ethyl (Z)-5-(1-tosyl-1H-indol-3-yl)pent-4-enoate (1.568 g, 3.94 mmol, 60%) as a colourless oil, and a second fraction containing a 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of (Z) and (E) ethyl-5-(1-tosyl-1H-indol-3-yl)pent-4-enoate (0.294 g, 0.739 mmol, 11%) also as a colourless oil.
1 mixture of (Z) and (E) ethyl-5-(1-tosyl-1H-indol-3-yl)pent-4-enoate (0.294 g, 0.739 mmol, 11%) also as a colourless oil.
Rf: 0.70 (Pet(40/60)–EA 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3); 1H NMR (400 MHz, CDCl3): δH 7.97 (1H, d, J = 7.9 Hz, 1H), 7.77 (2H, d, J = 7.8 Hz), 7.57 (1H, s), 7.49 (1H, d, J = 7.9 Hz), 7.32 (1H, t, J = 7.9 Hz), 7.25 (1H, t, J = 7.9 Hz), 7.20 (2H, d, J = 7.8 Hz), 6.44 (1H, d, J = 11.2 Hz), 5.78 (1H, dt, J = 11.2, 7.5 Hz), 4.14 (2H, q, J = 6.5 Hz), 2.67–2.62 (2H, m), 2.48 (2H, t, J = 6.9 Hz), 2.30 (3H, s), 1.24 (3H, t, J = 6.5 Hz); 13C NMR (100 MHz, CDCl3): δC 172.8, 145.1, 135.1, 134.6, 132.1, 130.8, 129.9, 126.8, 124.9, 123.6, 123.4, 119.5, 118.96, 113.6, 60.5, 34.1, 25.2, 21.6, 14.3; IR (neat): νmax/cm−1: 1727, 1597. MS (pNSI): 415.2 (100%, [M + NH4]+), 398.1 (20%, [M + H]+), 420.1 (10%, [M + Na]+); HRMS (pNSI): calcd C22H24NO4S [M + H]+: 398.14120; observed: 398.14120.
3); 1H NMR (400 MHz, CDCl3): δH 7.97 (1H, d, J = 7.9 Hz, 1H), 7.77 (2H, d, J = 7.8 Hz), 7.57 (1H, s), 7.49 (1H, d, J = 7.9 Hz), 7.32 (1H, t, J = 7.9 Hz), 7.25 (1H, t, J = 7.9 Hz), 7.20 (2H, d, J = 7.8 Hz), 6.44 (1H, d, J = 11.2 Hz), 5.78 (1H, dt, J = 11.2, 7.5 Hz), 4.14 (2H, q, J = 6.5 Hz), 2.67–2.62 (2H, m), 2.48 (2H, t, J = 6.9 Hz), 2.30 (3H, s), 1.24 (3H, t, J = 6.5 Hz); 13C NMR (100 MHz, CDCl3): δC 172.8, 145.1, 135.1, 134.6, 132.1, 130.8, 129.9, 126.8, 124.9, 123.6, 123.4, 119.5, 118.96, 113.6, 60.5, 34.1, 25.2, 21.6, 14.3; IR (neat): νmax/cm−1: 1727, 1597. MS (pNSI): 415.2 (100%, [M + NH4]+), 398.1 (20%, [M + H]+), 420.1 (10%, [M + Na]+); HRMS (pNSI): calcd C22H24NO4S [M + H]+: 398.14120; observed: 398.14120.
1c – 5-methoxy-1-tosyl-3-vinyl-1H-indole
To a stirred round bottomed flask was added 5-methoxy-1H-indole-3-carbaldehyde (0.70 g, 4.00 mmol) and DCM (20 mL) and the solution was cooled to 0 °C. To the stirred solution was added triethylamine (1.40 mL, 10.0 mmol) and the resulting solution was stirred at 0 °C for 1 hour. To the stirred solution was added p-toluenesulfonyl chloride (0.84 g, 4.40 mmol) in DCM (10 mL) and the solution was stirred at room temperature for 18 hours. The reaction was poured into water (50 mL) and extracted with DCM (3 × 20 mL). The combined organic extracts were dried over MgSO4, filtered and the solvent was removed under reduced pressure to leave the crude product as a pale orange solid. The product was purified using column chromatography (petrol (40/60)–ether–DCM 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, column diameter = 2 cm, silica = 15 cm) to give 5-methoxy-1-tosyl-1H-indole-3-carbaldehyde (1.14 g, 3.48 mmol, 87%) as a pale brown powder.
1, column diameter = 2 cm, silica = 15 cm) to give 5-methoxy-1-tosyl-1H-indole-3-carbaldehyde (1.14 g, 3.48 mmol, 87%) as a pale brown powder.
Mp: 126.1–128.4 °C; Rf: 0.71 (Pet(40/60)–Et2O–DCM 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (300 MHz, CDCl3): δH 10.07 (1H, s), 8.19 (1H, s), 7.86–7.83 (1H, m), 7.84 (2H, d, J = 8.5 Hz), 7.72 (1H, d, J = 2.6 Hz), 7.29 (2H, d, J = 8.5 Hz), 7.02 (1H, dd, J = 9.1, 2.6 Hz), 3.86 (3H, s), 2.38 (3H, s); 13C NMR (101 MHz, CDCl3): δC 185.6, 157.8, 146.2, 136.8, 134.4, 130.4, 129.8, 127.4, 127.2, 122.3, 116.2, 114.2, 104.1, 55.8, 21.8; IR (neat): νmax/cm−1 3128, 2832, 1671; anal. calcd for C17H15NO4S: C, 61.99; H, 4.59; N, 4.25. Found: C, 61.77; H, 4.70; N, 4.29.
1); 1H NMR (300 MHz, CDCl3): δH 10.07 (1H, s), 8.19 (1H, s), 7.86–7.83 (1H, m), 7.84 (2H, d, J = 8.5 Hz), 7.72 (1H, d, J = 2.6 Hz), 7.29 (2H, d, J = 8.5 Hz), 7.02 (1H, dd, J = 9.1, 2.6 Hz), 3.86 (3H, s), 2.38 (3H, s); 13C NMR (101 MHz, CDCl3): δC 185.6, 157.8, 146.2, 136.8, 134.4, 130.4, 129.8, 127.4, 127.2, 122.3, 116.2, 114.2, 104.1, 55.8, 21.8; IR (neat): νmax/cm−1 3128, 2832, 1671; anal. calcd for C17H15NO4S: C, 61.99; H, 4.59; N, 4.25. Found: C, 61.77; H, 4.70; N, 4.29.
In a Schlenk flask, methyltriphenylphosphonium iodide (1.35 g, 3.34 mmol) was dissolved in dry THF (30 mL). The solution was cooled to −78 °C and nBuLi (1.2 mL, 3.03 mmol) was added over 5 minutes. The yellow solution was warmed to 0 °C and was allowed to stir for 1 hour before being cooled to −78 °C. To the stirred solution, 5-methoxy-1-tosyl-1H-indole-3-carbaldehyde (1.00 g, 3.03 mmol) in DCM (10 mL) was added and the solution was stirred at room temperature for 3 hours. The reaction was poured into water (40 mL) and extracted with ethyl acetate (3 × 20 mL). The combined organic layers were dried over MgSO4, filtered and the solvent removed under pressure to leave the crude product an orange oil. The product was purified using column chromatography (petrol (40/60)–ethyl acetate 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, column diameter = 2 cm, silica = 16 cm) to give 5-methoxy-1-tosyl-3-vinyl-1H-indole (0.79 g, 2.42 mmol, 80%) as a brown powder.
1, column diameter = 2 cm, silica = 16 cm) to give 5-methoxy-1-tosyl-3-vinyl-1H-indole (0.79 g, 2.42 mmol, 80%) as a brown powder.
Mp: 101.4–103.9 °C; Rf: 0.66 (Pet(40/60)–EA 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (300 MHz, CDCl3): δH 7.87 (1H, d, J = 9.0 Hz), 7.73 (2H, d, J = 8.4 Hz), 7.55 (1H, s), 7.19 (2H, d, J = 8.3 Hz), 7.14 (1H, d, J = 2.5 Hz), 6.93 (1H, dd, J = 9.0, 2.5 Hz), 6.72 (1H, dd, J = 17.9, 11.3 Hz), 5.73 (1H, dd, J = 17.9, 1.1 Hz), 5.32 (1H, dd, J = 11.3, 1.1 Hz), 3.82 (3H, s), 2.31 (3H, s); 13C NMR (101 MHz, CDCl3): δC 156.7, 145.0, 135.1, 130.3, 130.1, 130.0, 127.6, 126.9, 124.9, 121.1, 115.2, 114.7, 113.7, 103.2, 55.8, 21.7; IR (neat): νmax/cm−1 3128, 2832, 1671; MS (pNSI): 328.1 (100%, (M + H)+), 350.1 (15%, (M + Na)+), 672.2 (2M + NH4)+; HRMS (pNSI): calcd for C18H18NO3S [M + H]+: 328.1002; observed: 328.1007.
1); 1H NMR (300 MHz, CDCl3): δH 7.87 (1H, d, J = 9.0 Hz), 7.73 (2H, d, J = 8.4 Hz), 7.55 (1H, s), 7.19 (2H, d, J = 8.3 Hz), 7.14 (1H, d, J = 2.5 Hz), 6.93 (1H, dd, J = 9.0, 2.5 Hz), 6.72 (1H, dd, J = 17.9, 11.3 Hz), 5.73 (1H, dd, J = 17.9, 1.1 Hz), 5.32 (1H, dd, J = 11.3, 1.1 Hz), 3.82 (3H, s), 2.31 (3H, s); 13C NMR (101 MHz, CDCl3): δC 156.7, 145.0, 135.1, 130.3, 130.1, 130.0, 127.6, 126.9, 124.9, 121.1, 115.2, 114.7, 113.7, 103.2, 55.8, 21.7; IR (neat): νmax/cm−1 3128, 2832, 1671; MS (pNSI): 328.1 (100%, (M + H)+), 350.1 (15%, (M + Na)+), 672.2 (2M + NH4)+; HRMS (pNSI): calcd for C18H18NO3S [M + H]+: 328.1002; observed: 328.1007.
1d – N,N-dimethyl-3-vinyl-1H-indole-1-sulfonamide
To a stirred round bottomed flask was added 1H-indole-3-carbaldehyde (3.0 g, 20.7 mmol) and THF (70 mL) and the solution was cooled to 0 °C. To the stirred solution was added sodium hydride (1.7 g, 41.4 mmol) in THF (30 mL) and the resulting solution was stirred at 0 °C for 1 hour. To the stirred solution was added dimethylsulfamoyl chloride (2.4 mL, 20.7 mmol) and the solution was stirred at room temperature for 18 hours. The reaction was poured into water (100 mL) and extracted with DCM (3 × 60 mL). The combined organic extracts were dried over MgSO4, filtered and the solvent was removed under reduced pressure to leave the crude product as a pale red pink solid. The product was purified by recrystallization from ethyl acetate to give 3-formyl-N,N-dimethyl-1H-indole-1-sulfonamide (97%, 5.07 g, 20.1 mmol) as a pink powder.Mp: 149.0–150.9 °C; Rf: 0.63 (Pet(40/60)–EA, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (300 MHz, CDCl3): δH 10.08 (1H, s), 8.31 (1H, app dd, J = 7.2, 1.4 Hz), 8.09 (1H, s), 7.94–7.88 (1H, m), 7.40 (2H, app ddd, J = 5.9, 3.3, 1.6 Hz), 2.91 (6H, s); 13C NMR (101 MHz, CDCl3): δC 185.5, 137.3, 136.0, 126.1, 125.9, 124.9, 122.6, 120.8, 113.6, 38.6; IR (neat): νmax/cm−1 3124, 2945, 1662; anal. calcd for C11H12N2O3S: C, 52.37; H, 4.79; N, 11.10. Found: C, 52.23; H, 4.91; N, 10.92.
1); 1H NMR (300 MHz, CDCl3): δH 10.08 (1H, s), 8.31 (1H, app dd, J = 7.2, 1.4 Hz), 8.09 (1H, s), 7.94–7.88 (1H, m), 7.40 (2H, app ddd, J = 5.9, 3.3, 1.6 Hz), 2.91 (6H, s); 13C NMR (101 MHz, CDCl3): δC 185.5, 137.3, 136.0, 126.1, 125.9, 124.9, 122.6, 120.8, 113.6, 38.6; IR (neat): νmax/cm−1 3124, 2945, 1662; anal. calcd for C11H12N2O3S: C, 52.37; H, 4.79; N, 11.10. Found: C, 52.23; H, 4.91; N, 10.92.
In a Schlenk flask, methyltriphenylphosphonium iodide (7.00 g, 17.4 mmol) was dissolved in dry THF (75 mL). The solution was cooled to −78 °C and nBuLi (6.4 mL, 15.9 mmol) was added over 10 minutes. The yellow solution was warmed to 0 °C and was left to stir for 1 hour before being cooled to −78 °C. To the stirred solution, 3-formyl-N,N-dimethyl-1H-indole-1-sulfonamide (4.00 g, 15.9 mmol) was added and the solution was stirred at room temperature for 3 hours. The reaction was poured into water (70 mL) and extracted with ethyl acetate (3 × 50 mL). The combined organic layers were dried over MgSO4, filtered and the solvent removed under pressure to leave the crude product as yellow oil. The product was purified using column chromatography (petrol (40/60)–diethyl ether 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, column diameter = 3 cm, silica = 14 cm) to give N,N-dimethyl-3-vinyl-1H-indole-1-sulfonamide (3.11 g, 12.4 mmol, 78%) as a pale orange powder.
1, column diameter = 3 cm, silica = 14 cm) to give N,N-dimethyl-3-vinyl-1H-indole-1-sulfonamide (3.11 g, 12.4 mmol, 78%) as a pale orange powder.
Mp: 68.7–67.8 °C; Rf: 0.63 (Pet(40/60)–EA 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (300 MHz, CDCl3): δH 7.98 (1H, dd, J = 8.0, 1.4 Hz), 7.85 (1H, dd, J = 7.7, 1.5 Hz), 7.35 (2H, app ddd, J = 7.0, 5.3, 1.6 Hz), 6.83 (1H, dd, J = 17.8, 11.2 Hz), 5.84 (1H, dd, J = 17.8, 1.2 Hz), 5.38 (1H, dd, J = 11.3, 1.2 Hz), 2.86 (6H, s); 13C NMR (101 MHz, CDCl3): δC 136.1, 128.2, 127.8, 125.2, 124.7, 123.1, 120.4, 118.8, 114.9, 114.0, 38.6; IR (neat): νmax/cm−1 3123, 2945; anal. calcd for C12H14N2O2S: C, 57.58; H, 5.64; N, 11.19. Found: C, 57.68; H, 5.75; N, 11.05.
1); 1H NMR (300 MHz, CDCl3): δH 7.98 (1H, dd, J = 8.0, 1.4 Hz), 7.85 (1H, dd, J = 7.7, 1.5 Hz), 7.35 (2H, app ddd, J = 7.0, 5.3, 1.6 Hz), 6.83 (1H, dd, J = 17.8, 11.2 Hz), 5.84 (1H, dd, J = 17.8, 1.2 Hz), 5.38 (1H, dd, J = 11.3, 1.2 Hz), 2.86 (6H, s); 13C NMR (101 MHz, CDCl3): δC 136.1, 128.2, 127.8, 125.2, 124.7, 123.1, 120.4, 118.8, 114.9, 114.0, 38.6; IR (neat): νmax/cm−1 3123, 2945; anal. calcd for C12H14N2O2S: C, 57.58; H, 5.64; N, 11.19. Found: C, 57.68; H, 5.75; N, 11.05.
1e – benzyl-3-vinyl-1H-indole-1-carboxylate
To a solution of 1H-indole-3-carbaldehyde (1.0 g, 6.9 mmol) in DCM (20 mL) at 0 °C was added triethylamine (1.8 mL, 17.3 mmol) dropwise. The solution was stirred at room temperature for 1 hour before benzyl chloroformate (1.4 mL, 8.3 mmol) was added. The solution was stirred for 18 hours after which it was poured into water and extracted with DCM (3 × 20 mL). The organic fractions were combined, dried with MgSO4, filtered and the solvent was removed under reduced pressure to give the crude product as an orange powder. The crude product was purified by column chromatography (column diameter = 2.5 cm, eluent = petrol (40/60)–ethyl acetate 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give benzyl-3-formyl-1H-indole-1-carboxylate (1.74 g, 6.35 mmol, 92%) as a pale orange powder.
1) to give benzyl-3-formyl-1H-indole-1-carboxylate (1.74 g, 6.35 mmol, 92%) as a pale orange powder.
Mp: 91–92 °C; Rf: 0.68 (Pet–EA, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (300 MHz, CDCl3): δH 10.06 (1H, s), 8.30–8.25 (1H, m), 8.23 (1H, s), 8.17 (1H, d, J = 8.0 Hz), 7.50 (2H, app dd, J = 7.7, 1.8 Hz), 7.42 (3H, app ddd, J = 6.6, 5.1, 1.5 Hz), 7.38 (1H, app t, J = 1.6 Hz), 7.37–7.34 (1H, m), 5.49 (2H, s); 13C NMR (101 MHz, CDCl3): δC 185.8, 150.2, 136.1, 136.1, 134.3, 129.3, 129.0, 128.9, 126.5, 126.1, 125.0, 122.3, 122.3, 115.2, 69.9; IR (neat): νmax/cm−1 3127, 3008, 1733; MS (pNSI): 280.1 (100%, (M + H)+), 302.1 (96%, (M + Na)+), 581.2 (25%, (2M + Na)+); HRMS (pNSI): calcd for C17H14NO3 [M + H]+: 280.0968; observed: 280.0970.
1); 1H NMR (300 MHz, CDCl3): δH 10.06 (1H, s), 8.30–8.25 (1H, m), 8.23 (1H, s), 8.17 (1H, d, J = 8.0 Hz), 7.50 (2H, app dd, J = 7.7, 1.8 Hz), 7.42 (3H, app ddd, J = 6.6, 5.1, 1.5 Hz), 7.38 (1H, app t, J = 1.6 Hz), 7.37–7.34 (1H, m), 5.49 (2H, s); 13C NMR (101 MHz, CDCl3): δC 185.8, 150.2, 136.1, 136.1, 134.3, 129.3, 129.0, 128.9, 126.5, 126.1, 125.0, 122.3, 122.3, 115.2, 69.9; IR (neat): νmax/cm−1 3127, 3008, 1733; MS (pNSI): 280.1 (100%, (M + H)+), 302.1 (96%, (M + Na)+), 581.2 (25%, (2M + Na)+); HRMS (pNSI): calcd for C17H14NO3 [M + H]+: 280.0968; observed: 280.0970.
To a stirred Schlenk flask, methylenetriphenylphosphorane (2.38 g, 5.90 mmol) was dissolved in dry THF (30 mL). The solution was cooled to −78 °C and nBuLi (2.15 mL, 5.35 mmol) was added over 10 minutes. The yellow solution was warmed to 0 °C and was left to stir for 1 hour before being cooled to −78 °C. To the stirred solution, benzyl 3-formyl-1H-indole-1-carboxylate (1.50 g, 5.35 mmol) was added and the solution was stirred at room temperature for 3 hours. The reaction was poured into water (50 mL) and extracted with ethyl acetate (3 × 40 mL). The combined organic layers were dried with MgSO4, filtered and the solvent removed under pressure to leave the crude product as yellow oil. The product was purified using column chromatography (petrol (40/60)–ethyl acetate 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, column diameter = 2 cm, silica = 15 cm) to give benzyl-3-vinyl-1H-indole-1-carboxylate (1.14 g, 4.07 mmol, 76%) as a yellow powder.
1, column diameter = 2 cm, silica = 15 cm) to give benzyl-3-vinyl-1H-indole-1-carboxylate (1.14 g, 4.07 mmol, 76%) as a yellow powder.
Mp: 43–45 °C; Rf: 0.73 (Pet(40/60)–EA, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (300 MHz, CDCl3): δH 8.33 (1H, d, J = 7.1 Hz), 7.91–7.86 (1H, m), 7.74 (1H, s), 7.58–7.55 (2H, m), 7.52–7.44 (4H, m), 7.43–7.36 (1H, m), 6.87 (1H, dd, J = 17.8, 11.3 Hz), 5.96–5.87 (1H, d, J = 17.8 Hz), 5.51 (2H, s), 5.44 (1H, d, J = 11.3 Hz); 13C NMR (101 MHz, CDCl3): δC 150.8, 135.1, 128.9, 128.9, 128.8, 128.6, 128.4, 128.0, 125.1, 123.6, 123.4, 120.2, 120.2, 115.5, 115.0, 68.9; IR (neat): νmax/cm−1 3153, 2962, 1729; MS (pAPCI): 181.1 (50%), 260.1 (100%), 278.1 (25%, (M + H)+); HRMS (pAPCI): calcd for C18H16NO2 [M + H]+: 278.1176; observed: 278.1173.
1); 1H NMR (300 MHz, CDCl3): δH 8.33 (1H, d, J = 7.1 Hz), 7.91–7.86 (1H, m), 7.74 (1H, s), 7.58–7.55 (2H, m), 7.52–7.44 (4H, m), 7.43–7.36 (1H, m), 6.87 (1H, dd, J = 17.8, 11.3 Hz), 5.96–5.87 (1H, d, J = 17.8 Hz), 5.51 (2H, s), 5.44 (1H, d, J = 11.3 Hz); 13C NMR (101 MHz, CDCl3): δC 150.8, 135.1, 128.9, 128.9, 128.8, 128.6, 128.4, 128.0, 125.1, 123.6, 123.4, 120.2, 120.2, 115.5, 115.0, 68.9; IR (neat): νmax/cm−1 3153, 2962, 1729; MS (pAPCI): 181.1 (50%), 260.1 (100%), 278.1 (25%, (M + H)+); HRMS (pAPCI): calcd for C18H16NO2 [M + H]+: 278.1176; observed: 278.1173.
1f – benzyl-5-methoxy-3-vinyl-1H-indole-1-carboxylate
To a stirred Schlenk flask, methylenetriphenylphosphorane (3.54 g, 8.70 mmol) was dissolved in dry THF (34 mL). The solution was cooled to −78 °C and nBuLi (3.1 mL, 7.87 mmol) was added over 10 minutes. The yellow solution was warmed to 0 °C and was left to stir for 1 hour before being cooled to −78 °C. In a separate Schlenk flask, 5-methoxy-1H-indole-3-carbaldehyde (1.38 g, 7.87 mmol) was dissolved in THF (10 mL) and to the solution sodium bis(trimethylsilyl)amide (7.87 mL, 7.87 mmol) was added. This solution was transferred into the first Schlenk flask and the red solution was allowed to stir at room temperature for 1 hour. The reaction was poured into water (50 mL) and extracted with ethyl acetate (2 × 30 mL). The combined organic layers were dried with MgSO4, filtered and the solvent removed under pressure to leave the crude product as yellow oil. The product was purified using column chromatography (petrol (40/60)–diethyl ether 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, column diameter = 2.5 cm, silica = 16 cm) to give 5-methoxy-3-vinyl-1H-indole (1.38 g, 7.6 mmol, 97%) as a yellow powder.
1, column diameter = 2.5 cm, silica = 16 cm) to give 5-methoxy-3-vinyl-1H-indole (1.38 g, 7.6 mmol, 97%) as a yellow powder.
Mp: 190–193 °C; Rf: 0.49 (Pet–Et2O, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (300 MHz, CDCl3): δH 8.28 (1H, s), 7.66 (1H, d, J = 2.4 Hz), 7.29 (1H, d, J = 8.8 Hz), 7.24 (1H, d, J = 2.7 Hz), 7.20–7.10 (2H, m), 7.18 (1H, d, J = 2.5 Hz), 7.15 (2H, dd, J = 4.5, 2.1 Hz), 7.10 (1H, s), 5.95 (1H, dd, J = 17.8, 1.5 Hz), 5.46 (1H, dd, J = 11.2, 1.5 Hz), 4.07 (3H, s); 13C NMR (101 MHz, CDCl3): δC 154.4, 132.5, 130.8, 126.4, 126.1, 114.2, 113.0, 112.0, 109.1, 102.0, 55.9; IR (neat): νmax/cm−1 3410, 2925, 2836; MS (pNSI): 174.1 (100%, (M + H)+), 520.3 (100%, (3M + H)+); HRMS (pNSI): calcd for C11H12NO [M + H]+: 174.0913; observed: 174.0912.
1); 1H NMR (300 MHz, CDCl3): δH 8.28 (1H, s), 7.66 (1H, d, J = 2.4 Hz), 7.29 (1H, d, J = 8.8 Hz), 7.24 (1H, d, J = 2.7 Hz), 7.20–7.10 (2H, m), 7.18 (1H, d, J = 2.5 Hz), 7.15 (2H, dd, J = 4.5, 2.1 Hz), 7.10 (1H, s), 5.95 (1H, dd, J = 17.8, 1.5 Hz), 5.46 (1H, dd, J = 11.2, 1.5 Hz), 4.07 (3H, s); 13C NMR (101 MHz, CDCl3): δC 154.4, 132.5, 130.8, 126.4, 126.1, 114.2, 113.0, 112.0, 109.1, 102.0, 55.9; IR (neat): νmax/cm−1 3410, 2925, 2836; MS (pNSI): 174.1 (100%, (M + H)+), 520.3 (100%, (3M + H)+); HRMS (pNSI): calcd for C11H12NO [M + H]+: 174.0913; observed: 174.0912.
To a stirred Schlenk flask, 5-methoxy-3-vinyl-1H-indole (1.15 g, 6.61 mmol) was dissolved in THF (30 mL) and the solution was cooled to 0 °C. To the stirred solution, sodium bis(trimethylsilyl)amide (7.27 mL, 7.27 mmol) was added and the solution was stirred for 30 minutes before benzyl chloroformate (0.90 mL, 6.61 mmol) was added. The solution was stirred for 30 minutes at room temperature before being added to water (50 mL) and extracted with ethyl acetate (3 × 30 mL). The combined organic washings were dried with MgSO4, filtered and the solvent was removed under reduced pressure to give the crude product as an orange oil. The product was purified using column chromatography (petrol (40/60)–ethyl acetate 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, column diameter = 2.0 cm, silica = 15 cm) to give benzyl-5-methoxy-3-vinyl-1H-indole-1-carboxylate (1.78 g, 4.7 mmol, 71%) as a pale yellow oil.
1, column diameter = 2.0 cm, silica = 15 cm) to give benzyl-5-methoxy-3-vinyl-1H-indole-1-carboxylate (1.78 g, 4.7 mmol, 71%) as a pale yellow oil.
Rf: 0.82 (Pet(40/60)–EA, 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz), δH 8.09 (1H, s), 7.66 (1H, s), 7.50 (2H, d, J = 7.2 Hz), 7.45–4.37 (1H, m), 7.25 (1H, d, J = 2.5 Hz), 6.96 (1H, dd, J = 9.0, 2.2 Hz), 6.79 (1H, dd, J = 17.8, 11.4 Hz), 5.80 (1H, d, J = 17.8 Hz), 5.42 (2H, s), 5.34 (1H, d, J = 11.4 Hz), 3.85 (3H, s); 13C NMR (101 MHz, CDCl3): δC 156.4, 135.2, 129.7, 128.9, 128.9, 128.7, 128.6, 128.0, 124.1, 124.1, 120.0, 116.1, 114.8, 113.3, 103.3, 68.8, 55.8; IR (neat): νmax/cm−1 2955, 2834, 1726; MS (pAPCI): 181.1 (32%), 260.1 (100%), 308.1 (28%, (M + H)+); HRMS (pAPCI): calcd for C19H18NO3 [M + H]+: 308.1281; observed: 308.1277.
1); 1H NMR (400 MHz), δH 8.09 (1H, s), 7.66 (1H, s), 7.50 (2H, d, J = 7.2 Hz), 7.45–4.37 (1H, m), 7.25 (1H, d, J = 2.5 Hz), 6.96 (1H, dd, J = 9.0, 2.2 Hz), 6.79 (1H, dd, J = 17.8, 11.4 Hz), 5.80 (1H, d, J = 17.8 Hz), 5.42 (2H, s), 5.34 (1H, d, J = 11.4 Hz), 3.85 (3H, s); 13C NMR (101 MHz, CDCl3): δC 156.4, 135.2, 129.7, 128.9, 128.9, 128.7, 128.6, 128.0, 124.1, 124.1, 120.0, 116.1, 114.8, 113.3, 103.3, 68.8, 55.8; IR (neat): νmax/cm−1 2955, 2834, 1726; MS (pAPCI): 181.1 (32%), 260.1 (100%), 308.1 (28%, (M + H)+); HRMS (pAPCI): calcd for C19H18NO3 [M + H]+: 308.1281; observed: 308.1277.
2a – (3aS*,10bS*)-2-methyl-10-tosyl-4,10,10a,10b-tetrahydropyrrolo[3,4-a]carbazole-1,3(2H,3aH)-dione
Into a round bottomed flask, 1-tosyl-3-vinyl-1H-indole (2.0 g, 6.7 mmol) and DCM (10 mL) was added. To the stirred solution, N-methylmalemide (0.75 g, 6.7 mmol) was added and the solution was stirred at 40 °C for 48 hours. The solvent was removed under reduced pressure to leave the crude product as orange oil. The product was purified by column chromatography (petrol (40/60)–ethyl acetate, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, column diameter = 4 cm, silica = 15 cm) to give (3aS*,10bS*)-2-methyl-10-tosyl-4,10,10a,10b-tetrahydropyrrolo[3,4-a]carbazole-1,3(2H,3aH)-dione (2.01 g, 5.0 mmol, 76%) as a white powder.
1, column diameter = 4 cm, silica = 15 cm) to give (3aS*,10bS*)-2-methyl-10-tosyl-4,10,10a,10b-tetrahydropyrrolo[3,4-a]carbazole-1,3(2H,3aH)-dione (2.01 g, 5.0 mmol, 76%) as a white powder.
Mp: 204.2–208.0 °C; Rf: 0.09 (Pet(40/60)–EA, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (300 MHz, CDCl3): δH 7.72 (2H, d, J = 8.0 Hz), 7.61 (1H, d, J = 8.5 Hz), 7.21–7.19 (2H, d, J = 8.0), 7.21–7.16 (1H, m), 6.92 (1H, app t, J = 7.5 Hz), 6.01–5.96 (1H, m), 4.47 (1H, dd, J = 7.0, 3.3 Hz), 3.99 (1H, app t, J = 8.1 Hz), 3.12 (1H, app t, J = 8.1 Hz), 2.99–2.92 (1H, m), 2.76 (3H, s), 2.30 (3H, s), 2.11 (1H, ddd, J = 18.0, 6.4, 2.4 Hz); 13C NMR (101 MHz, CDCl3): δC 178.9, 174.2, 144.7, 144.6, 137.4, 134.3, 130.4, 129.9, 127.5, 126.4, 123.9, 121.0, 115.4, 112.9, 61.6, 43.3, 37.2, 25.3, 25.1, 21.7; IR (neat): νmax/cm−1 2981, 2889, 1694; MS (pNSI): 409.2 (61%, (M + H)+), 426.1 (100%, (M + (NH4))+), 834.3 (52%, (2M + (NH4))+); HRMS (pNSI): calcd C22H21N2O4S [M + H]+: 409.1217; observed: 409.1218.
1); 1H NMR (300 MHz, CDCl3): δH 7.72 (2H, d, J = 8.0 Hz), 7.61 (1H, d, J = 8.5 Hz), 7.21–7.19 (2H, d, J = 8.0), 7.21–7.16 (1H, m), 6.92 (1H, app t, J = 7.5 Hz), 6.01–5.96 (1H, m), 4.47 (1H, dd, J = 7.0, 3.3 Hz), 3.99 (1H, app t, J = 8.1 Hz), 3.12 (1H, app t, J = 8.1 Hz), 2.99–2.92 (1H, m), 2.76 (3H, s), 2.30 (3H, s), 2.11 (1H, ddd, J = 18.0, 6.4, 2.4 Hz); 13C NMR (101 MHz, CDCl3): δC 178.9, 174.2, 144.7, 144.6, 137.4, 134.3, 130.4, 129.9, 127.5, 126.4, 123.9, 121.0, 115.4, 112.9, 61.6, 43.3, 37.2, 25.3, 25.1, 21.7; IR (neat): νmax/cm−1 2981, 2889, 1694; MS (pNSI): 409.2 (61%, (M + H)+), 426.1 (100%, (M + (NH4))+), 834.3 (52%, (2M + (NH4))+); HRMS (pNSI): calcd C22H21N2O4S [M + H]+: 409.1217; observed: 409.1218.
2b – 2-phenyl-11-tosyl-11,11a-dihydro-1H,5H-[1,2,4]triazolo[1′,2′:1,2]pyridazino[3,4-b]indole-1,3(2H)-dione
To a stirred round bottomed flask was added 1-tosyl-3-vinyl-1H-indole (100 mg, 0.34 mmol) and DCM (7 mL) and the solution was cooled to −78 °C. To the stirred solution was added 4-phenyl-3H-1,2,4-triazole-3,5(4H)-dione (60 mg, 0.34 mmol) and the resulting solution was stirred at −78 °C for 3.5 hours before the solvent was removed under reduced pressure to leave the crude product as a pale red solid. The product was purified by column chromatography (petrol (40/60)–ether–DCM 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, column diameter = 1 cm, silica = 20 cm) to give 2-phenyl-11-tosyl-11,11a-dihydro-1H,5H-[1,2,4]triazolo[1′,2′:1,2]pyridazino[3,4-b]indole-1,3(2H)-dione (88%, 140 mg, 0.30 mmol) as a pale red powder.
1, column diameter = 1 cm, silica = 20 cm) to give 2-phenyl-11-tosyl-11,11a-dihydro-1H,5H-[1,2,4]triazolo[1′,2′:1,2]pyridazino[3,4-b]indole-1,3(2H)-dione (88%, 140 mg, 0.30 mmol) as a pale red powder.
Mp: 160.1–162.8 °C; Rf: 0.14 (Pet(40/60)–Et2O–DCM 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (300 MHz, CDCl3): δH 7.86 (2H, d, J = 8.4 Hz), 7.63–7.55 (2H, m), 7.51–7.46 (2H, m), 7.46–7.42 (1H, m), 7.42–7.39 (1H, m), 7.38–7.34 (2H, m), 7.26–7.22 (2H, m), 7.09 (1H, app td, J = 7.5, 1.0 Hz), 6.26 (1H, td, J = 2.6, 1.8 Hz), 6.18 (1H, app dt, J = 5.3, 2.7 Hz), 4.56–4.46 (1H, app td, J = 17.6, 2.8 Hz), 4.39 (1H, ddd, J = 17.6, 5.2, 1.9 Hz), 2.37 (3H, s); 13C NMR (75 MHz, CDCl3): δC 152.7, 150.8, 144.7, 143.7, 135.6, 134.8, 131.5, 130.4, 129.6, 128.9, 128.5, 128.1, 126.8, 125.7, 125.2, 120.9, 117.4, 113.8, 74.8, 44.6, 21.4; IR (neat): νmax/cm−1 3070, 2926, 1719; MS (pNSI): 473.1 (100%, (M + H)+), 522.2 (30%); HRMS (pNSI): calcd for C25H21N4O4S [M + H]+: 473.1278; observed: 473.1277.
1); 1H NMR (300 MHz, CDCl3): δH 7.86 (2H, d, J = 8.4 Hz), 7.63–7.55 (2H, m), 7.51–7.46 (2H, m), 7.46–7.42 (1H, m), 7.42–7.39 (1H, m), 7.38–7.34 (2H, m), 7.26–7.22 (2H, m), 7.09 (1H, app td, J = 7.5, 1.0 Hz), 6.26 (1H, td, J = 2.6, 1.8 Hz), 6.18 (1H, app dt, J = 5.3, 2.7 Hz), 4.56–4.46 (1H, app td, J = 17.6, 2.8 Hz), 4.39 (1H, ddd, J = 17.6, 5.2, 1.9 Hz), 2.37 (3H, s); 13C NMR (75 MHz, CDCl3): δC 152.7, 150.8, 144.7, 143.7, 135.6, 134.8, 131.5, 130.4, 129.6, 128.9, 128.5, 128.1, 126.8, 125.7, 125.2, 120.9, 117.4, 113.8, 74.8, 44.6, 21.4; IR (neat): νmax/cm−1 3070, 2926, 1719; MS (pNSI): 473.1 (100%, (M + H)+), 522.2 (30%); HRMS (pNSI): calcd for C25H21N4O4S [M + H]+: 473.1278; observed: 473.1277.
2c – ethyl-3-((3aS*,4R*,10bS*)-2-methyl-1,3-dioxo-10-tosyl-1,2,3,3a,4,10,10a,10b-octahydropyrrolo[3,4-a]carbazol-4-yl)propanoate
Dimethylaluminum chloride (1.0 M in hexane, 9.38 ml, 9.38 mmol) was added dropwise to a solution of N-methylmaleimide (0.521 g, 4.69 mmol) in dry DCM (15 mL) at −78 °C. The mixture left to stir for 30 min. A solution of ethyl-(Z)-5-(1-tosyl-1H-indol-3-yl) pent-4-enoate (4.69 mmol, 1.863 g) in dry DCM (15 mL) was added dropwise at −78 °C. The reaction mixture was then warmed to reflux for 48 hours and quenched with saturated NaHCO3(aq.) (20 mL) and extracted with DCM (2 × 100 mL). The combined organic layers were washed with brine, dried over MgSO4 and filtered. The solvent was removed under reduced pressure to give the crude yellow solid. The product was purified by column chromatography (petrol (40/60)–ethyl acetate 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to yield ethyl 3-((3aS*,4R*,10bS*)-2-methyl-1,3-dioxo-10-tosyl-1,2,3,3a,4,10,10a,10b-octahydropyrrolo[3,4-a]carbazol-4-yl)propanoate in (2.038 g, 4.01 mmol, 85%) a bright yellow solid.
1) to yield ethyl 3-((3aS*,4R*,10bS*)-2-methyl-1,3-dioxo-10-tosyl-1,2,3,3a,4,10,10a,10b-octahydropyrrolo[3,4-a]carbazol-4-yl)propanoate in (2.038 g, 4.01 mmol, 85%) a bright yellow solid.
Mp: 187–188 °C; Rf: 0.3 (Pet(40/60)–EA 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CDCl3): δH 7.79 (2H, d, J = 7.3 Hz), 7.62 (1H, d, J = 7.0 Hz), 7.25–7.21 (4H, m), 6.95 (1H, t, J = 7.0 Hz), 6.09 (1H, dd, J = 3.7, 6.7 Hz), 4.85 (1H, dd, J = 3.7, 6.9 Hz), 4.15 (1H, t, J = 6.9 Hz), 4.07 (2H, m), 3.15–3.12 (1H, m), 3.05–3.02 (1H, m), 2.83 (3H, s), 2.43–2.36 (2H, m), 2.35 (3H, s), 1.91–1.75 (2H, m), 1.19 (3H, t, J = 7.3 Hz); 13C NMR (101 MHz, CDCl3): δC 178.6, 174.0, 172.79, 144.6, 144.0, 136.6, 134.0, 130.5, 130.0, 127.5, 126.6, 123.9, 121.0, 116.3, 115.4, 60.6, 59.9, 44.22, 42.98, 37.2, 33.1, 28.3, 25.3, 21.70, 14.3; IR (neat): νmax/cm−1: 1776, 1698; HRMS (pNSI): calcd C27H29N2O6S [M + H]+: 509.1741; observed: 509.1731.
1); 1H NMR (400 MHz, CDCl3): δH 7.79 (2H, d, J = 7.3 Hz), 7.62 (1H, d, J = 7.0 Hz), 7.25–7.21 (4H, m), 6.95 (1H, t, J = 7.0 Hz), 6.09 (1H, dd, J = 3.7, 6.7 Hz), 4.85 (1H, dd, J = 3.7, 6.9 Hz), 4.15 (1H, t, J = 6.9 Hz), 4.07 (2H, m), 3.15–3.12 (1H, m), 3.05–3.02 (1H, m), 2.83 (3H, s), 2.43–2.36 (2H, m), 2.35 (3H, s), 1.91–1.75 (2H, m), 1.19 (3H, t, J = 7.3 Hz); 13C NMR (101 MHz, CDCl3): δC 178.6, 174.0, 172.79, 144.6, 144.0, 136.6, 134.0, 130.5, 130.0, 127.5, 126.6, 123.9, 121.0, 116.3, 115.4, 60.6, 59.9, 44.22, 42.98, 37.2, 33.1, 28.3, 25.3, 21.70, 14.3; IR (neat): νmax/cm−1: 1776, 1698; HRMS (pNSI): calcd C27H29N2O6S [M + H]+: 509.1741; observed: 509.1731.
2d – ethyl-3-((3aS*,4R*,10bS*)-1,3-dioxo-10-tosyl-1,2,3,3a,4,10,10a,10b-octahydropyrrolo[3,4-a]carbazol-4-yl)propanoate
Dimethyl aluminum chloride (1.0 M in hexane, 2.86 mL, 2.86 mmol) was added dropwise to a solution of N-maleimide (0.14 g, 1.43 mmol) in dry DCM (5 mL) at −78 °C. The mixture left to stir for 30 min. A solution of ethyl-(Z)-5-(1-tosyl-1H-indol-3-yl)-pent-4-enoate (0.57 g, 1.43 mmol) in dry DCM (10 mL) was added. The reaction mixture was slowly heated to reflux for 48 h, quenched with saturated NaHCO3(aq.) (5 mL). The organic layer was extracted with DCM (1 × 100 mL), washed with brine, dried over MgSO4 and filtered. The solvent was removed under reduced pressure to give the crude yellow solid product which was purified by column chromatography (petrol (40/60)–ethyl acetate 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 gradient to 1
1 gradient to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 petrol–ethyl acetate) to yield ethyl 3-((3aS*,4R*,10bS*)-1,3-dioxo-10-tosyl-1,2,3,3a,4,10,10a,10b-octahydropyrrolo[3,4-a]carbazol-4-yl)propanoate in (0.452 g, 0.91 mmol, 64%) as a white solid.
1 petrol–ethyl acetate) to yield ethyl 3-((3aS*,4R*,10bS*)-1,3-dioxo-10-tosyl-1,2,3,3a,4,10,10a,10b-octahydropyrrolo[3,4-a]carbazol-4-yl)propanoate in (0.452 g, 0.91 mmol, 64%) as a white solid.
Mp: 218–220 °C; Rf: 0.14 (Pet(40/60)–EA 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CDCl3): δH 7.78 (2H, d, J = 6.9 Hz), 7.64 (1H, d, J = 6.8 Hz), 7.27–7.22 (4H, m), 6.98 (1H, t, J = 7.8 Hz), 6.16 (1H, dd, J = 3.6, 7.1 Hz), 4.84 (1H, dd, J = 3.6, 7.3 Hz), 4.18 (1H, t, J = 7.3 Hz), 4.12–4.05 (2H, m), 3.16–1.12 (1H, m), 3.10 (1H, t, J = 7.3 Hz), 2.40 (2H, t, J = 7.3 Hz), 2.35 (3H, s), 1.89–1.76 (2H, m), 1.20 (3H, t, J = 7.5 Hz). 13C NMR (101 MHz, CDCl3): δC 178.3, 173.6, 172.7, 144.5, 144.1, 136.6, 133.8, 130.5, 129.8, 127.5, 126.6, 123.8, 120.0, 116.4, 115.5, 60.8, 59.6, 45.2, 44.0, 36.9, 33.0, 27.8, 21.5, 14.1; IR (neat): νmax/cm−1: 3657, 2981, 1776, 1703; MS (pNSI): 512.18 (100%, [M + NH4]+), 495.15 (55%, [M + H]+), 340.14 (31%, [M − Ts]); HRMS (pNSI): calcd C26H27N2O6S [M + H]+: 495.1584; observed: 495.1585.
1); 1H NMR (400 MHz, CDCl3): δH 7.78 (2H, d, J = 6.9 Hz), 7.64 (1H, d, J = 6.8 Hz), 7.27–7.22 (4H, m), 6.98 (1H, t, J = 7.8 Hz), 6.16 (1H, dd, J = 3.6, 7.1 Hz), 4.84 (1H, dd, J = 3.6, 7.3 Hz), 4.18 (1H, t, J = 7.3 Hz), 4.12–4.05 (2H, m), 3.16–1.12 (1H, m), 3.10 (1H, t, J = 7.3 Hz), 2.40 (2H, t, J = 7.3 Hz), 2.35 (3H, s), 1.89–1.76 (2H, m), 1.20 (3H, t, J = 7.5 Hz). 13C NMR (101 MHz, CDCl3): δC 178.3, 173.6, 172.7, 144.5, 144.1, 136.6, 133.8, 130.5, 129.8, 127.5, 126.6, 123.8, 120.0, 116.4, 115.5, 60.8, 59.6, 45.2, 44.0, 36.9, 33.0, 27.8, 21.5, 14.1; IR (neat): νmax/cm−1: 3657, 2981, 1776, 1703; MS (pNSI): 512.18 (100%, [M + NH4]+), 495.15 (55%, [M + H]+), 340.14 (31%, [M − Ts]); HRMS (pNSI): calcd C26H27N2O6S [M + H]+: 495.1584; observed: 495.1585.
2e – ethyl 3-((3aS*,4R*,10bS*)-1,3-dioxo-2-phenyl-10-tosyl-1,2,3,3a,4,10,10a,10b-octahydropyrrolo[3,4-a]carbazol-4-yl)propanoate
Dimethyl aluminum chloride (1.0 M in THF, 2.39 mL, 2.39 mmol) was added dropwise to a solution of N-phenylmaleimide (0.207 g, 1.19 mmol) in dry DCM (5 mL) at −78 °C. The mixture left to stir for 30 min. A solution of ethyl (Z)-5-(1-tosyl-1H-indol-3-yl) pent-4-enoate (0.475 g, 1.19 mmol) in dry DCM (10 mL) was added. The reaction mixture was heated to reflux for 48 h, quenched with saturated NaHCO3(aq.) (10 mL) and extracted with DCM (100 mL). The organic layer was washed with brine, dried over MgSO4 and filtered. The solvent was removed under reduced pressure to give the crude yellow solid product which was purified by column chromatography (petrol (40/60)–ethyl acetate 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to yield ethyl 3-((3aS*,4R*,10bS*)-1,3-dioxo-2-phenyl-10-tosyl-1,2,3,3a,4,10,10a,10b-octahydropyrrolo[3,4-a]carbazol-4-yl)propanoate in (0.5095 g, 0.84 mmol, 71%) as a white solid.
1) to yield ethyl 3-((3aS*,4R*,10bS*)-1,3-dioxo-2-phenyl-10-tosyl-1,2,3,3a,4,10,10a,10b-octahydropyrrolo[3,4-a]carbazol-4-yl)propanoate in (0.5095 g, 0.84 mmol, 71%) as a white solid.
Mp: 201–203 °C; Rf: 0.2 (Pet(40/60)–EA 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CDCl3): δH 7.81 (2H, d, J = 7.3 Hz), 7.60 (1H, d, J = 7.3 Hz), 7.34–7.21 (7H, m), 7.06 (2H, d, J = 7.3 Hz), 6.97 (1H, t, J = 7.3 Hz), 6.20 (1H, dd, J = 3.4, 7.1 Hz), 4.59 (1H, dd, J = 3.4, 7.1 Hz), 4.18 (1H, t, J = 7.3 Hz), 4.13–4.05 (2H, m), 3.26–3.20 (2H, m), 2.45–2.40 (2H, m), 2.35 (3H, s), 1.89–1.85 (2H, m), 1.20 (3H, t, J = 6.9 Hz); 13C NMR (101 MHz, CDCl3): δC 177.5, 172.9, 172.7, 144.6, 144.3, 137.0, 134.1, 131.7, 130.7, 130.0, 129.0, 128.5, 127.6, 126.7, 126.3, 124.0, 120.9, 116.2, 115.5, 60.8, 60.0, 44.3, 43.1, 37.7, 33.2, 28.3, 21.6, 14.2; IR (neat): νmax/cm−1: 1776, 1703; MS (pNSI): 588.21 (100%, [M + NH4]+), 1158.39 (33%, [2M + NH4]+); HRMS (pNSI): calcd C32H31N2O6S [M + H]+: 571.18; observed: 571.1891.
1); 1H NMR (400 MHz, CDCl3): δH 7.81 (2H, d, J = 7.3 Hz), 7.60 (1H, d, J = 7.3 Hz), 7.34–7.21 (7H, m), 7.06 (2H, d, J = 7.3 Hz), 6.97 (1H, t, J = 7.3 Hz), 6.20 (1H, dd, J = 3.4, 7.1 Hz), 4.59 (1H, dd, J = 3.4, 7.1 Hz), 4.18 (1H, t, J = 7.3 Hz), 4.13–4.05 (2H, m), 3.26–3.20 (2H, m), 2.45–2.40 (2H, m), 2.35 (3H, s), 1.89–1.85 (2H, m), 1.20 (3H, t, J = 6.9 Hz); 13C NMR (101 MHz, CDCl3): δC 177.5, 172.9, 172.7, 144.6, 144.3, 137.0, 134.1, 131.7, 130.7, 130.0, 129.0, 128.5, 127.6, 126.7, 126.3, 124.0, 120.9, 116.2, 115.5, 60.8, 60.0, 44.3, 43.1, 37.7, 33.2, 28.3, 21.6, 14.2; IR (neat): νmax/cm−1: 1776, 1703; MS (pNSI): 588.21 (100%, [M + NH4]+), 1158.39 (33%, [2M + NH4]+); HRMS (pNSI): calcd C32H31N2O6S [M + H]+: 571.18; observed: 571.1891.
3a – (3aS*,5S*,10bS*)-5-(hydroxy(phenyl)amino)-2-methyl-10-tosyl-4,5,10,10b-tetrahydropyrrolo[3,4-a]carbazole-1,3(2H,3aH)-dione
To a stirred round bottomed flask was added 1-tosyl-3-vinyl-1H-indole (100 mg, 0.34 mmol), DCM (5 mL) and 1-methyl-1H-pyrrole-2,5-dione (38 mg, 0.34 mmol). The resulting solution was heated at reflux for 48 hours. The reaction was allowed to cool to room temperature, nitrosobenzene (40 mg, 0.34 mmol) was added, and the solution was stirred at room temperature for 18 hours. The solvent was removed under reduced pressure to leave the crude product as a pale yellow solid which was purified by column chromatography (petrol (40/60)–ethyl acetate 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, column diameter = 1 cm, silica = 16 cm) to give (3aS*,5S*,10bS*)-5-(hydroxy(phenyl)amino)-2-methyl-10-tosyl-4,5,10,10b-tetrahydropyrrolo[3,4-a]carbazole-1,3(2H,3aH)-dione as a yellow powder (71%, 128 mg, 0.24 mmol).
1, column diameter = 1 cm, silica = 16 cm) to give (3aS*,5S*,10bS*)-5-(hydroxy(phenyl)amino)-2-methyl-10-tosyl-4,5,10,10b-tetrahydropyrrolo[3,4-a]carbazole-1,3(2H,3aH)-dione as a yellow powder (71%, 128 mg, 0.24 mmol).
Mp: 196.8–199.5 °C; Rf: 0.64 (Pet(40/60)–EA, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (500 MHz, CD2Cl2): δH 7.94 (1H, d, J = 8.4 Hz), 7.69 (2H, d, J = 8.2 Hz), 7.60 (1H, d, J = 7.9 Hz), 7.32–7.25 (3H, m), 7.23 (2H, d, J = 8.2 Hz), 7.18–7.13 (3H, m), 7.02 (1H, t, J = 7.3 Hz), 5.06 (1H, d, J = 8.0 Hz), 4.75 (1H, app. t, J = 5.9 Hz), 4.72 (1H, s), 3.64 (1H, app q, J = 7.2 Hz), 2.95 (3H, s), 2.43 (1H, app dt, J = 13.6, 6.4 Hz), 2.35 (3H, s), 2.06 (1H, ddd, J = 13.6, 7.2, 4.9); 13C NMR (101 MHz, CD2Cl2): δC 178.1, 173.6, 150.7, 145.3, 137.5, 134.9, 131.4, 129.7, 128.9, 128.9, 126.8, 125.2, 124.2, 122.6, 121.7, 120.2, 117.2, 115.4, 58.0, 40.5, 39.5, 25.0, 23.3, 21.4; IR (neat): νmax/cm−1 3661, 2990, 2886, 1690; MS (pNSI): 407.1 (66%, (M − (C6H5NOH))+), 516.2 (49%, (M + H)+), 533.2 (100%, (M + NH4)+), 1031.3 (57%, (2M + H)+), 1053.3 (13%, (2M + Na)+); HRMS (pNSI): calcd for C28H26N3O5S [M + H]+: 516.1588; observed: 516.1584.
1); 1H NMR (500 MHz, CD2Cl2): δH 7.94 (1H, d, J = 8.4 Hz), 7.69 (2H, d, J = 8.2 Hz), 7.60 (1H, d, J = 7.9 Hz), 7.32–7.25 (3H, m), 7.23 (2H, d, J = 8.2 Hz), 7.18–7.13 (3H, m), 7.02 (1H, t, J = 7.3 Hz), 5.06 (1H, d, J = 8.0 Hz), 4.75 (1H, app. t, J = 5.9 Hz), 4.72 (1H, s), 3.64 (1H, app q, J = 7.2 Hz), 2.95 (3H, s), 2.43 (1H, app dt, J = 13.6, 6.4 Hz), 2.35 (3H, s), 2.06 (1H, ddd, J = 13.6, 7.2, 4.9); 13C NMR (101 MHz, CD2Cl2): δC 178.1, 173.6, 150.7, 145.3, 137.5, 134.9, 131.4, 129.7, 128.9, 128.9, 126.8, 125.2, 124.2, 122.6, 121.7, 120.2, 117.2, 115.4, 58.0, 40.5, 39.5, 25.0, 23.3, 21.4; IR (neat): νmax/cm−1 3661, 2990, 2886, 1690; MS (pNSI): 407.1 (66%, (M − (C6H5NOH))+), 516.2 (49%, (M + H)+), 533.2 (100%, (M + NH4)+), 1031.3 (57%, (2M + H)+), 1053.3 (13%, (2M + Na)+); HRMS (pNSI): calcd for C28H26N3O5S [M + H]+: 516.1588; observed: 516.1584.
Note: 1H NMR run at 35 °C, broad signals observed at room temperature.
3b – (3aS*,5S*,10bS*)-5-(hydroxy(o-tolyl)amino)-2-methyl-10-tosyl-4,5,10,10b-tetrahydropyrrolo[3,4-a]carbazole-1,3(2H,3aH)-dione
To a stirred round bottomed flask was added 1-tosyl-3-vinyl-1H-indole (100 mg, 0.34 mmol), DCM (5 mL) and 1-methyl-1H-pyrrole-2,5-dione (38 mg, 0.34 mmol). The resulting solution was heated at reflux for 48 hours. The reaction was allowed to cool to room temperature, 1-methyl-2-nitrosobenzene (42 mg, 0.17 mmol) was added and the solution was stirred at room temperature for 18 hours. The solvent was removed under reduced pressure to leave the crude product as a pale yellow solid. The product was purified by column chromatography (petrol (40/60)–ethyl acetate 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, column diameter = 1 cm, silica = 14 cm) to give (3aS*,5S*,10bS*)-5-(hydroxy(o-tolyl)amino)-2-methyl-10-tosyl-4,5,10,10b-tetrahydropyrrolo[3,4-a]carbazole-1,3(2H,3aH)-dione (71%, 128 mg, 0.24 mmol) as a yellow powder.
1, column diameter = 1 cm, silica = 14 cm) to give (3aS*,5S*,10bS*)-5-(hydroxy(o-tolyl)amino)-2-methyl-10-tosyl-4,5,10,10b-tetrahydropyrrolo[3,4-a]carbazole-1,3(2H,3aH)-dione (71%, 128 mg, 0.24 mmol) as a yellow powder.
Mp: 193.0–196.7 °C; Rf: 0.59 (Pet(40/60)–EA 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CD2Cl2): δH 7.89 (1H, d, J = 8.3 Hz), 7.65 (2H, d, J = 8.4 Hz), 7.40 (1H, d, J = 7.9 Hz), 7.28 (1H, d, J = 7.7 Hz), 7.21–7.18 (3H, m), 7.13–6.99 (4H, m), 5.04 (1H, d, J = 8.1 Hz), 4.87 (1H, s), 4.27 (1H, app t, J = 5.4 Hz), 3.73 (1H, app td, J = 8.0, 6.1 Hz), 2.91 (3H, s), 2.58 (1H, app dt, J = 12.9, 6.2 Hz), 2.32 (3H, s), 2.25 (3H, s), 1.95 (1H, ddd, J = 12.9, 7.8, 4.5 Hz); 13C NMR (101 MHz, CD2Cl2): δC 178.2, 173.6, 149.3, 145.3, 137.3, 134.9, 131.6, 130.9, 129.7, 129.7, 129.2, 126.8, 126.2, 125.0, 124.9, 124.1, 121.4, 120.5, 115.3, 57.3, 40.6, 39.4, 25.0, 24.6, 21.4, 18.3; IR (neat): νmax/cm−1 3662, 2990, 2886, 1701; MS (pNSI): 407.1 (98%, (M − ((o-CH3) − C6H4NOH))+), 530.2 (52%, (M + H)+), 547.2 (65%, (M + NH4)+), 1059.3 (100%, (2M + H)+); HRMS (pNSI): calcd for C29H28N3O5S [M + H]+: 530.1744; observed: 530.1743.
1); 1H NMR (400 MHz, CD2Cl2): δH 7.89 (1H, d, J = 8.3 Hz), 7.65 (2H, d, J = 8.4 Hz), 7.40 (1H, d, J = 7.9 Hz), 7.28 (1H, d, J = 7.7 Hz), 7.21–7.18 (3H, m), 7.13–6.99 (4H, m), 5.04 (1H, d, J = 8.1 Hz), 4.87 (1H, s), 4.27 (1H, app t, J = 5.4 Hz), 3.73 (1H, app td, J = 8.0, 6.1 Hz), 2.91 (3H, s), 2.58 (1H, app dt, J = 12.9, 6.2 Hz), 2.32 (3H, s), 2.25 (3H, s), 1.95 (1H, ddd, J = 12.9, 7.8, 4.5 Hz); 13C NMR (101 MHz, CD2Cl2): δC 178.2, 173.6, 149.3, 145.3, 137.3, 134.9, 131.6, 130.9, 129.7, 129.7, 129.2, 126.8, 126.2, 125.0, 124.9, 124.1, 121.4, 120.5, 115.3, 57.3, 40.6, 39.4, 25.0, 24.6, 21.4, 18.3; IR (neat): νmax/cm−1 3662, 2990, 2886, 1701; MS (pNSI): 407.1 (98%, (M − ((o-CH3) − C6H4NOH))+), 530.2 (52%, (M + H)+), 547.2 (65%, (M + NH4)+), 1059.3 (100%, (2M + H)+); HRMS (pNSI): calcd for C29H28N3O5S [M + H]+: 530.1744; observed: 530.1743.
3c – (3aS*,5S*,10bS*)-5-(3,5-dioxo-4-phenyl-1,2,4-triazolidin-1-yl)-2-methyl-10-tosyl-4,5,10,10b-tetrahydropyrrolo[3,4-a]carbazole-1,3(2H,3aH)-dione
To a stirred round bottomed flask was added 1-tosyl-3-vinyl-1H-indole (100 mg, 0.34 mmol), DCM (5 mL) and 1-methyl-1H-pyrrole-2,5-dione (38 mg, 0.34 mmol) and the resulting solution was heated at reflux for 48 hours. The reaction was cooled to 0 °C before PTAD (60 mg, 0.34 mmol) was added. The reaction was stirred at 0 °C for 4 hours. The solvent was removed under reduced pressure to leave the crude product as a pale red powder. The product was purified by column chromatography (petrol (40/60)–ethyl acetate 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, column diameter = 1 cm, silica = 14 cm) to give (3aS*,5S*,10bS*)-5-(3,5-dioxo-4-phenyl-1,2,4-triazolidin-1-yl)-2-methyl-10-tosyl-4,5,10,10b-tetrahydropyrrolo[3,4-a]carbazole-1,3(2H,3aH)-dione (76%, 150 mg, 0.26 mmol) as a white powder.
1, column diameter = 1 cm, silica = 14 cm) to give (3aS*,5S*,10bS*)-5-(3,5-dioxo-4-phenyl-1,2,4-triazolidin-1-yl)-2-methyl-10-tosyl-4,5,10,10b-tetrahydropyrrolo[3,4-a]carbazole-1,3(2H,3aH)-dione (76%, 150 mg, 0.26 mmol) as a white powder.
Mp: 183.4–187.7 °C; Rf: 0.05 (Pet(40/60)–EA 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CDCl3): δH 7.84 (2H, d, J = 8.4 Hz), 7.67 (1H, d, J = 8.2 Hz), 7.52 (1H, d, J = 7.6 Hz), 7.46–7.31 (5H, m), 7.29–7.19 (2H, m), 7.17 (2H, d, J = 8.3 Hz), 5.55 (1H, app t, J = 4.7 Hz), 5.08 (1H, d, J = 7.7 Hz), 3.66 (1H, ddd, J = 10.5, 7.7, 5.8 Hz), 2.96 (3H, s), 2.49 (1H, app dt, J = 14.8, 5.3 Hz), 2.28 (3H, s), 2.14 (1H, ddd, J = 14.8, 10.5, 5.5 Hz); 13C NMR (101 MHz, CDCl3): δC 177.4, 173.4, 153.6, 152.8, 145.5, 137.0, 135.3, 132.3, 130.8, 130.0, 129.3, 128.5, 127.5, 126.9, 125.7, 125.6, 124.3, 119.4, 115.4, 114.9, 47.8, 40.3, 39.0, 28.4, 25.4, 21.7; IR (neat): νmax/cm−1 3665, 2984, 2884, 1699; MS (pNSI): 601.2 (100%, (M + NH4)+), 1184.3 (13%, (2M + NH4)+); HRMS (pNSI): calcd for C30H29N6O6S [M + NH4]+: 601.1864; observed: 601.1861.
1); 1H NMR (400 MHz, CDCl3): δH 7.84 (2H, d, J = 8.4 Hz), 7.67 (1H, d, J = 8.2 Hz), 7.52 (1H, d, J = 7.6 Hz), 7.46–7.31 (5H, m), 7.29–7.19 (2H, m), 7.17 (2H, d, J = 8.3 Hz), 5.55 (1H, app t, J = 4.7 Hz), 5.08 (1H, d, J = 7.7 Hz), 3.66 (1H, ddd, J = 10.5, 7.7, 5.8 Hz), 2.96 (3H, s), 2.49 (1H, app dt, J = 14.8, 5.3 Hz), 2.28 (3H, s), 2.14 (1H, ddd, J = 14.8, 10.5, 5.5 Hz); 13C NMR (101 MHz, CDCl3): δC 177.4, 173.4, 153.6, 152.8, 145.5, 137.0, 135.3, 132.3, 130.8, 130.0, 129.3, 128.5, 127.5, 126.9, 125.7, 125.6, 124.3, 119.4, 115.4, 114.9, 47.8, 40.3, 39.0, 28.4, 25.4, 21.7; IR (neat): νmax/cm−1 3665, 2984, 2884, 1699; MS (pNSI): 601.2 (100%, (M + NH4)+), 1184.3 (13%, (2M + NH4)+); HRMS (pNSI): calcd for C30H29N6O6S [M + NH4]+: 601.1864; observed: 601.1861.
3d – (3aS*,5S*,10bS*)-5-((S*)-hydroxy(perfluorophenyl)methyl)-2-methyl-10-tosyl-4,5,10,10b-tetrahydropyrrolo[3,4-a]carbazole-1,3(2H,3aH)-dione
To a stirred round bottomed flask was added 1-tosyl-3-vinyl-1H-indole (100 mg, 0.34 mmol) DCM (5 mL) and 1-methyl-1H-pyrrole-2,5-dione (38 mg, 0.34 mmol) and the resulting solution was heated at reflux for 48 hours. The reaction was cooled to −78 °C and 2,3,4,5,6-pentafluorobenzaldehyde (0.04 mL, 0.34 mmol) was added followed by DMAC (1 M in hexane, 0.34 mL, 0.34 mmol). The reaction was stirred at −78 °C for 15 minutes before being allowed to warm to room temperature. The reaction was stirred at room temperature for 18 hours. The reaction was poured into saturated sodium bicarbonate solution (10 mL) and extracted with DCM (2 × 10 mL). The combined organic layers were dried with MgSO4, filtered and the solvent was removed under reduced pressure to give the crude product as a pale brown solid. The product was purified by column chromatography (petrol (40/60)–ethyl acetate 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, column diameter = 2 cm, silica = 15 cm) to give a separable 5
1, column diameter = 2 cm, silica = 15 cm) to give a separable 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of (3aS*,5S*,10bS*)-5-((S*)-hydroxy(perfluorophenyl)methyl)-2-methyl-10-tosyl-4,5,10,10b-tetrahydropyrrolo[3,4-a]carbazole-1,3(2H,3aH)-dione and (3aS*,5S*,10bS*)-5-((R*)-hydroxy(perfluorophenyl)methyl)-2-methyl-10-tosyl-4,5,10,10b-tetrahydropyrrolo[3,4-a]carbazole-1,3(2H,3aH)-dione (72%, 149 mg, 0.25 mmol).
1 mixture of (3aS*,5S*,10bS*)-5-((S*)-hydroxy(perfluorophenyl)methyl)-2-methyl-10-tosyl-4,5,10,10b-tetrahydropyrrolo[3,4-a]carbazole-1,3(2H,3aH)-dione and (3aS*,5S*,10bS*)-5-((R*)-hydroxy(perfluorophenyl)methyl)-2-methyl-10-tosyl-4,5,10,10b-tetrahydropyrrolo[3,4-a]carbazole-1,3(2H,3aH)-dione (72%, 149 mg, 0.25 mmol).
Major diastereomer: Mp: 120.4–121.7 °C; Rf: 0.24 (Pet(40/60)–EA 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (300 MHz, CDCl3): δH δ 7.85 (3H, app d, J = 8.4 Hz), 7.31–7.22 (4H, m), 7.22–7.14 (1H, m), 5.13 (1H, d, J = 8.1 Hz), 4.99 (1H, d, J = 7.5 Hz), 3.71–3.52 (2H, m), 3.01 (3H, s), 2.38 (3H, s), 2.20–2.10 (1H, m), 1.67 (1H, app td, J = 13.8, 5.3 Hz); 13C NMR (101 MHz, CDCl3): δC 177.7, 173.3, 145.3, 137.4, 135.4, 129.8, 129.7, 129.6, 127.2, 125.3, 123.9, 120.1, 119.9, 115.2, 70.2, 41.5, 39.1, 36.9, 28.7, 25.2, 21.7; IR (neat): νmax/cm−1 3371, 2981, 2889, 1690; MS (pNSI): 605.1 (40%, (M + H)+), 622.1 (88%, (M + NH4)+), 627.1 (100%, (M + Na)+), 643.1 (17%), 709.1 (15%); HRMS (pNSI): calcd for C29H21F5N2NaO5S [M + Na]+: 627.0984; observed: 627.0968. Note: 13C NMR missing peaks due to C–F coupling.
1); 1H NMR (300 MHz, CDCl3): δH δ 7.85 (3H, app d, J = 8.4 Hz), 7.31–7.22 (4H, m), 7.22–7.14 (1H, m), 5.13 (1H, d, J = 8.1 Hz), 4.99 (1H, d, J = 7.5 Hz), 3.71–3.52 (2H, m), 3.01 (3H, s), 2.38 (3H, s), 2.20–2.10 (1H, m), 1.67 (1H, app td, J = 13.8, 5.3 Hz); 13C NMR (101 MHz, CDCl3): δC 177.7, 173.3, 145.3, 137.4, 135.4, 129.8, 129.7, 129.6, 127.2, 125.3, 123.9, 120.1, 119.9, 115.2, 70.2, 41.5, 39.1, 36.9, 28.7, 25.2, 21.7; IR (neat): νmax/cm−1 3371, 2981, 2889, 1690; MS (pNSI): 605.1 (40%, (M + H)+), 622.1 (88%, (M + NH4)+), 627.1 (100%, (M + Na)+), 643.1 (17%), 709.1 (15%); HRMS (pNSI): calcd for C29H21F5N2NaO5S [M + Na]+: 627.0984; observed: 627.0968. Note: 13C NMR missing peaks due to C–F coupling.
3e – 6-(hydroxy(phenyl)amino)-2-phenyl-11-tosyl-5,6-dihydro-[1,2,4]triazolo[1′,2′:1,2]pyridazino[3,4-b]indole-1,3(2H,11H)-dione
To a stirred round bottomed flask was added 1-tosyl-3-vinyl-1H-indole (100 mg, 0.34 mmol) and DCM (5 mL) and the solution was cooled to −78 °C. To this solution PTAD (70 mg, 0.34 mmol) was added and the reaction was stirred at −78 °C for 3.5 hours. The reaction was warmed to room temperature, nitrosobenzene (44 mg, 0.34 mmol) was added and the reaction was stirred for 18 hours. The solvent was removed under reduced pressure to leave the crude product as a pale yellow oil. The product was purified by column chromatography (column diameter = 1 cm, silica = 16 cm, eluent = petrol (40/60)–ether–DCM 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give 6-(hydroxy(phenyl)amino)-2-phenyl-11-tosyl-5,6-dihydro-[1,2,4]triazolo[1′,2′:1,2]pyridazino[3,4-b]indole-1,3(2H,11H)-dione (72%, 54 mg, 0.09 mmol) as a white powder.
1) to give 6-(hydroxy(phenyl)amino)-2-phenyl-11-tosyl-5,6-dihydro-[1,2,4]triazolo[1′,2′:1,2]pyridazino[3,4-b]indole-1,3(2H,11H)-dione (72%, 54 mg, 0.09 mmol) as a white powder.
Mp: 176.1–180.0 °C; Rf: 0.48 (Pet(40/60)–Et2O 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CDCl3): δH 8.03 (1H, d, J = 8.3 Hz), 7.62 (2H, d, J = 8.1 Hz), 7.56 (2H, d, J = 7.6 Hz), 7.46 (2H, app t, J = 7.7 Hz), 7.42–7.36 (1H, m), 7.23–7.07 (7H, m), 7.05–6.99 (2H, m), 6.59 (1H, d, J = 7.4 Hz), 5.81 (1H, br s), 5.18 (1H, d, J = 13.5 Hz), 4.59 (1H, s), 3.23 (1H, d, J = 13.5 Hz), 2.30 (3H, s); 13C NMR (101 MHz, DMSO-d6): δC 152.7, 152.2, 150.5, 146.0, 134.9, 132.9, 132.5, 131.8, 130.4, 129.8, 129.3, 129.2, 128.5, 127.3, 127.3, 125.5, 125.4, 122.5, 119.8, 117.6, 116.6, 108.6, 55.9, 44.6, 21.6; IR (neat): νmax/cm−1 2981, 2884, 1714; MS (pAPCI): 138.1 (100%), 157.0 (95%), 213.1 (50%), 248.1 (86%), 279.1 (62%), 317.1 (33%), 333.1 (29%), 471.1 (31%), 564.2 (11%), 580.2 (10%, (M + H)+); HRMS (pAPCI): calcd for C31H26N5O5S [M + H]+: 580.1649; observed: 580.1640.
1); 1H NMR (400 MHz, CDCl3): δH 8.03 (1H, d, J = 8.3 Hz), 7.62 (2H, d, J = 8.1 Hz), 7.56 (2H, d, J = 7.6 Hz), 7.46 (2H, app t, J = 7.7 Hz), 7.42–7.36 (1H, m), 7.23–7.07 (7H, m), 7.05–6.99 (2H, m), 6.59 (1H, d, J = 7.4 Hz), 5.81 (1H, br s), 5.18 (1H, d, J = 13.5 Hz), 4.59 (1H, s), 3.23 (1H, d, J = 13.5 Hz), 2.30 (3H, s); 13C NMR (101 MHz, DMSO-d6): δC 152.7, 152.2, 150.5, 146.0, 134.9, 132.9, 132.5, 131.8, 130.4, 129.8, 129.3, 129.2, 128.5, 127.3, 127.3, 125.5, 125.4, 122.5, 119.8, 117.6, 116.6, 108.6, 55.9, 44.6, 21.6; IR (neat): νmax/cm−1 2981, 2884, 1714; MS (pAPCI): 138.1 (100%), 157.0 (95%), 213.1 (50%), 248.1 (86%), 279.1 (62%), 317.1 (33%), 333.1 (29%), 471.1 (31%), 564.2 (11%), 580.2 (10%, (M + H)+); HRMS (pAPCI): calcd for C31H26N5O5S [M + H]+: 580.1649; observed: 580.1640.
3f – 6-(hydroxy(o-tolyl)amino)-2-phenyl-11-tosyl-5,6-dihydro-[1,2,4]triazolo[1′,2′:1,2]pyridazino[3,4-b]indole-1,3(2H,11H)-dione
To a stirred round bottomed flask was added 1-tosyl-3-vinyl-1H-indole (100 mg, 0.34 mmol) and DCM (5 mL) and the solution cooled to −78 °C. To this solution PTAD (70 mg, 0.34 mmol) was added and the reaction was stirred at −78 °C for 3.5 hours. The reaction was warmed to room temperature and 1-methyl-2-nitrosobenzene (42 mg, 0.34 mmol) was added and the reaction was stirred for 18 hours. The solvent was removed under reduced pressure to leave the crude product as a pale yellow oil. The product was purified by column chromatography (column diameter = 1 cm, silica = 14 cm, eluent = petrol (40/60)–ether–DCM 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give 6-(hydroxy(o-tolyl)amino)-2-phenyl-11-tosyl-5,6-dihydro-[1,2,4]triazolo[1′,2′:1,2]pyridazino[3,4-b]indole-1,3(2H,11H)-dione as a white powder (78%, 60 mg, 0.10 mmol).
1) to give 6-(hydroxy(o-tolyl)amino)-2-phenyl-11-tosyl-5,6-dihydro-[1,2,4]triazolo[1′,2′:1,2]pyridazino[3,4-b]indole-1,3(2H,11H)-dione as a white powder (78%, 60 mg, 0.10 mmol).
Mp: 149.7–153.1 °C; Rf: 0.32 (Pet(40/60)–Et2O–DCM 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (300 MHz, CDCl3): δH 7.99 (1H, d, J = 8.3 Hz), 7.61–7.58 (5H, m), 7.43–7.40 (1H, m), 7.47 (2H, app t, J = 7.7 Hz), 7.40–7.37 (1H, m), 7.18 (2H, app t, J = 7.8 Hz), 7.11 (2H, d, J = 8.2 Hz), 6.97 (2H, app q, J = 7.2 Hz), 6.85 (1H, d, J = 7.5 Hz), 6.50 (1H, d, J = 7.8 Hz), 5.93 (1H, s), 5.24 (1H, d, J = 13.5 Hz), 4.44 (1H, s), 3.11 (1H, d, J = 13.5 Hz), 2.29 (3H, s), 1.91 (3H, s); 13C NMR (101 MHz, CDCl3): δC 153.3, 150.3, 148.7, 145.4, 135.1, 133.7, 132.0, 131.7, 131.3, 130.6, 129.6, 129.3, 128.7, 127.9, 127.2, 127.0, 126.8, 125.9, 124.9, 124.8, 122.6, 118.0, 116.8, 107.0, 59.1, 44.5, 21.7, 17.8; IR (neat): νmax/cm−1 3068, 2981, 1713; MS (pAPCI): 138.1 (100%), 157.0 (82%), 262.1 (55%), 279.1 (76%), 317.1 (50%), 391.3 (37%), 471.1 (21%), 594.2 (10%, (M + H)+); HRMS (pAPCI): calcd for C32H28N5O5S [M + H]+: 594.1806; observed: 594.1801.
1); 1H NMR (300 MHz, CDCl3): δH 7.99 (1H, d, J = 8.3 Hz), 7.61–7.58 (5H, m), 7.43–7.40 (1H, m), 7.47 (2H, app t, J = 7.7 Hz), 7.40–7.37 (1H, m), 7.18 (2H, app t, J = 7.8 Hz), 7.11 (2H, d, J = 8.2 Hz), 6.97 (2H, app q, J = 7.2 Hz), 6.85 (1H, d, J = 7.5 Hz), 6.50 (1H, d, J = 7.8 Hz), 5.93 (1H, s), 5.24 (1H, d, J = 13.5 Hz), 4.44 (1H, s), 3.11 (1H, d, J = 13.5 Hz), 2.29 (3H, s), 1.91 (3H, s); 13C NMR (101 MHz, CDCl3): δC 153.3, 150.3, 148.7, 145.4, 135.1, 133.7, 132.0, 131.7, 131.3, 130.6, 129.6, 129.3, 128.7, 127.9, 127.2, 127.0, 126.8, 125.9, 124.9, 124.8, 122.6, 118.0, 116.8, 107.0, 59.1, 44.5, 21.7, 17.8; IR (neat): νmax/cm−1 3068, 2981, 1713; MS (pAPCI): 138.1 (100%), 157.0 (82%), 262.1 (55%), 279.1 (76%), 317.1 (50%), 391.3 (37%), 471.1 (21%), 594.2 (10%, (M + H)+); HRMS (pAPCI): calcd for C32H28N5O5S [M + H]+: 594.1806; observed: 594.1801.
3g – ethyl 3-((3aS*,4S*,5S*,10bS*)-5-(hydroxy(o-tolyl)amino)-2-methyl-1,3-dioxo-10-tosyl-1,2,3,3a,4,5,10,10b-octahydropyrrolo[3,4-a]carbazol-4-yl)propanoate
A solution of 2-nitrosotoluene (0.035 g, 0.29 mmol) and ethyl 3-((3aS*,4R*,10bS*)-2-methyl-1,3-dioxo-10-tosyl-1,2,3,3a,4,10,10a,10b-octahydropyrrolo[3,4-a]carbazol-4-yl)propanoate (0.150 g, 0.29 mmol) in dry DCM (10 mL) was stirred at room temperature for 24 hours. The solvent was removed under reduced pressure to give the crude green solid product which was purified by column chromatography (petrol (40/60)–ethyl acetate 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give ethyl 3-((3aS*,4S*,5S*,10bS*)-5-(hydroxy(o-tolyl)amino)-2-methyl-1,3-dioxo-10-tosyl-1,2,3,3a,4,5,10,10b-octahydropyrrolo[3,4-a]carbazol-4-yl)propanoate (0.109 g, 0.17 mmol, 59%) as a bright yellow solid.
1) to give ethyl 3-((3aS*,4S*,5S*,10bS*)-5-(hydroxy(o-tolyl)amino)-2-methyl-1,3-dioxo-10-tosyl-1,2,3,3a,4,5,10,10b-octahydropyrrolo[3,4-a]carbazol-4-yl)propanoate (0.109 g, 0.17 mmol, 59%) as a bright yellow solid.
Mp: 190–192 °C; Rf: 0.26 (Pet(40/60)–EA 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CDCl3): δH 7.95 (1H, d, J = 7.2 Hz), 7.61 (2H, d, J = 7.2 Hz), 7.18 (2H, d, J = 7.2 Hz), 7.10 (1H, d, J = 7.2 Hz), 7.05 (1H, t, J = 7.2 Hz), 6.81 (1H, t, J = 7.2 Hz), 6.65 (1H, t, J = 7.5 Hz), 6.19 (1H, t, J = 7.5 Hz), 5.88 (1H, d, J = 7.2 Hz), 5.46 (1H, d, J = 7.2 Hz), 4.99 (1H, br s), 4.95 (1H, d, J = 7.2 Hz), 4.30 (1H, d, J = 4.6 Hz), 4.11 (2H, q, J = 7.0 Hz), 3.80–3.75 (1H, m), 3.01 (3H, s), 2.70–2.54 (4H, m), 2.41 (3H, s), 2.33 (3H, s), 1.92–1.85 (1H, m), 1.24 (3H, t, J = 7.0 Hz). 13C NMR (101 MHz, CDCl3): δC 178.2, 173.3, 149.9, 144.6, 139.9, 137.1, 134.1, 131.9, 130.6, 130.3, 129.4, 128.7, 127.0, 125.7, 124.8, 124.4, 123.6, 122.1, 118.9, 118.0, 115.7, 60.6, 57.8, 45.1, 42.4, 40.0, 32.5, 25.2, 23.3, 21.6, 18.5, 14.3; IR (neat): νmax/cm−1: 3655, 2980, 1702; MS (pNSI): 507.15 (100%, [M − (Tol-N-OH)]), 652.20 (55%, [M + Na]+); HRMS (pNSI): calcd C34H35N3O7S [M + Na]+: 652.2088; observed: 652.2082.
1); 1H NMR (400 MHz, CDCl3): δH 7.95 (1H, d, J = 7.2 Hz), 7.61 (2H, d, J = 7.2 Hz), 7.18 (2H, d, J = 7.2 Hz), 7.10 (1H, d, J = 7.2 Hz), 7.05 (1H, t, J = 7.2 Hz), 6.81 (1H, t, J = 7.2 Hz), 6.65 (1H, t, J = 7.5 Hz), 6.19 (1H, t, J = 7.5 Hz), 5.88 (1H, d, J = 7.2 Hz), 5.46 (1H, d, J = 7.2 Hz), 4.99 (1H, br s), 4.95 (1H, d, J = 7.2 Hz), 4.30 (1H, d, J = 4.6 Hz), 4.11 (2H, q, J = 7.0 Hz), 3.80–3.75 (1H, m), 3.01 (3H, s), 2.70–2.54 (4H, m), 2.41 (3H, s), 2.33 (3H, s), 1.92–1.85 (1H, m), 1.24 (3H, t, J = 7.0 Hz). 13C NMR (101 MHz, CDCl3): δC 178.2, 173.3, 149.9, 144.6, 139.9, 137.1, 134.1, 131.9, 130.6, 130.3, 129.4, 128.7, 127.0, 125.7, 124.8, 124.4, 123.6, 122.1, 118.9, 118.0, 115.7, 60.6, 57.8, 45.1, 42.4, 40.0, 32.5, 25.2, 23.3, 21.6, 18.5, 14.3; IR (neat): νmax/cm−1: 3655, 2980, 1702; MS (pNSI): 507.15 (100%, [M − (Tol-N-OH)]), 652.20 (55%, [M + Na]+); HRMS (pNSI): calcd C34H35N3O7S [M + Na]+: 652.2088; observed: 652.2082.
3h – ethyl 3-((3aS*,4S*,5S*,10bS*)-5-(hydroxy(o-tolyl)amino)-1,3-dioxo-2-phenyl-10-tosyl-1,2,3,3a,4,5,10,10b-octahydropyrrolo[3,4-a]carbazol-4-yl)propanoate
A solution of 2-nitrosotoluene (0.016 g, 0.13 mmol) and ethyl 3-((3aS*,4R*,10bS*)-1,3-dioxo-2-phenyl-10-tosyl-1,2,3,3a,4,10,10a,10b-octahydropyrrolo[3,4-a]carbazol-4-yl)propanoate (0.08 g, 0.13 mmol) in dry DCM (10 mL) was stirred at room temperature for 24 hours. The solvent was removed under reduced pressure to give the crude yellow product which was purified by column chromatography (petrol (40/60)–ethyl acetate 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give ethyl 3-((3aS*,4S*,5S*,10bS*)-5-(hydroxy(o-tolyl)amino)-1,3-dioxo-2-phenyl-10-tosyl-1,2,3,3a,4,5,10,10b-octahydropyrrolo[3,4-a]carbazol-4-yl)propanoate in (0.415 g, 0.059 mmol, 58%) as a bright yellow solid.
1) to give ethyl 3-((3aS*,4S*,5S*,10bS*)-5-(hydroxy(o-tolyl)amino)-1,3-dioxo-2-phenyl-10-tosyl-1,2,3,3a,4,5,10,10b-octahydropyrrolo[3,4-a]carbazol-4-yl)propanoate in (0.415 g, 0.059 mmol, 58%) as a bright yellow solid.
Mp: 182–184 °C; Rf: 0.34 (Pet(40/60)–EA 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CDCl3): δH 7.94 (1H, d, J = 6.7 Hz), 7.64 (2H, d, J = 6.7 Hz), 7.45–7.37 (5H, m), 7.20–7.00 (4H, m), 6.84 (1H, t, J = 6.9 Hz), 6.66 (1H, t, J = 6.9 Hz), 6.24 (1H, t, J = 6.2 Hz), 5.95 (1H, d, J = 6.9 Hz), 5.66 (1H, d, J = 6.4 Hz), 5.20 (1H, d, J = 7.4 Hz), 4.99 (1H, br s), 4.39 (1H, d, J = 4.0 Hz), 4.08 (2H, q, J = 7.2 Hz), 4.03–3.98 (1H, m), 2.72–2.61 (4H, m), 2.42 (3H, s), 2.33 (3H, s), 2.14–2.08 (1H, m). 1.20 (3H, t, J = 7.2 Hz); 13C NMR (101 MHz, CDCl3): δC 177.0, 173.3, 172.2, 150.1, 144.6, 137.1, 134.2, 131.0, 131.8, 130.5, 130.3, 129.4, 129.0, 128.6, 128.5, 127.0, 126.5, 125.8, 124.8, 124.5123.5, 122.2, 119.1, 118.1, 115.6, 60.0, 57.7, 45.3, 42.6, 40.0, 32.4, 23.1, 21.6, 18.5, 14.5; IR (neat): νmax/cm−1: 3858, 3826, 1709, 1595; MS (pNSI): 569.17 (100%, [M − N(OH)(o-Tol)]), 692.24 (30%, [M + H]+); HRMS (pNSI): calcd C39H36N3O7S [M − H]+: 690.2268; observed: 690.2266.
1); 1H NMR (400 MHz, CDCl3): δH 7.94 (1H, d, J = 6.7 Hz), 7.64 (2H, d, J = 6.7 Hz), 7.45–7.37 (5H, m), 7.20–7.00 (4H, m), 6.84 (1H, t, J = 6.9 Hz), 6.66 (1H, t, J = 6.9 Hz), 6.24 (1H, t, J = 6.2 Hz), 5.95 (1H, d, J = 6.9 Hz), 5.66 (1H, d, J = 6.4 Hz), 5.20 (1H, d, J = 7.4 Hz), 4.99 (1H, br s), 4.39 (1H, d, J = 4.0 Hz), 4.08 (2H, q, J = 7.2 Hz), 4.03–3.98 (1H, m), 2.72–2.61 (4H, m), 2.42 (3H, s), 2.33 (3H, s), 2.14–2.08 (1H, m). 1.20 (3H, t, J = 7.2 Hz); 13C NMR (101 MHz, CDCl3): δC 177.0, 173.3, 172.2, 150.1, 144.6, 137.1, 134.2, 131.0, 131.8, 130.5, 130.3, 129.4, 129.0, 128.6, 128.5, 127.0, 126.5, 125.8, 124.8, 124.5123.5, 122.2, 119.1, 118.1, 115.6, 60.0, 57.7, 45.3, 42.6, 40.0, 32.4, 23.1, 21.6, 18.5, 14.5; IR (neat): νmax/cm−1: 3858, 3826, 1709, 1595; MS (pNSI): 569.17 (100%, [M − N(OH)(o-Tol)]), 692.24 (30%, [M + H]+); HRMS (pNSI): calcd C39H36N3O7S [M − H]+: 690.2268; observed: 690.2266.
3i – ethyl 3-((3aS*,4S*,5S*,10bS*)-5-(3,5-dioxo-4-phenyl-1,2,4-triazolidin-1-yl)-2-methyl-1,3-dioxo-10-tosyl-1,2,3,3a,4,5,10,10b-octahydropyrrolo[3,4-a]carbazol-4-yl)propanoate
A solution of 4-phenyl-3H-1,2,4-triazole-3,5(4H)-dione (0.052 g, 0.29 mmol) and ethyl 3-((3aS*,4R*,10bS*)-2-methyl-1,3-dioxo-10-tosyl-1,2,3,3a,4,10,10a,10b-octahydropyrrolo[3,4-a]carbazol-4-yl)propanoate (0.150 g, 0.29 mmol) in dry DCM (10 mL) was stirred at 0 °C for 6 hours. The solvent was removed under reduced pressure to give the crude product which was purified by column chromatography (petrol (40/60)–ethyl acetate 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give ethyl 3-((3aS*,4S*,5S*,10bS*)-5-(3,5-dioxo-4-phenyl-1,2,4-triazolidin-1-yl)-2-methyl-1,3-dioxo-10-tosyl-1,2,3,3a,4,5,10,10b-octahydropyrrolo[3,4-a]carbazol-4-yl)propanoate in (56%, 0.110 g, 0.161 mmol) as a white solid.
1) to give ethyl 3-((3aS*,4S*,5S*,10bS*)-5-(3,5-dioxo-4-phenyl-1,2,4-triazolidin-1-yl)-2-methyl-1,3-dioxo-10-tosyl-1,2,3,3a,4,5,10,10b-octahydropyrrolo[3,4-a]carbazol-4-yl)propanoate in (56%, 0.110 g, 0.161 mmol) as a white solid.
Mp: 264–265 °C; Rf: 0.30 (Pet(40/60)–EA 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CDCl3): δH 8.86 (1H, br s), 7.78 (2H, d, J = 7.0 Hz), 7.71 (1H, d, J = 7.9 Hz), 7.47 (1H, d, J = 7.9 Hz), 7.44–7.40 (1H, m), 7.36–7.30 (3H, m), 7.23–7.17 (2H, m), 7.02 (2H, d, J = 7.0 Hz), 5.66 (1H, d, J = 6.2 Hz), 5.04 (1H, d, J = 6.5 Hz), 4.08 (2H, q, J = 6.2 Hz), 3.44 (1H, dd, J = 6.5, 11.9 Hz), 3.02 (3H, s), 2.68–2.51 (3H, m), 2.15 (3H, s), 2.14–2.09 (1H, m), 1.90–1.82 (1H, m), 1.20 (3H, t, J = 6.2 Hz); 13C NMR (101 MHz, CDCl3): δC 176.6, 173.4, 173.2, 153.6, 152.6, 145.2, 136.8, 135.5, 132.1, 130.7, 129.7, 129.3, 128.6, 127.2, 126.8, 125.6, 124.2, 119.0, 114.8, 114.4, 60.8, 44.3, 42.0, 39.3, 30.9, 25.4, 23.0, 21.5, 14.2; IR (neat): νmax/cm−1: 3659, 1775, 1691; MS (pNSI): 701.23 (100%, [M + NH4]+), 1384.44 (17%, [2M + NH4]+); HRMS (pNSI): calcd C35H34N5O8S [M + H]+: 684.2123; observed: 684.2115.
1); 1H NMR (400 MHz, CDCl3): δH 8.86 (1H, br s), 7.78 (2H, d, J = 7.0 Hz), 7.71 (1H, d, J = 7.9 Hz), 7.47 (1H, d, J = 7.9 Hz), 7.44–7.40 (1H, m), 7.36–7.30 (3H, m), 7.23–7.17 (2H, m), 7.02 (2H, d, J = 7.0 Hz), 5.66 (1H, d, J = 6.2 Hz), 5.04 (1H, d, J = 6.5 Hz), 4.08 (2H, q, J = 6.2 Hz), 3.44 (1H, dd, J = 6.5, 11.9 Hz), 3.02 (3H, s), 2.68–2.51 (3H, m), 2.15 (3H, s), 2.14–2.09 (1H, m), 1.90–1.82 (1H, m), 1.20 (3H, t, J = 6.2 Hz); 13C NMR (101 MHz, CDCl3): δC 176.6, 173.4, 173.2, 153.6, 152.6, 145.2, 136.8, 135.5, 132.1, 130.7, 129.7, 129.3, 128.6, 127.2, 126.8, 125.6, 124.2, 119.0, 114.8, 114.4, 60.8, 44.3, 42.0, 39.3, 30.9, 25.4, 23.0, 21.5, 14.2; IR (neat): νmax/cm−1: 3659, 1775, 1691; MS (pNSI): 701.23 (100%, [M + NH4]+), 1384.44 (17%, [2M + NH4]+); HRMS (pNSI): calcd C35H34N5O8S [M + H]+: 684.2123; observed: 684.2115.
3j – ethyl 3-((3aS*,4S*,5S*,10bS*)-5-(3,5-dioxo-4-phenyl-1,2,4-triazolidin-1-yl)-1,3-dioxo-10-tosyl-1,2,3,3a,4,5,10,10b-octahydropyrrolo[3,4-a]carbazol-4-yl)propanoate
A solution of 4-phenyl-3H-1,2,4-triazole-3,5(4H)-dione (0.037 g, 0.21 mmol) and ethyl 3-((3aS*,4R*,10bS*)-1,3-dioxo-10-tosyl-1,2,3,3a,4,10,10a,10b-octahydropyrrolo[3,4-a]carbazol-4-yl)propanoate (0.105 g, 0.21 mmol) in dry DCM (10 mL) was stirred at 0 °C for 6 h. The solvent was removed to give the crude white solid product which was purified by trituration from DCM to yield ethyl 3-((3aS*,4S*,5S*,10bS*)-5-(3,5-dioxo-4-phenyl-1,2,4-triazolidin-1-yl)-1,3-dioxo-10-tosyl-1,2,3,3a,4,5,10,10b-octahydropyrrolo[3,4-a]carbazol-4-yl)propanoate in (57%, 0.08 g, 0.119 mmol) as a white solid.Mp: 269–271 °C; 1H NMR (300 MHz, d6-DMSO): δH 11.44 (1H, br s), 10.68 (1H, br s), 7.87–7.76 (3H, m), 7.51–7.39 (4H, m), 7.29–7.25 (6H, m), 5.69 (1H, d, J = 6.1 Hz), 5.28 (1H, d, J = 6.8 Hz), 4.05 (2H, q, J = 6.8 Hz), 3.45 (1H, dd, J = 6.8, 11.3 Hz), 3.58–3.52 (1H, m), 2.67–2.65 (1H, m), 2.25 (3H, s), 2.49–2.44 (2H, m), 1.80–1.70 (1H, m), 1.18 (3H, t, J = 6.8 Hz); 13C NMR (101 MHz, d6-DMSO): 178.6, 175.0, 172.99, 154.4, 153.1, 145.5, 136.6, 134.9, 133.1, 131.7, 130.4, 129.4, 128.6, 127.6, 127.1, 126.3, 125.6, 124.5, 119.3, 115.9, 114.9, 60.3, 45.2, 43.0, 38.0, 30.7, 23.6, 21.4, 14.5; IR (neat): νmax/cm−1: 3659, 1775, 1692; MS (pNSI): 687.22 (100%, [M + NH4]+), 1356.41 (27%, [2M + NH4]+), 692.17 (12%, [M + Na]+); HRMS (pNSI): calcd C34H35N6O8S [M + H]+: 687.2232; observed: 687.2229.
3k – (3aS*,5S*,10bS*)-5-(hydroxy(phenyl)amino)-10-tosyl-4,5,10,10b-tetrahydropyrrolo[3,4-a]carbazole-1,3(2H,3aH)-dione
To a stirred round bottomed flask was added 1-tosyl-3-vinyl-1H-indole (100 mg, 0.34 mmol), DCM (5 mL) and 1H-pyrrole-2,5-dione (33 mg, 0.34 mmol) and the resulting solution was heated at reflux for 48 hours. The reaction was cooled to room temperature and nitrosobenzene (36 mg, 0.34 mmol) was added. The reaction was stirred at room temperature for 4 hours before the solvent was removed under reduced pressure to leave the crude product as a white solid. The product was purified by trituration from DCM to give (3aS*,5S*,10bS*)-5-(hydroxy(phenyl)amino)-10-tosyl-4,5,10,10b-tetrahydropyrrolo[3,4-a]carbazole-1,3(2H,3aH)-dione (89%, 151 mg, 0.30 mmol) as a white powder.Mp: 203.7–206.9 °C; Rf: 0.15 (Pet(40/60)–EA 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (300 MHz, DMSO-d6): δH 11.26 (1H, s), 8.45 (1H, s), 7.88 (1H, d, J = 8.3 Hz). 7.76 (2H, d, J = 8.2 Hz), 7.42 (1H, d, J = 7.8 Hz), 7.33 (2H, d, J = 8.2 Hz), 7.29–7.04 (5H, m), 6.89 (1H, t, J = 7.2 Hz), 5.17 (1H, d, J = 7.8 Hz), 4.88 (1H, app t, J = 4.4 Hz), 3.65 (1H, app td, J = 8.8, 5.9 Hz), 2.32 (3H, s), 2.36–2.27 (1H, m), 1.81 (1H, ddd, J = 14.0, 9.4, 5.0 Hz); 13C NMR (101 MHz, DMSO-d6): δC 179.4, 174.7, 152.0, 144.7, 136.4, 135.0, 131.5, 129.8, 128.9, 128.4, 126.6, 124.4, 123.4, 121.2, 120.7, 120.6, 117.0, 114.4, 56.8, 41.5, 40.6, 25.2, 20.9; IR (neat): νmax/cm−1 3452, 2981, 1715; MS (pNSI): 393.1, (100%, (M − (C6H5NOH)+)), 502.1 (14%, (M + H)+), 519.2 (96%, (M + NH4)+), 524.1 (17%, (M + Na)+), 1003.3 (40%, (2M + H)+), 1025.3 (15%, (2M + Na)+); HRMS (pNSI): calcd for C27H24N3O5S [M + H]+: 502.1431; observed: 502.1428.
1); 1H NMR (300 MHz, DMSO-d6): δH 11.26 (1H, s), 8.45 (1H, s), 7.88 (1H, d, J = 8.3 Hz). 7.76 (2H, d, J = 8.2 Hz), 7.42 (1H, d, J = 7.8 Hz), 7.33 (2H, d, J = 8.2 Hz), 7.29–7.04 (5H, m), 6.89 (1H, t, J = 7.2 Hz), 5.17 (1H, d, J = 7.8 Hz), 4.88 (1H, app t, J = 4.4 Hz), 3.65 (1H, app td, J = 8.8, 5.9 Hz), 2.32 (3H, s), 2.36–2.27 (1H, m), 1.81 (1H, ddd, J = 14.0, 9.4, 5.0 Hz); 13C NMR (101 MHz, DMSO-d6): δC 179.4, 174.7, 152.0, 144.7, 136.4, 135.0, 131.5, 129.8, 128.9, 128.4, 126.6, 124.4, 123.4, 121.2, 120.7, 120.6, 117.0, 114.4, 56.8, 41.5, 40.6, 25.2, 20.9; IR (neat): νmax/cm−1 3452, 2981, 1715; MS (pNSI): 393.1, (100%, (M − (C6H5NOH)+)), 502.1 (14%, (M + H)+), 519.2 (96%, (M + NH4)+), 524.1 (17%, (M + Na)+), 1003.3 (40%, (2M + H)+), 1025.3 (15%, (2M + Na)+); HRMS (pNSI): calcd for C27H24N3O5S [M + H]+: 502.1431; observed: 502.1428.
3l – (3aS*,5S*,10bS*)-5-(hydroxy(o-tolyl)amino)-10-tosyl-4,5,10,10b-tetrahydropyrrolo[3,4-a]carbazole-1,3(2H,3aH)-dione
To a stirred round bottomed flask was added 1-tosyl-3-vinyl-1H-indole (100 mg, 0.34 mmol), DCM (5 mL) and 1H-pyrrole-2,5-dione (33 mg, 0.34 mmol) was added and the resulting solution was heated at reflux for 48 hours. The reaction was cooled to room temperature and 1-methyl-2-nitrosobenzene (41 mg, 0.34 mmol) was added. The reaction was stirred at room temperature for 4 hours before the solvent was removed under reduced pressure to leave the crude product as a pale yellow solid. The product was purified by column chromatography (petrol (40/60)–ether–DCM 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, column diameter = 2 cm, silica = 14 cm) to give (3aS*,5S*,10bS*)-5-(hydroxy(o-tolyl)amino)-10-tosyl-4,5,10,10b-tetrahydropyrrolo[3,4-a]carbazole-1,3(2H,3aH)-dione (82%, 143 mg, 0.28 mmol) as a yellow powder.
1, column diameter = 2 cm, silica = 14 cm) to give (3aS*,5S*,10bS*)-5-(hydroxy(o-tolyl)amino)-10-tosyl-4,5,10,10b-tetrahydropyrrolo[3,4-a]carbazole-1,3(2H,3aH)-dione (82%, 143 mg, 0.28 mmol) as a yellow powder.
Mp: 171.1–174.0 °C; Rf: 0.13 (Pet(40/60)–EA 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CDCl3): δ 7.90 (1H, d, J = 8.4 Hz), 7.73 (2H, d, J = 8.2 Hz), 7.77 (1H, s), 7.35 (2H, dd, J = 15.7, 7.9 Hz), 7.20 (3H, app d, J = 8.2 Hz), 7.09 (2H, d, J = 7.5 Hz), 7.07–7.00 (2H, m), 5.17 (1H, d, J = 8.1 Hz), 4.75 (1H, s), 4.39 (1H, app t, J = 5.1 Hz), 3.80 (1H, app q, J = 8.3, 5.7 Hz), 2.64 (1H, app dt, J = 13.1, 5.5 Hz), 2.34 (3H, s), 2.26 (3H, s), 1.96 (1H, ddd, J = 15.9, 7.9, 3.7 Hz); 13C NMR (75 MHz, CDCl3): δC 178.3, 173.4, 149.3, 144.7, 137.4, 135.8, 131.3, 130.9, 129.8, 129.6, 129.5, 129.0, 127.0, 126.3, 125.1, 124.7, 123.7, 121.5, 120.1, 115.3, 57.3, 42.0, 40.8, 26.0, 21.4, 18.3; IR (neat): νmax/cm−1 3294, 2981, 1713; MS (pAPCI): 293.1 (16%), 332.1 (13%), 342.1 (16%), 393.1 (100%, (M − (o-Tol)N(OH))+), 489.1 (54%, (M − H2O)+), 516.2 (26%, (M + H)+); HRMS (pAPCI): calcd for C28H26N3O5S [M + H]+: 516.1588; observed: 516.1576.
1); 1H NMR (400 MHz, CDCl3): δ 7.90 (1H, d, J = 8.4 Hz), 7.73 (2H, d, J = 8.2 Hz), 7.77 (1H, s), 7.35 (2H, dd, J = 15.7, 7.9 Hz), 7.20 (3H, app d, J = 8.2 Hz), 7.09 (2H, d, J = 7.5 Hz), 7.07–7.00 (2H, m), 5.17 (1H, d, J = 8.1 Hz), 4.75 (1H, s), 4.39 (1H, app t, J = 5.1 Hz), 3.80 (1H, app q, J = 8.3, 5.7 Hz), 2.64 (1H, app dt, J = 13.1, 5.5 Hz), 2.34 (3H, s), 2.26 (3H, s), 1.96 (1H, ddd, J = 15.9, 7.9, 3.7 Hz); 13C NMR (75 MHz, CDCl3): δC 178.3, 173.4, 149.3, 144.7, 137.4, 135.8, 131.3, 130.9, 129.8, 129.6, 129.5, 129.0, 127.0, 126.3, 125.1, 124.7, 123.7, 121.5, 120.1, 115.3, 57.3, 42.0, 40.8, 26.0, 21.4, 18.3; IR (neat): νmax/cm−1 3294, 2981, 1713; MS (pAPCI): 293.1 (16%), 332.1 (13%), 342.1 (16%), 393.1 (100%, (M − (o-Tol)N(OH))+), 489.1 (54%, (M − H2O)+), 516.2 (26%, (M + H)+); HRMS (pAPCI): calcd for C28H26N3O5S [M + H]+: 516.1588; observed: 516.1576.
3m – (3aS*,5S*,10bS*)-5-((S*)-hydroxy(perfluorophenyl)methyl)-10-tosyl-4,5,10,10b-tetrahydropyrrolo[3,4-a]carbazole-1,3(2H,3aH)-dione
To a stirred round bottomed flask was added 1-tosyl-3-vinyl-1H-indole (100 mg, 0.34 mmol), DCM (5 mL) and 1H-pyrrole-2,5-dione (33 mg, 0.34 mmol). The resulting solution was heated at reflux for 48 hours. The reaction was cooled to 0 °C and pentafluorobenzaldehyde (67 mg, 0.34 mmol) and DMAC (1 M in hexane, 0.34 mL, 0.34 mmol) were added. The solution was stirred at 0 °C for 1 hour and then warmed to room temperature for 18 hours. The reaction was poured into sodium bicarbonate (15 mL) and extracted with DCM. The combined organic layers were dried with MgSO4, filtered and the solvent was removed under reduced pressure to give the crude product as an off white solid. The product was purified by column chromatography (diameter = 1.5 cm, silica = 15 cm, eluent = petrol (40/60)–EA 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give (3aS*,5S*,10bS*)-5-((S*)-hydroxy(perfluorophenyl)methyl)-10-tosyl-4,5,10,10b-tetrahydropyrrolo[3,4-a]carbazole-1,3(2H,3aH)-dione (71%, 0.1427 g, 0.24 mmol) as an off white solid.
1) to give (3aS*,5S*,10bS*)-5-((S*)-hydroxy(perfluorophenyl)methyl)-10-tosyl-4,5,10,10b-tetrahydropyrrolo[3,4-a]carbazole-1,3(2H,3aH)-dione (71%, 0.1427 g, 0.24 mmol) as an off white solid.
Mp: 181.3–185.1 °C; Rf: 0.22 (Pet(40/60)–EA 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CDCl3): δH 8.03 (1H, br s), 7.81 (3H, app d, J = 7.9 Hz), 7.28 (1H, d, J = 8.0 Hz), 7.25–7.22 (3H, m), 7.16 (1H, app t, J = 7.6 Hz), 5.08 (1H, d, J = 8.3 Hz), 5.05 (1H, d, J = 7.4 Hz), 3.61–3.66 (1H, m), 3.49–3.57 (1H, m), 2.34 (3H, s), 2.09 (1H, dd, J = 14.0, 4.5 Hz), 1.74 (1H, app td, J = 13.9, 5.3 Hz); 13C NMR (101 MHz, CDCl3): δC 177.4, 173.0, 145.3, 137.4, 135.5, 129.8, 129.7, 129.1, 127.1, 125.5, 123.9, 120.2, 120.0, 115.3, 70.2, 42.5, 40.3, 36.8, 28.5, 21.7; IR (neat): νmax/cm−1 3240, 2981, 1717; MS (pAPCI): 157.0 (79%), 221.1 (61%), 393.1 (15%, (M − (C6F5COH)+)), 443.1 (51%), 573.1 (8%, (M − H2O)+), 591.1 (100%, (M + H)+); HRMS (pAPCI): calcd for C28H20F5N2O5S [M + H]+: 591.1008; observed: 591.1001. Note: 13C NMR missing peaks due to C–F coupling.
1); 1H NMR (400 MHz, CDCl3): δH 8.03 (1H, br s), 7.81 (3H, app d, J = 7.9 Hz), 7.28 (1H, d, J = 8.0 Hz), 7.25–7.22 (3H, m), 7.16 (1H, app t, J = 7.6 Hz), 5.08 (1H, d, J = 8.3 Hz), 5.05 (1H, d, J = 7.4 Hz), 3.61–3.66 (1H, m), 3.49–3.57 (1H, m), 2.34 (3H, s), 2.09 (1H, dd, J = 14.0, 4.5 Hz), 1.74 (1H, app td, J = 13.9, 5.3 Hz); 13C NMR (101 MHz, CDCl3): δC 177.4, 173.0, 145.3, 137.4, 135.5, 129.8, 129.7, 129.1, 127.1, 125.5, 123.9, 120.2, 120.0, 115.3, 70.2, 42.5, 40.3, 36.8, 28.5, 21.7; IR (neat): νmax/cm−1 3240, 2981, 1717; MS (pAPCI): 157.0 (79%), 221.1 (61%), 393.1 (15%, (M − (C6F5COH)+)), 443.1 (51%), 573.1 (8%, (M − H2O)+), 591.1 (100%, (M + H)+); HRMS (pAPCI): calcd for C28H20F5N2O5S [M + H]+: 591.1008; observed: 591.1001. Note: 13C NMR missing peaks due to C–F coupling.
3n – (3aS*,5S*,10bS*)-5-(3,5-dioxo-4-phenyl-1,2,4-triazolidin-1-yl)-10-tosyl-4,5,10,10b-tetrahydropyrrolo[3,4-a]carbazole-1,3(2H,3aH)-dione
To a stirred round bottomed flask was added 1-tosyl-3-vinyl-1H-indole (100 mg, 0.34 mmol), DCM (5 mL) and 1H-pyrrole-2,5-dione (33 mg, 0.34 mmol). The resulting solution was heated at reflux for 48 hours. The reaction was cooled to 0 °C and PTAD was added. The solution was stirred at 0 °C for 4 hours and the solvent was removed under reduced pressure to give the crude product as pale red. The product was purified by column chromatography (diameter = 1.5 cm, silica = 17 cm, eluent = petrol (40/60)–EA 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give (3aS*,5S*,10bS*)-5-(3,5-dioxo-4-phenyl-1,2,4-triazolidin-1-yl)-10-tosyl-4,5,10,10b-tetrahydropyrrolo[3,4-a]carbazole-1,3(2H,3aH)-dione (23%, 0.044 g, 0.08 mmol) as an off white solid.
1) to give (3aS*,5S*,10bS*)-5-(3,5-dioxo-4-phenyl-1,2,4-triazolidin-1-yl)-10-tosyl-4,5,10,10b-tetrahydropyrrolo[3,4-a]carbazole-1,3(2H,3aH)-dione (23%, 0.044 g, 0.08 mmol) as an off white solid.
Mp: 206.4–209.7 °C; Rf: 0.10 (Pet(40/60)–EA 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, DMSO-d6): δC 11.37 (1H, s), 10.77 (1H, s), 7.82 (2H, d, J = 8.3 Hz), 7.72 (1H, d, J = 7.9 Hz), 7.48–7.43 (2H, m), 7.42–7.35 (4H, m), 7.28 (2H, d, J = 8.5 Hz), 7.25–7.18 (2H, m), 5.46 (1H, app t, J = 4.9 Hz), 5.19 (1H, d, J = 7.7 Hz), 3.77–3.65 (1H, m), 2.41 (1H, app dt, J = 9.8, 5.1 Hz), 2.26 (3H, s), 2.24–2.16 (1H, m); 13C NMR (101 MHz, CDCl3): δH 178.8, 173.6, 153.6, 152.4, 145.4, 136.9, 135.3, 132.2, 130.9, 129.9, 129.2, 128.4, 127.5, 126.9, 125.7, 125.6, 124.2, 119.3, 115.1, 114.8, 47.3, 41.5, 40.2, 28.8, 21.7; IR (neat): νmax/cm−1 3194, 2981, 2980, 1699; MS (pNSI): 587.2 (100% (M + NH4)+), 592.1 (30% (M + Na)+); HRMS (pNSI): calcd for C29H27N6O6S [M + NH4]+: 587.1707; observed: 587.1706.
1); 1H NMR (400 MHz, DMSO-d6): δC 11.37 (1H, s), 10.77 (1H, s), 7.82 (2H, d, J = 8.3 Hz), 7.72 (1H, d, J = 7.9 Hz), 7.48–7.43 (2H, m), 7.42–7.35 (4H, m), 7.28 (2H, d, J = 8.5 Hz), 7.25–7.18 (2H, m), 5.46 (1H, app t, J = 4.9 Hz), 5.19 (1H, d, J = 7.7 Hz), 3.77–3.65 (1H, m), 2.41 (1H, app dt, J = 9.8, 5.1 Hz), 2.26 (3H, s), 2.24–2.16 (1H, m); 13C NMR (101 MHz, CDCl3): δH 178.8, 173.6, 153.6, 152.4, 145.4, 136.9, 135.3, 132.2, 130.9, 129.9, 129.2, 128.4, 127.5, 126.9, 125.7, 125.6, 124.2, 119.3, 115.1, 114.8, 47.3, 41.5, 40.2, 28.8, 21.7; IR (neat): νmax/cm−1 3194, 2981, 2980, 1699; MS (pNSI): 587.2 (100% (M + NH4)+), 592.1 (30% (M + Na)+); HRMS (pNSI): calcd for C29H27N6O6S [M + NH4]+: 587.1707; observed: 587.1706.
3o – (3aS*,5S*,10bS*)-5-(hydroxy(o-tolyl)amino)-7-methoxy-10-tosyl-4,5,10,10b-tetrahydropyrrolo[3,4-a]carbazole-1,3(2H,3aH)-dione
To a stirred round bottomed flask was added 6-methoxy-1-tosyl-3-vinyl-1H-indole (111 mg, 0.34 mmol), DCM (5 mL) and 1H-pyrrole-2,5-dione (33 mg, 0.34 mmol). The resulting solution was heated at reflux for 48 hours. The reaction was cooled to room temperature and 1-methyl-2-nitrosobenzene (41 mg, 0.34 mmol) was added. The reaction was stirred at room temperature for 24 hours before the solvent was removed under reduced pressure to leave the crude product as a pale yellow solid. The product was purified by column chromatography (petrol (40/60)–ethyl acetate 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give (3aS*,5S*,10bS*)-5-(hydroxy(o-tolyl)amino)-7-methoxy-10-tosyl-4,5,10,10b-tetrahydropyrrolo[3,4-a]carbazole-1,3(2H,3aH)-dione (76%, 141 mg, 0.26 mmol) as a light brown solid.
1) to give (3aS*,5S*,10bS*)-5-(hydroxy(o-tolyl)amino)-7-methoxy-10-tosyl-4,5,10,10b-tetrahydropyrrolo[3,4-a]carbazole-1,3(2H,3aH)-dione (76%, 141 mg, 0.26 mmol) as a light brown solid.
Mp: 185 °C decomposed; Rf: 0.26 (Pet(40/60)–EA 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3); 1H NMR (400 MHz, DMSO-d6): δH 11.19 (1H, s), 8.34 (1H, s), 7.69 (1H, d, J = 9.1 Hz), 7.61 (2H, d, J = 8.2 Hz), 7.27 (2H, d, J = 8.2 Hz), 7.08–6.97 (2H, m), 6.95–6.85 (2H, m), 6.72 (1H, dd, J = 9.1, 2.4 Hz), 6.40 (1H, s), 5.07 (1H, d, J = 7.9 Hz), 4.20 (1H, app t, J = 4.4 Hz), 3.76 (1H, app q, J = 8.4 Hz), 3.47 (3H, s), 2.53–2.47 (1H, m), 2.28 (s, 3H), 2.08 (s, 3H), 1.75 (1H, ddd, J = 13.6, 9.8, 4.4 Hz); 13C NMR (101 MHz, DMSO-d6): δC 180.5, 175.4, 156.3, 151.7, 145.3, 134.7, 133.1, 131.1, 131.0, 130.8, 130.3, 129.7, 127.1, 126.4, 124.6, 122.1, 121.2, 115.9, 113.8, 103.3, 57.1, 55.3, 42.2, 26.9, 21.5, 21.3, 18.5; IR (neat): ν(max)/cm−1 3388, 3071, 2552, 1727; MS (pNSI): 355.1 (50%), 371.1 (100%), 423.1 (57%), 445.1 (30%), 546.2 (5%, (M + H)+), 568.2 (16%, (M + Na)+), 584.1 (21%); HRMS (pNSI): calcd for C29H28N3O6S1 [M + H]+: 546.1693; observed: 546.1690.
3); 1H NMR (400 MHz, DMSO-d6): δH 11.19 (1H, s), 8.34 (1H, s), 7.69 (1H, d, J = 9.1 Hz), 7.61 (2H, d, J = 8.2 Hz), 7.27 (2H, d, J = 8.2 Hz), 7.08–6.97 (2H, m), 6.95–6.85 (2H, m), 6.72 (1H, dd, J = 9.1, 2.4 Hz), 6.40 (1H, s), 5.07 (1H, d, J = 7.9 Hz), 4.20 (1H, app t, J = 4.4 Hz), 3.76 (1H, app q, J = 8.4 Hz), 3.47 (3H, s), 2.53–2.47 (1H, m), 2.28 (s, 3H), 2.08 (s, 3H), 1.75 (1H, ddd, J = 13.6, 9.8, 4.4 Hz); 13C NMR (101 MHz, DMSO-d6): δC 180.5, 175.4, 156.3, 151.7, 145.3, 134.7, 133.1, 131.1, 131.0, 130.8, 130.3, 129.7, 127.1, 126.4, 124.6, 122.1, 121.2, 115.9, 113.8, 103.3, 57.1, 55.3, 42.2, 26.9, 21.5, 21.3, 18.5; IR (neat): ν(max)/cm−1 3388, 3071, 2552, 1727; MS (pNSI): 355.1 (50%), 371.1 (100%), 423.1 (57%), 445.1 (30%), 546.2 (5%, (M + H)+), 568.2 (16%, (M + Na)+), 584.1 (21%); HRMS (pNSI): calcd for C29H28N3O6S1 [M + H]+: 546.1693; observed: 546.1690.
3p – (3aS*,5S*,10bS*)-5-((S*)-hydroxy(perfluorophenyl)methyl)-7-methoxy-2-methyl-10-tosyl-4,5,10,10b-tetrahydropyrrolo[3,4-a]carbazole-1,3(2H,3aH)-dione
To a stirred round bottomed flask was added 5-methoxy-1-tosyl-3-vinyl-1H-indole (112 mg, 0.34 mmol), DCM (5 mL) and 1-methyl-1H-pyrrole-2,5-dione (38 mg, 0.34 mmol). The reaction was heated at reflux for 48 hours. The reaction was cooled to −78 °C and pentafluorobenzaldehyde (0.04 mL, 0.34 mmol) and DMAC (1 M in hexane, 0.34 mL, 0.34 mmol) were added. The solution was stirred at −78 °C for 15 minutes and then at room temperature for 18 hours. The solvent was removed under reduced pressure to give the crude product as an off white solid. The product was purified by column chromatography (column diameter = 2 cm, silica = 15 cm, eluent = petrol (40/60)–EA 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give a 8
1) to give a 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of (3aS*,5S*,10bS*)-5-((S*)-hydroxy(perfluorophenyl)methyl)-7-methoxy-2-methyl-10-tosyl-4,5,10,10b-tetrahydropyrrolo[3,4-a]carbazole-1,3(2H,3aH)-dione and (3aS*,5S*,10bS*)-5-((R*)-hydroxy(perfluorophenyl)methyl)-7-methoxy-2-methyl-10-tosyl-4,5,10,10b-tetrahydropyrrolo[3,4-a]carbazole-1,3(2H,3aH)-dione (70%, 0.151 mg, 0.24 mmol) as a white powder.
1 mixture of (3aS*,5S*,10bS*)-5-((S*)-hydroxy(perfluorophenyl)methyl)-7-methoxy-2-methyl-10-tosyl-4,5,10,10b-tetrahydropyrrolo[3,4-a]carbazole-1,3(2H,3aH)-dione and (3aS*,5S*,10bS*)-5-((R*)-hydroxy(perfluorophenyl)methyl)-7-methoxy-2-methyl-10-tosyl-4,5,10,10b-tetrahydropyrrolo[3,4-a]carbazole-1,3(2H,3aH)-dione (70%, 0.151 mg, 0.24 mmol) as a white powder.
Major diastereomer: Mp: 136.7–139.0 °C; Rf: 0.40 (Pet(40/60)–EA 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CDCl3): δH 7.76 (2H, d, J = 8.3 Hz), 7.69 (1H, d, J = 9.1 Hz), 7.22 (2H, d, J = 8.3 Hz), 6.83 (1H, dd, J = 9.1, 2.5 Hz), 6.72 (1H, d, J = 2.5 Hz), 5.08 (1H, d, J = 8.3 Hz), 4.87 (1H, d, J = 7.4 Hz), 3.71 (3H, s), 3.58–3.47 (2H, m), 2.94 (3H, s), 2.34 (3H, s), 2.11–2.04 (1H, m), 1.60 (1H, td, J = 13.9, 5.3 Hz); 13C NMR (101 MHz, CDCl3): δC 177.7, 173.2, 156.8, 145.2, 135.2, 132.0, 130.9, 130.3, 129.8, 127.0, 120.6, 116.2, 114.0, 102.6, 70.2, 55.6, 41.5, 39.0, 36.9, 28.6, 25.3, 21.7; IR (neat): νmax/cm−1 2981, 2884, 1709; MS (pAPCI): 157.0 (80%), 221.1 (92%), 281.1 (49%) 475.1 (94%), 635.1 (100%, (M + H)+); HRMS (pAPCI): calcd for C30H24F5N2O6S [M + H]+: 635.1270; observed: 635.1266. Note: 13C NMR missing peaks due to C–F coupling.
1); 1H NMR (400 MHz, CDCl3): δH 7.76 (2H, d, J = 8.3 Hz), 7.69 (1H, d, J = 9.1 Hz), 7.22 (2H, d, J = 8.3 Hz), 6.83 (1H, dd, J = 9.1, 2.5 Hz), 6.72 (1H, d, J = 2.5 Hz), 5.08 (1H, d, J = 8.3 Hz), 4.87 (1H, d, J = 7.4 Hz), 3.71 (3H, s), 3.58–3.47 (2H, m), 2.94 (3H, s), 2.34 (3H, s), 2.11–2.04 (1H, m), 1.60 (1H, td, J = 13.9, 5.3 Hz); 13C NMR (101 MHz, CDCl3): δC 177.7, 173.2, 156.8, 145.2, 135.2, 132.0, 130.9, 130.3, 129.8, 127.0, 120.6, 116.2, 114.0, 102.6, 70.2, 55.6, 41.5, 39.0, 36.9, 28.6, 25.3, 21.7; IR (neat): νmax/cm−1 2981, 2884, 1709; MS (pAPCI): 157.0 (80%), 221.1 (92%), 281.1 (49%) 475.1 (94%), 635.1 (100%, (M + H)+); HRMS (pAPCI): calcd for C30H24F5N2O6S [M + H]+: 635.1270; observed: 635.1266. Note: 13C NMR missing peaks due to C–F coupling.
3q – (3aS*,5S*,10bS*)-5-(3,5-dioxo-4-phenyl-1,2,4-triazolidin-1-yl)-7-methoxy-10-tosyl-4,5,10,10b-tetrahydropyrrolo[3,4-a]carbazole-1,3(2H,3aH)-dione
To a stirred round bottomed flask was added 5-methoxy-1-tosyl-3-vinyl-1H-indole (112 mg, 0.34 mmol), DCM (5 mL) and 1H-pyrrole-2,5-dione (33 mg, 0.34 mmol). The reaction was heated at reflux for 48 hours. The reaction was cooled to 0 °C and PTAD (60 mg, 0.34 mmol) was added. The reaction was stirred at 0 °C for 4 hours before the solvent was removed under reduced pressure to give the crude product as a pale red solid. The product was purified by column chromatography (column diameter = 2 cm, silica = 16 cm, eluent = petrol (40/60)–EA 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give (3aS*,5S*,10bS*)-5-(3,5-dioxo-4-phenyl-1,2,4-triazolidin-1-yl)-7-methoxy-10-tosyl-4,5,10,10b-tetrahydropyrrolo[3,4-a]carbazole-1,3(2H,3aH)-dione (75%, 0.152 mg, 0.25 mmol) as a pale yellow powder.
1) to give (3aS*,5S*,10bS*)-5-(3,5-dioxo-4-phenyl-1,2,4-triazolidin-1-yl)-7-methoxy-10-tosyl-4,5,10,10b-tetrahydropyrrolo[3,4-a]carbazole-1,3(2H,3aH)-dione (75%, 0.152 mg, 0.25 mmol) as a pale yellow powder.
Mp: 189.9–193.3 °C; Rf: 0.07 (Pet(40/60)–EA 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CDCl3): δH 9.31 (1H, br), 7.67 (2H, d, J = 7.5 Hz), 7.55 (1H, d, J = 8.8 Hz), 7.38–7.23 (5H, m), 7.00 (2H, d, J = 7.8 Hz), 6.90 (1H, s), 6.80 (1H, d, J = 8.9 Hz), 5.53 (1H, br s), 5.18 (1H, d, J = 6.7 Hz), 3.74–3.69 (1H, m), 3.64 (3H, s), 2.48–2.55 (1H, m), 2.17 (3H, s), 2.12–2.02 (2H, m); 13C NMR (101 MHz, CDCl3): δC 179.0, 173.9, 156.9, 153.7, 152.7, 145.2, 135.1, 132.7, 131.5, 131.0, 129.8, 129.2, 128.4, 128.0, 127.2, 125.6, 115.9, 115.5, 114.3, 101.9, 55.7, 47.6, 41.6, 40.1, 28.8, 21.6; IR (neat): νmax/cm−1 2972, 2885, 1781, 1709; MS (pNSI): 617.2 (69%, (M + NH4)+), 622.1 (100%, (M + Na)+), 644.1 (48%); HRMS (pNSI): calcd for C30H29N6O7S [M + NH4]+: 617.1813; observed: 617.1817.
1); 1H NMR (400 MHz, CDCl3): δH 9.31 (1H, br), 7.67 (2H, d, J = 7.5 Hz), 7.55 (1H, d, J = 8.8 Hz), 7.38–7.23 (5H, m), 7.00 (2H, d, J = 7.8 Hz), 6.90 (1H, s), 6.80 (1H, d, J = 8.9 Hz), 5.53 (1H, br s), 5.18 (1H, d, J = 6.7 Hz), 3.74–3.69 (1H, m), 3.64 (3H, s), 2.48–2.55 (1H, m), 2.17 (3H, s), 2.12–2.02 (2H, m); 13C NMR (101 MHz, CDCl3): δC 179.0, 173.9, 156.9, 153.7, 152.7, 145.2, 135.1, 132.7, 131.5, 131.0, 129.8, 129.2, 128.4, 128.0, 127.2, 125.6, 115.9, 115.5, 114.3, 101.9, 55.7, 47.6, 41.6, 40.1, 28.8, 21.6; IR (neat): νmax/cm−1 2972, 2885, 1781, 1709; MS (pNSI): 617.2 (69%, (M + NH4)+), 622.1 (100%, (M + Na)+), 644.1 (48%); HRMS (pNSI): calcd for C30H29N6O7S [M + NH4]+: 617.1813; observed: 617.1817.
3r – 6-(3,5-dioxo-4-phenyl-1,2,4-triazolidin-1-yl)-2-phenyl-11-tosyl-5,6-dihydro-[1,2,4]triazolo[1′,2′:1,2]pyridazino[3,4-b]indole-1,3(2H,11H)-dione
To a stirred round bottomed flask was added 1-tosyl-3-vinyl-1H-indole (100 mg, 0.34 mmol) and DCM (5 mL). The resulting solution was cooled to −78 °C and then PTAD (70 mg, 0.34 mmol) was added. The reaction was stirred at −78 °C for 3.5 hours. The reaction was warmed to 0 °C and a further equivalent of PTAD (60 mg, 0.34 mmol) was added. The reaction was stirred 0 °C for 4 hours, resulting in the formation of a white precipitate. The reaction mixture was filtered and 6-(3,5-dioxo-4-phenyl-1,2,4-triazolidin-1-yl)-2-phenyl-11-tosyl-5,6-dihydro-[1,2,4]triazolo[1′,2′:1,2]pyridazino[3,4-b]indole-1,3(2H,11H)-dione (65%, 134 mg, 0.207 mmol) was recovered as a white powder.Mp: 227.8–230.6 °C; 1H NMR (400 MHz, DMSO-d6): δH 10.76 (1H, s, NH), 7.91 (1H, d, J = 7.8 Hz), 7.61 (2H, d, J = 8.3 Hz), 7.48–7.24 (15H, m), 5.52 (1H, s), 4.76 (1H, d, J = 13.8 Hz), 3.86 (1H, d, J = 13.8 Hz), 2.24 (3H, s); 13C NMR (101 MHz, DMSO-d6): δC 154.3, 153.5, 150.6, 149.5, 146.0, 135.1, 133.3, 133.0, 131.7, 131.5, 130.4, 129.6, 129.5, 129.4, 128.8, 128.1, 127.6, 127.5, 126.9, 125.9, 125.4, 119.3, 116.8, 104.1, 48.8, 43.3, 21.6; IR (neat): νmax/cm−1 2971, 2883, 1714; MS (pNSI): 263.0 (36%), 345.0 (51%), 371.1 (42%), 665.2 (89%, (M + NH4)+), 670.1 (100%, (M + Na)+); HRMS (pNSI): calcd for C33H25N7NaO6S [M + Na]+: 670.1479; observed: 670.1475.
3s – 6-(hydroxy(phenyl)amino)-2-phenyl-11-tosyl-5,6-dihydro-[1,2,4]triazolo[1′,2′:1,2]pyridazino[3,4-b]indole-1,3(2H,11H)-dione
To a stirred round bottomed flask was added 1-tosyl-3-vinyl-1H-indole (100 mg, 0.34 mmol) and DCM (5 mL) and the solution was cooled to −78 °C. To this solution PTAD (70 mg, 0.34 mmol) was added and the reaction was stirred at −78 °C for 3.5 hours. The reaction was warmed to room temperature nitrosobenzene (44 mg, 0.34 mmol) was added and the reaction was stirred for 18 hours. The solvent was removed under reduced pressure to leave the crude product as a pale yellow oil. The product was purified by column chromatography (column diameter = 1 cm, silica = 16 cm, eluent = petrol (40/60)–ether–DCM 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give 6-(hydroxy(phenyl)amino)-2-phenyl-11-tosyl-5,6-dihydro-[1,2,4]triazolo[1′,2′:1,2]pyridazino[3,4-b]indole-1,3(2H,11H)-dione (72%, 141 mg, 0.24 mmol) as a white powder.
1) to give 6-(hydroxy(phenyl)amino)-2-phenyl-11-tosyl-5,6-dihydro-[1,2,4]triazolo[1′,2′:1,2]pyridazino[3,4-b]indole-1,3(2H,11H)-dione (72%, 141 mg, 0.24 mmol) as a white powder.
Mp: 176.1–180.0 °C; Rf: 0.48 (Pet(40/60)–Et2O 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CDCl3): δH 8.03 (1H, d, J = 8.3 Hz), 7.62 (2H, d, J = 8.1 Hz), 7.56 (2H, d, J = 7.6 Hz), 7.46 (2H, app t, J = 7.7 Hz), 7.42–7.36 (1H, m), 7.23–7.07 (7H, m), 7.05–6.99 (2H, m), 6.59 (1H, d, J = 7.4 Hz), 5.81 (1H, br s), 5.18 (1H, d, J = 13.5 Hz), 4.59 (1H, s), 3.23 (1H, d, J = 13.5 Hz), 2.30 (3H, s); 13C NMR (101 MHz, DMSO-d6): δC 152.7, 152.2, 150.5, 146.0, 134.9, 132.9, 132.5, 131.8, 130.4, 129.8, 129.3, 129.2, 128.5, 127.3, 127.3, 125.5, 125.4, 122.5, 119.8, 117.6, 116.6, 108.6, 55.9, 44.6, 21.6; IR (neat): νmax/cm−1 2981, 2884, 1714; MS (pAPCI): 138.1 (100%), 157.0 (95%), 213.1 (50%), 248.1 (86%), 279.1 (62%), 317.1 (33%), 333.1 (29%), 471.1 (31%), 564.2 (11%), 580.2 (10%, (M + H)+); HRMS (pAPCI): calcd for C31H26N5O5S [M + H]+: 580.1649; observed: 580.1640.
1); 1H NMR (400 MHz, CDCl3): δH 8.03 (1H, d, J = 8.3 Hz), 7.62 (2H, d, J = 8.1 Hz), 7.56 (2H, d, J = 7.6 Hz), 7.46 (2H, app t, J = 7.7 Hz), 7.42–7.36 (1H, m), 7.23–7.07 (7H, m), 7.05–6.99 (2H, m), 6.59 (1H, d, J = 7.4 Hz), 5.81 (1H, br s), 5.18 (1H, d, J = 13.5 Hz), 4.59 (1H, s), 3.23 (1H, d, J = 13.5 Hz), 2.30 (3H, s); 13C NMR (101 MHz, DMSO-d6): δC 152.7, 152.2, 150.5, 146.0, 134.9, 132.9, 132.5, 131.8, 130.4, 129.8, 129.3, 129.2, 128.5, 127.3, 127.3, 125.5, 125.4, 122.5, 119.8, 117.6, 116.6, 108.6, 55.9, 44.6, 21.6; IR (neat): νmax/cm−1 2981, 2884, 1714; MS (pAPCI): 138.1 (100%), 157.0 (95%), 213.1 (50%), 248.1 (86%), 279.1 (62%), 317.1 (33%), 333.1 (29%), 471.1 (31%), 564.2 (11%), 580.2 (10%, (M + H)+); HRMS (pAPCI): calcd for C31H26N5O5S [M + H]+: 580.1649; observed: 580.1640.
3t – (3aS*,5S*,10bS*)-5-(hydroxy(o-tolyl)amino)-N,N,2-trimethyl-1,3-dioxo-1,3,3a,4,5,10b-hexahydropyrrolo[3,4-a]carbazole-10(2H)-sulfonamide
To a stirred bottomed flask was added N,N-dimethyl-3-vinyl-1H-indole-1-sulfonamide (85 mg, 0.34 mmol), DCM (5 mL) and 1-methyl-1H-pyrrole-2,5-dione (38 mg, 0.34 mmol). The solution was heated at reflux for 48 hours. The reaction was cooled to room temperature and 1-methyl-2-nitrosobenzene (41 mg, 0.34 mmol) was added. The reaction was stirred for 3 hours at room temperature before the solvent was removed under reduced pressure to give the crude product. The crude product was purified by column chromatography (column diameter = 2 cm, silica = 16 cm, eluent = petrol (40/60)–ethyl acetate 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give (3aS*,5S*,10bS*)-5-(hydroxy(o-tolyl)amino)-N,N,2-trimethyl-1,3-dioxo-1,3,3a,4,5,10b-hexahydropyrrolo[3,4-a]carbazole-10(2H)-sulfonamide (74%, 0.122 g, 0.25 mmol) as an off white solid.
1) to give (3aS*,5S*,10bS*)-5-(hydroxy(o-tolyl)amino)-N,N,2-trimethyl-1,3-dioxo-1,3,3a,4,5,10b-hexahydropyrrolo[3,4-a]carbazole-10(2H)-sulfonamide (74%, 0.122 g, 0.25 mmol) as an off white solid.
Mp: 169.3–171.9 °C; Rf: 0.32 (Pet(40/60)–EA 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CDCl3): δH 7.84 (1H, d, J = 8.4 Hz), 7.60 (1H, d, J = 7.9 Hz), 7.50 (1H, d, J = 8.0 Hz), 7.28 (1H, app t, J = 7.8 Hz), 7.21–7.16 (2H, m), 7.13 (1H, d, J = 8.1 Hz), 7.07–7.03 (1H, m), 4.98–4.96 (2H, m), 4.35–4.31 (1H, m), 3.69 (1H, app t, J = 7.7 Hz), 2.93 (9H, s), 2.60 (1H, app dt, J = 13.1, 6.2 Hz), 2.33 (3H, s), 1.97 (1H, ddd, J = 13.1, 7.7, 4.6 Hz); 13C NMR (101 MHz, CDCl3): δC 178.4, 173.8, 149.2, 137.4, 131.9, 131.1, 129.6, 128.4, 126.6, 125.3, 124.7, 123.7, 121.5, 120.4, 118.8, 115.0, 57.5, 40.5, 39.4, 38.4, 25.2, 24.7, 18.7; IR (neat): νmax/cm−1 3426, 2981, 1712; MS (pAPCI): 221.1 (9%), 251.1 (13%), 360.1 (100%, (M − (o-Tol)N(OH))+), 465.2 (15%, (M − H2O)+), 483.2 (15%, (M + H)+); HRMS (pAPCI): calcd for C24H27N4O5S1 [M + H]+: 483.1697; observed: 483.1685.
1); 1H NMR (400 MHz, CDCl3): δH 7.84 (1H, d, J = 8.4 Hz), 7.60 (1H, d, J = 7.9 Hz), 7.50 (1H, d, J = 8.0 Hz), 7.28 (1H, app t, J = 7.8 Hz), 7.21–7.16 (2H, m), 7.13 (1H, d, J = 8.1 Hz), 7.07–7.03 (1H, m), 4.98–4.96 (2H, m), 4.35–4.31 (1H, m), 3.69 (1H, app t, J = 7.7 Hz), 2.93 (9H, s), 2.60 (1H, app dt, J = 13.1, 6.2 Hz), 2.33 (3H, s), 1.97 (1H, ddd, J = 13.1, 7.7, 4.6 Hz); 13C NMR (101 MHz, CDCl3): δC 178.4, 173.8, 149.2, 137.4, 131.9, 131.1, 129.6, 128.4, 126.6, 125.3, 124.7, 123.7, 121.5, 120.4, 118.8, 115.0, 57.5, 40.5, 39.4, 38.4, 25.2, 24.7, 18.7; IR (neat): νmax/cm−1 3426, 2981, 1712; MS (pAPCI): 221.1 (9%), 251.1 (13%), 360.1 (100%, (M − (o-Tol)N(OH))+), 465.2 (15%, (M − H2O)+), 483.2 (15%, (M + H)+); HRMS (pAPCI): calcd for C24H27N4O5S1 [M + H]+: 483.1697; observed: 483.1685.
3u – (3aS*,5S*,10bS*)-5-((2,6-dibromophenyl) (hydroxy)amino)-N,N-dimethyl-1,3-dioxo-2-phenyl-1,3,3a,4,5,10b-hexahydropyrrolo[3,4-a]carbazole-10(2H)-sulfonamide
To a stirred round bottomed flask was added N,N-dimethyl-3-vinyl-1H-indole-1-sulfonamide (85 mg, 0.34 mmol), DCM (5 mL) and 1-phenyl-1H-pyrrole-2,5-dione (59 mg, 0.34 mmol) and the resulting solution was heated at reflux for 48 hours. The reaction was cooled to room temperature and 2,6-dibromonitrosobenzene (90 mg, 0.34 mmol) was added. The reaction was stirred at room temperature for 18 hours and the solvent was removed under reduced pressure to give the crude product as a pale yellow solid. The product was purified by column chromatography (petrol (40/60)–ethyl acetate 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, column diameter = 2 cm, silica = 17 cm) to give (3aS*,5S*,10bS*)-5-((2,6-dibromophenyl) (hydroxy)amino)-N,N-dimethyl-1,3-dioxo-2-phenyl-2,3,3a,4,5,10b-hexahydropyrrolo[3,4-a]carbazole-10(1H)-sulfonamide (69%, 162 mg, 0.23 mmol) as a pale orange powder.
1, column diameter = 2 cm, silica = 17 cm) to give (3aS*,5S*,10bS*)-5-((2,6-dibromophenyl) (hydroxy)amino)-N,N-dimethyl-1,3-dioxo-2-phenyl-2,3,3a,4,5,10b-hexahydropyrrolo[3,4-a]carbazole-10(1H)-sulfonamide (69%, 162 mg, 0.23 mmol) as a pale orange powder.
Mp: 241–242 °C; Rf: 0.43 (Pet(40/60)–Et2O 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CDCl3): δ 7.53 (2H, d, J = 7.8 Hz), 7.46–7.30 (7H, m), 7.15 (1H, app t, J = 7.4 Hz), 7.02 (1H, app t, J = 7.5 Hz), 6.71 (1H, app td, J = 8.0, 2.1 Hz), 6.05 (1H, s), 5.49–5.47 (1H, m), 5.17–5.12 (1H, m), 4.35–4.27 (1H, m), 3.18–3.11 (1H, m), 2.92 (6H, s), 1.80 (1H, app t, J = 13.1 Hz); 13C NMR (101 MHz, CD2Cl2): δC 177.9, 172.5, 144.6, 136.1, 134.3, 132.7, 132.1, 132.0, 129.5, 129.1, 128.6, 128.2, 126.6, 124.0, 122.8, 119.2, 115.1, 113.6, 77.6, 54.7, 42.7, 39.0, 38.0, 30.0; IR (neat): νmax/cm−1 3431, 2927, 1780, 1715; MS (pNSI): 422.1 (25%, (M − N(OH)C6H3Br2)+), 689.0 (76% (M + H)+), 711.0 (54%, (M + Na)+), HRMS (pNSI): calcd C28H25Br2N4O5S [M + H]+: 688.9888; observed: 688.9886.
1); 1H NMR (400 MHz, CDCl3): δ 7.53 (2H, d, J = 7.8 Hz), 7.46–7.30 (7H, m), 7.15 (1H, app t, J = 7.4 Hz), 7.02 (1H, app t, J = 7.5 Hz), 6.71 (1H, app td, J = 8.0, 2.1 Hz), 6.05 (1H, s), 5.49–5.47 (1H, m), 5.17–5.12 (1H, m), 4.35–4.27 (1H, m), 3.18–3.11 (1H, m), 2.92 (6H, s), 1.80 (1H, app t, J = 13.1 Hz); 13C NMR (101 MHz, CD2Cl2): δC 177.9, 172.5, 144.6, 136.1, 134.3, 132.7, 132.1, 132.0, 129.5, 129.1, 128.6, 128.2, 126.6, 124.0, 122.8, 119.2, 115.1, 113.6, 77.6, 54.7, 42.7, 39.0, 38.0, 30.0; IR (neat): νmax/cm−1 3431, 2927, 1780, 1715; MS (pNSI): 422.1 (25%, (M − N(OH)C6H3Br2)+), 689.0 (76% (M + H)+), 711.0 (54%, (M + Na)+), HRMS (pNSI): calcd C28H25Br2N4O5S [M + H]+: 688.9888; observed: 688.9886.
3v – (3aS*,5S*,10bS*)-5-((S*)-hydroxy(perfluorophenyl)methyl)-N,N-dimethyl-1,3-dioxo-1,3,3a,4,5,10b-hexahydropyrrolo[3,4-a]carbazole-10(2H)-sulfonamide
To a stirred round bottomed flask was added N,N-dimethyl-3-vinyl-1H-indole-1-sulfonamide (85 mg, 0.34 mmol), DCM (5 mL) and 1-methyl-1H-pyrrole-2,5-dione (38 mg, 0.34 mmol). The reaction was heated at reflux for 48 hours. The reaction was cooled to −78 °C and pentafluorobenzaldehyde (66 mg, 0.34 mmol) and DMAC (1 M in hexane, 0.34 mL, 0.34 mmol) were added and the reaction was stirred for 1 hour. The reaction was poured into a solution of sodium bicarbonate (10 mL) and extracted with DCM (3 × 10 mL). The combined organic extracts were dried with MgSO4, filtered and the solvent was removed under reduced pressure to leave the crude product as a pale pink solid. The product was purified by column chromatography (diameter = 2 cm, silica = 17 cm, eluent = petrol (40/60)–EA 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give (3aS*,5S*,10bS*)-5-((S*)-hydroxy(perfluorophenyl)methyl)-N,N-dimethyl-1,3-dioxo-1,3,3a,4,5,10b-hexahydropyrrolo[3,4-a]carbazole-10(2H)-sulfonamide (77%, 0.145 g, 0.26 mmol) as a pale pink solid.
1) to give (3aS*,5S*,10bS*)-5-((S*)-hydroxy(perfluorophenyl)methyl)-N,N-dimethyl-1,3-dioxo-1,3,3a,4,5,10b-hexahydropyrrolo[3,4-a]carbazole-10(2H)-sulfonamide (77%, 0.145 g, 0.26 mmol) as a pale pink solid.
Mp: 255.8–257.2 °C; Rf: 0.30 (Pet(40/60)–EA 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CDCl3): δH 7.77 (1H, d, J = 8.3 Hz), 7.39 (1H, d, J = 7.8 Hz), 7.33–7.28 (1H, m), 7.21 (1H, app t, J = 7.8 Hz), 5.14 (1H, d, J = 8.0 Hz), 4.80 (1H, d, J = 7.3 Hz), 3.67–3.62 (1H, m), 3.48 (1H, ddd, J = 12.8, 7.2, 5.0 Hz), 2.98 (6H, s), 2.93 (3H, s), 2.49 (1H, br s), 2.06 (1H, app dd, J = 14.9, 4.1 Hz), 1.59 (1H, app dt, J = 13.8, 6.9 Hz); 13C NMR (101 MHz, CDCl3): δC 177.8, 173.4, 137.2, 130.0, 129.3, 125.0, 123.5, 120.0, 118.2, 114.6, 70.3, 41.5, 39.2, 38.2, 36.7, 28.6, 25.2; IR (neat): νmax/cm−1 3415, 2972, 2884, 1713; MS (pNSI): 371.1 (22%), 558.1 (81% (M + H)+), 580.1 (100%, (M + Na)+), HRMS (pNSI): calcd C24H20F5N3O5S [M + H]+: 558.1117; observed: 558.1118.
1); 1H NMR (400 MHz, CDCl3): δH 7.77 (1H, d, J = 8.3 Hz), 7.39 (1H, d, J = 7.8 Hz), 7.33–7.28 (1H, m), 7.21 (1H, app t, J = 7.8 Hz), 5.14 (1H, d, J = 8.0 Hz), 4.80 (1H, d, J = 7.3 Hz), 3.67–3.62 (1H, m), 3.48 (1H, ddd, J = 12.8, 7.2, 5.0 Hz), 2.98 (6H, s), 2.93 (3H, s), 2.49 (1H, br s), 2.06 (1H, app dd, J = 14.9, 4.1 Hz), 1.59 (1H, app dt, J = 13.8, 6.9 Hz); 13C NMR (101 MHz, CDCl3): δC 177.8, 173.4, 137.2, 130.0, 129.3, 125.0, 123.5, 120.0, 118.2, 114.6, 70.3, 41.5, 39.2, 38.2, 36.7, 28.6, 25.2; IR (neat): νmax/cm−1 3415, 2972, 2884, 1713; MS (pNSI): 371.1 (22%), 558.1 (81% (M + H)+), 580.1 (100%, (M + Na)+), HRMS (pNSI): calcd C24H20F5N3O5S [M + H]+: 558.1117; observed: 558.1118.
Note: 13C NMR missing peaks due to C–F coupling.
3w – 6-(hydroxy(o-tolyl)amino)-N,N-dimethyl-1,3-dioxo-2-phenyl-2,3,5,6-tetrahydro-[1,2,4]triazolo[1′,2′:1,2]pyridazino[3,4-b]indole-11(1H)-sulfonamide
To a stirred round bottomed flask was added N,N-dimethyl-3-vinyl-1H-indole-1-sulfonamide (85 mg, 0.34 mmol), DCM (5 mL) and cooled to −78 °C. To this solution PTAD (60 mg, 0.34 mmol) was added and the reaction stirred at −78 °C for 1 hour. 1-Methyl-2-nitrosobenzene (41 mg, 0.34 mmol) was added and the reaction was stirred 4 hours before the solvent was removed under reduced pressure to give the crude product. The crude product was purified by column chromatography (column diameter = 2 cm, silica = 16 cm, eluent = petrol (40/60)–ethyl acetate 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give 6-(hydroxy(o-tolyl)amino)-N,N-dimethyl-1,3-dioxo-2-phenyl-2,3,5,6-tetrahydro-[1,2,4]triazolo[1′,2′:1,2]pyridazino[3,4-b]indole-11(1H)-sulfonamide (76%, 0.141 g) as an off white solid.
1) to give 6-(hydroxy(o-tolyl)amino)-N,N-dimethyl-1,3-dioxo-2-phenyl-2,3,5,6-tetrahydro-[1,2,4]triazolo[1′,2′:1,2]pyridazino[3,4-b]indole-11(1H)-sulfonamide (76%, 0.141 g) as an off white solid.
Mp: 168.5–172.8 °C; Rf: 0.25 (Pet(40/60)–EA 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (300 MHz, CDCl3): δH 7.87 (1H, d, J = 8.3 Hz), 7.73 (1H, d, J = 8.1 Hz), 7.60 (2H, d, J = 7.4 Hz), 7.51 (2H, t, J = 7.6 Hz), 7.46–7.38 (1H, m), 7.30–7.18 (2H, m), 7.04 (2H, t, J = 7.5 Hz), 6.90 (1H, d, J = 7.6 Hz), 6.71 (1H, d, J = 7.8 Hz), 5.76 (1H, s), 5.37 (1H, d, J = 14.1 Hz), 4.66 (1H, s), 3.46 (1H, d, J = 14.1 Hz), 2.90 (6H, s), 1.94 (3H, s); 13C NMR (101 MHz, CDCl3): δC 153.5, 150.5, 148.7, 135.1, 132.1, 131.9, 131.2, 130.6, 129.4, 128.7, 127.1, 126.9, 126.8, 125.9, 124.4, 124.2, 122.7, 118.1, 115.9, 104.7, 59.4, 44.7, 38.7, 17.9; IR (neat): νmax/cm−1 3322, 2971, 1707; MS (pNSI): 339.1 (33%), 424.1 (94%, (M − MeC6H4NOH)+), 547.2 (72%, (M + H)+), 569.2 (100%, (M + Na)+); HRMS (pNSI): calcd C27H27N6O5S [M + H]+: 547.1758; observed: 547.1761.
1); 1H NMR (300 MHz, CDCl3): δH 7.87 (1H, d, J = 8.3 Hz), 7.73 (1H, d, J = 8.1 Hz), 7.60 (2H, d, J = 7.4 Hz), 7.51 (2H, t, J = 7.6 Hz), 7.46–7.38 (1H, m), 7.30–7.18 (2H, m), 7.04 (2H, t, J = 7.5 Hz), 6.90 (1H, d, J = 7.6 Hz), 6.71 (1H, d, J = 7.8 Hz), 5.76 (1H, s), 5.37 (1H, d, J = 14.1 Hz), 4.66 (1H, s), 3.46 (1H, d, J = 14.1 Hz), 2.90 (6H, s), 1.94 (3H, s); 13C NMR (101 MHz, CDCl3): δC 153.5, 150.5, 148.7, 135.1, 132.1, 131.9, 131.2, 130.6, 129.4, 128.7, 127.1, 126.9, 126.8, 125.9, 124.4, 124.2, 122.7, 118.1, 115.9, 104.7, 59.4, 44.7, 38.7, 17.9; IR (neat): νmax/cm−1 3322, 2971, 1707; MS (pNSI): 339.1 (33%), 424.1 (94%, (M − MeC6H4NOH)+), 547.2 (72%, (M + H)+), 569.2 (100%, (M + Na)+); HRMS (pNSI): calcd C27H27N6O5S [M + H]+: 547.1758; observed: 547.1761.
3x – (3aS*,5S*,10bS*)-5-(hydroxy(o-tolyl)amino)-N,N-dimethyl-1,3-dioxo-1,3,3a,4,5,10b-hexahydropyrrolo[3,4-a]carbazole-10(2H)-sulfonamide
To a round bottomed flask was added N,N-dimethyl-3-vinyl-1H-indole-1-sulfonamide (85 mg, 0.34 mmol), DCM (5 mL) and 1H-pyrrole-2,5-dione (33 mg, 0.34 mmol). The reaction was heated at reflux for 48 hours and then cooled to room temperature. 1-Methyl-2-nitrosobenzene (41 mg, 0.34 mmol) was added and the reaction is stirred for 4.5 hours. The solvent was removed to give the crude product as pale yellow solid. The crude product was purified by trituration from DCM to give (3aS*,5S*,10bS*)-5-(hydroxy(o-tolyl)amino)-N,N-dimethyl-1,3-dioxo-1,3,3a,4,5,10b-hexahydropyrrolo[3,4-a]carbazole-10(2H)-sulfonamide (77%, 123 mg, 0.26 mmol) as a white solid.Mp: 199.9–201.0 °C; Rf: 0.64 (Pet(40/60)–EA 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (300 MHz, DMSO-d6): δH 11.15 (1H, s), 8.38 (1H, s), 7.88 (1H, d, J = 8.4 Hz), 7.36 (1H, d, J = 7.9 Hz), 7.31–7.20 (2H, m), 7.13 (3H, app dt, J = 14.3, 7.3 Hz), 7.01 (1H, d, J = 7.3 Hz), 4.98 (1H, d, J = 7.8 Hz), 4.38 (1H, app t, J = 4.8 Hz), 3.73 (1H, app q, J = 7.8 Hz), 2.70 (6H, s), 2.45–2.37 (1H, m), 2.36 (3H, s), 1.77–1.65, 1.77 (1H, m); 13C NMR (101 MHz, DMSO-d6): δC 180.4, 175.5, 151.6, 137.2, 133.2, 131.1, 129.3, 129.1, 126.6, 124.4, 124.3, 123.4, 121.8, 121.1, 118.6, 115.3, 56.5, 42.2, 41.1, 38.7, 26.6, 18.9; IR (neat): νmax/cm−1 3426, 2981, 1712; MS (pAPCI): 237.1 (60%), 346.1 (100%, (M − MeC6H4NOH)+), 451.1 (25%, (M − H2O)+), 469.2 (22%, (M + H)+); HRMS (pAPCI): calcd C23H25N4O5S [M + H]+: 469.1540; observed: 469.1537.
1); 1H NMR (300 MHz, DMSO-d6): δH 11.15 (1H, s), 8.38 (1H, s), 7.88 (1H, d, J = 8.4 Hz), 7.36 (1H, d, J = 7.9 Hz), 7.31–7.20 (2H, m), 7.13 (3H, app dt, J = 14.3, 7.3 Hz), 7.01 (1H, d, J = 7.3 Hz), 4.98 (1H, d, J = 7.8 Hz), 4.38 (1H, app t, J = 4.8 Hz), 3.73 (1H, app q, J = 7.8 Hz), 2.70 (6H, s), 2.45–2.37 (1H, m), 2.36 (3H, s), 1.77–1.65, 1.77 (1H, m); 13C NMR (101 MHz, DMSO-d6): δC 180.4, 175.5, 151.6, 137.2, 133.2, 131.1, 129.3, 129.1, 126.6, 124.4, 124.3, 123.4, 121.8, 121.1, 118.6, 115.3, 56.5, 42.2, 41.1, 38.7, 26.6, 18.9; IR (neat): νmax/cm−1 3426, 2981, 1712; MS (pAPCI): 237.1 (60%), 346.1 (100%, (M − MeC6H4NOH)+), 451.1 (25%, (M − H2O)+), 469.2 (22%, (M + H)+); HRMS (pAPCI): calcd C23H25N4O5S [M + H]+: 469.1540; observed: 469.1537.
3y – benzyl (3aS*,5S*,10bS*)-5-(hydroxy(phenyl)amino)-2-methyl-1,3-dioxo-2,3,3a,4,5,10b-hexahydropyrrolo[3,4-a]carbazole-10(1H)-carboxylate
To a stirred Schlenk flask was added benzyl 3-vinyl-1H-indole-1-carboxylate (94 mg, 0.34 mmol), DCM (5 mL) and 1-methyl-1H-pyrrole-2,5-dione (33 mg, 0.34 mmol). The resulting solution was heated at reflux for 24 hours. The reaction was allowed to cool to room temperature and to the stirred solution nitrosobenzene (36 mg, 0.34 mmol) was added and the solution was stirred for 18 hours. The solvent was removed under reduced pressure to leave the crude product as a pale yellow solid. The product was purified by column chromatography (petrol (40/60)–ethyl acetate 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, column diameter = 2 cm, silica = 14 cm) to give benzyl (3aS*,5S*,10bS*)-5-(hydroxy(phenyl)amino)-2-methyl-1,3-dioxo-2,3,3a,4,5,10b-hexahydropyrrolo[3,4-a]carbazole-10(1H)-carboxylate (74%, 124 mg, 0.25 mmol) as a yellow powder.
1, column diameter = 2 cm, silica = 14 cm) to give benzyl (3aS*,5S*,10bS*)-5-(hydroxy(phenyl)amino)-2-methyl-1,3-dioxo-2,3,3a,4,5,10b-hexahydropyrrolo[3,4-a]carbazole-10(1H)-carboxylate (74%, 124 mg, 0.25 mmol) as a yellow powder.
Mp: 105.7–109.2 °C; Rf: 0.34 (Pet(40/60)–EA 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CD2Cl2): δH 8.10 (1H, br d, J = 8.1 Hz), 7.70 (1H, br d, J = 7.3 Hz), 7.49 (2H, br d, J = 6.6 Hz), 7.39 (3H, br app q, J = 6.9, 6.4 Hz), 7.35–7.25 (3H, br m), 7.22–7.13 (3H, br m), 7.01 (1H, br app t, J = 6.7 Hz), 5.55 (1H, d, J = 11.8 Hz), 5.41 (1H, d, J = 11.8 Hz), 4.88 (1H, br d, J = 6.8 Hz), 4.87 (1H, br s), 4.80–4.77 (1H, br m), 3.54–3.41 (1H, br m), 2.85 (3H, s), 2.40–2.25 (1H, br m), 2.08–1.93 (1H, br m); 13C NMR (101 MHz, CD2Cl2): δC 178.4, 174.6, 151.6, 151.0, 137.0, 135.0, 130.0, 129.0, 128.9, 128.8, 128.8, 127.6, 125.0, 123.3, 122.3, 120.0, 118.3, 117.1, 115.1, 69.5, 57.7, 40.2, 39.2, 24.9, 22.6; IR (neat): νmax/cm−1 3433, 2953, 1699; MS (pNSI): 387.1 (97%, (M − N(OH)Ph)+), 494.2 (100%, (M − H)+), 518.2 (30%, (M + Na)+), 991.4 (15%, (2M + H)+), 1013.3 (10%, (2M + Na)+); HRMS (pNSI): calcd C29H25N3O5Na [M + Na]+: 518.1686; observed: 518.1676.
1); 1H NMR (400 MHz, CD2Cl2): δH 8.10 (1H, br d, J = 8.1 Hz), 7.70 (1H, br d, J = 7.3 Hz), 7.49 (2H, br d, J = 6.6 Hz), 7.39 (3H, br app q, J = 6.9, 6.4 Hz), 7.35–7.25 (3H, br m), 7.22–7.13 (3H, br m), 7.01 (1H, br app t, J = 6.7 Hz), 5.55 (1H, d, J = 11.8 Hz), 5.41 (1H, d, J = 11.8 Hz), 4.88 (1H, br d, J = 6.8 Hz), 4.87 (1H, br s), 4.80–4.77 (1H, br m), 3.54–3.41 (1H, br m), 2.85 (3H, s), 2.40–2.25 (1H, br m), 2.08–1.93 (1H, br m); 13C NMR (101 MHz, CD2Cl2): δC 178.4, 174.6, 151.6, 151.0, 137.0, 135.0, 130.0, 129.0, 128.9, 128.8, 128.8, 127.6, 125.0, 123.3, 122.3, 120.0, 118.3, 117.1, 115.1, 69.5, 57.7, 40.2, 39.2, 24.9, 22.6; IR (neat): νmax/cm−1 3433, 2953, 1699; MS (pNSI): 387.1 (97%, (M − N(OH)Ph)+), 494.2 (100%, (M − H)+), 518.2 (30%, (M + Na)+), 991.4 (15%, (2M + H)+), 1013.3 (10%, (2M + Na)+); HRMS (pNSI): calcd C29H25N3O5Na [M + Na]+: 518.1686; observed: 518.1676.
3z – benzyl (3aS*,5S*,10bS*)-5-(hydroxy(o-tolyl)amino)-2-methyl-1,3-dioxo-2,3,3a,4,5,10b-hexahydropyrrolo[3,4-a]carbazole-10(1H)-carboxylate
To a stirred Schlenk flask was added benzyl 3-vinyl-1H-indole-1-carboxylate (94 mg, 0.34 mmol), DCM (5 mL) and 1-methyl-1H-pyrrole-2,5-dione (33 mg, 0.34 mmol). The resulting solution was heated at reflux for 24 hours. The reaction was allowed to cool to room temperature and to the stirred solution 1-methyl-2-nitrosobenzene (41 mg, 0.34 mmol) was added and the solution was stirred for 18 hours. The solvent was removed under reduced pressure to leave the crude product as a pale yellow solid. The product was purified by column chromatography (petrol (40/60)–ethyl acetate 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, column diameter = 2 cm, silica = 16 cm) to give benzyl (3aS*,5S*,10bS*)-5-(hydroxy(o-tolyl)amino)-2-methyl-1,3-dioxo-2,3,3a,4,5,10b-hexahydropyrrolo[3,4-a]carbazole-10(1H)-carboxylateas (78%, 135 mg, 0.26 mmol) as a yellow powder.
1, column diameter = 2 cm, silica = 16 cm) to give benzyl (3aS*,5S*,10bS*)-5-(hydroxy(o-tolyl)amino)-2-methyl-1,3-dioxo-2,3,3a,4,5,10b-hexahydropyrrolo[3,4-a]carbazole-10(1H)-carboxylateas (78%, 135 mg, 0.26 mmol) as a yellow powder.
Mp: 128.4–131.5 °C; Rf: 0.45 (Pet(40/60)–EA 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CDCl3): δH 8.11 (1H, d, J = 8.3 Hz), 7.59 (1H, d, J = 7.8 Hz), 7.55 (1H, d, J = 8.1 Hz), 7.50–7.48 (2H, m), 7.45–7.38 (3H, m), 7.29–7.23 (1H, m), 7.20 (1H, app t, J = 7.6 Hz), 7.14 (1H, d, J = 7.6 Hz), 7.10 (1H, d, J = 7.8 Hz), 7.05 (1H, d, J = 7.3 Hz), 5.56 (1H, d, J = 11.8 Hz), 5.46 (1H, d, J = 11.8 Hz), 5.02 (1H, s), 4.93 (1H, d, J = 7.6 Hz), 4.38 (1H, t, J = 5.2 Hz), 3.63 (1H, app q, J = 6.9 Hz), 2.91 (3H, s), 2.59 (1H, app dt, J = 12.3, 6.0 Hz), 2.29 (3H, s), 1.92 (1H, ddd, J = 12.5, 7.5, 4.6 Hz); 13C NMR (101 MHz, CDCl3): δC 178.5, 174.6, 151.7, 149.2, 136.8, 134.8, 131.1, 129.9, 129.0, 129.0, 128.9, 128.9, 127.7, 126.6, 125.3, 125.0, 123.3, 121.5, 120.1, 118.1, 115.3, 69.6, 57.4, 40.4, 39.1, 25.2, 24.1, 18.6; IR (neat): νmax/cm−1 3450, 2954, 1699; MS (pNSI): 343.1 (40%), 387.1 (82%, (M − (N(OH)(o-Tol)))+), 508.2 (100%, (M − (H2) + H)+), 532.2 (59%, (M + Na)+); HRMS (pNSI): calcd C30H27N3O5Na [M + Na]+: 532.1843; observed: 532.1834.
1); 1H NMR (400 MHz, CDCl3): δH 8.11 (1H, d, J = 8.3 Hz), 7.59 (1H, d, J = 7.8 Hz), 7.55 (1H, d, J = 8.1 Hz), 7.50–7.48 (2H, m), 7.45–7.38 (3H, m), 7.29–7.23 (1H, m), 7.20 (1H, app t, J = 7.6 Hz), 7.14 (1H, d, J = 7.6 Hz), 7.10 (1H, d, J = 7.8 Hz), 7.05 (1H, d, J = 7.3 Hz), 5.56 (1H, d, J = 11.8 Hz), 5.46 (1H, d, J = 11.8 Hz), 5.02 (1H, s), 4.93 (1H, d, J = 7.6 Hz), 4.38 (1H, t, J = 5.2 Hz), 3.63 (1H, app q, J = 6.9 Hz), 2.91 (3H, s), 2.59 (1H, app dt, J = 12.3, 6.0 Hz), 2.29 (3H, s), 1.92 (1H, ddd, J = 12.5, 7.5, 4.6 Hz); 13C NMR (101 MHz, CDCl3): δC 178.5, 174.6, 151.7, 149.2, 136.8, 134.8, 131.1, 129.9, 129.0, 129.0, 128.9, 128.9, 127.7, 126.6, 125.3, 125.0, 123.3, 121.5, 120.1, 118.1, 115.3, 69.6, 57.4, 40.4, 39.1, 25.2, 24.1, 18.6; IR (neat): νmax/cm−1 3450, 2954, 1699; MS (pNSI): 343.1 (40%), 387.1 (82%, (M − (N(OH)(o-Tol)))+), 508.2 (100%, (M − (H2) + H)+), 532.2 (59%, (M + Na)+); HRMS (pNSI): calcd C30H27N3O5Na [M + Na]+: 532.1843; observed: 532.1834.
3aa – (3aS*,5S*,10bS*)-5-(3,5-dioxo-4-phenyl-1,2,4-triazolidin-1-yl)-2-methyl-1,3-dioxo-2,3,3a,4,5,10b-hexahydropyrrolo[3,4-a]carbazole-10(1H)-carboxylate
To a stirred Schlenk flask was added benzyl 3-vinyl-1H-indole-1-carboxylate (189 mg, 0.68 mmol), 1-methyl-1H-pyrrole-2,5-dione (76 mg, 0.68 mmol) and DCM (10 mL). The reaction mixture was heated at reflux for 24 hours. The reaction was cooled to 0 °C and then 4-phenyl-1,2,4-triazolidine-3,5-dione (120 mg, 0.68 mmol) was added. The reaction was stirred at 0 °C for 1 hour then at room temperature for 18 hours. The solvent was removed under reduced pressure to leave the crude product as an orange powder. The product was purified by column chromatography (petrol (40/60)–ethyl acetate 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, column diameter = 2 cm, silica = 20 cm) to give (3aS*,5S*,10bS*)-5-(3,5-dioxo-4-phenyl-1,2,4-triazolidin-1-yl)-2-methyl-1,3-dioxo-2,3,3a,4,5,10b-hexahydropyrrolo[3,4-a]carbazole-10(1H)-carboxylate (54%, 207 mg, 0.37 mmol) as an off-white powder and (3aS*,5S*,10bS*)-5-(3,5-dioxo-4-phenyl-1,2,4-triazolidin-1-yl)-2-methyl-1,3-dioxo-2,3,3a,4,5,10b-hexahydropyrrolo[3,4-a]carbazole-10(1H)-carboxylate (27%, 76 mg, 0.19 mmol) as a white powder.
1, column diameter = 2 cm, silica = 20 cm) to give (3aS*,5S*,10bS*)-5-(3,5-dioxo-4-phenyl-1,2,4-triazolidin-1-yl)-2-methyl-1,3-dioxo-2,3,3a,4,5,10b-hexahydropyrrolo[3,4-a]carbazole-10(1H)-carboxylate (54%, 207 mg, 0.37 mmol) as an off-white powder and (3aS*,5S*,10bS*)-5-(3,5-dioxo-4-phenyl-1,2,4-triazolidin-1-yl)-2-methyl-1,3-dioxo-2,3,3a,4,5,10b-hexahydropyrrolo[3,4-a]carbazole-10(1H)-carboxylate (27%, 76 mg, 0.19 mmol) as a white powder.
Mp: 202.3–203.9 °C; Rf: 0.15 (Pet(40/60)–EA 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CDCl3): δH 8.38 (1H, s), 8.07 (1H, d, J = 8.4 Hz), 7.51 (1H, app dt, J = 7.6, 0.9 Hz), 7.49–7.37 (4H, m), 7.39–7.33 (6H, m), 7.30 (1H, ddd, J = 8.6, 7.3, 1.3 Hz), 7.21 (1H, app td, J = 7.5, 1.0 Hz), 5.53 (1H, app t, J = 5.2 Hz), 5.44 (1H, d, J = 11.8 Hz), 5.38 (1H, d, J = 11.8 Hz), 4.89 (1H, d, J = 8.0 Hz), 3.50 (1H, ddd, J = 9.6, 8.0, 5.5 Hz), 2.87 (3H, s), 2.41 (1H, app dt, J = 14.2, 5.5 Hz), 2.14 (1H, ddd, J = 14.2, 9.5, 5.5 Hz); 13C NMR (101 MHz, CDCl3): δC 177.4, 173.6, 153.6, 152.2, 151.3, 136.9, 134.5, 130.9, 130.8, 129.3, 129.1, 128.9, 128.9, 128.4, 126.1, 125.8, 125.3, 123.8, 118.8, 115.7, 113.8, 69.8, 47.5, 40.0, 38.4, 27.5, 25.2; IR (neat): νmax cm−1 3462, 2969, 1699; MS (pNSI): 199.2 (16%), 387.1 (19%, (M − PTAD)+), 564.2 (59%, (M + H)+), 581.2 (100%, (M + NH4)+), 643.2 (15%), 1144.4 (39%, (2M + NH4)+); HRMS (pNSI): calcd C31H26N5O6 [M + H]+: 564.1878; observed: 564.1873.
1); 1H NMR (400 MHz, CDCl3): δH 8.38 (1H, s), 8.07 (1H, d, J = 8.4 Hz), 7.51 (1H, app dt, J = 7.6, 0.9 Hz), 7.49–7.37 (4H, m), 7.39–7.33 (6H, m), 7.30 (1H, ddd, J = 8.6, 7.3, 1.3 Hz), 7.21 (1H, app td, J = 7.5, 1.0 Hz), 5.53 (1H, app t, J = 5.2 Hz), 5.44 (1H, d, J = 11.8 Hz), 5.38 (1H, d, J = 11.8 Hz), 4.89 (1H, d, J = 8.0 Hz), 3.50 (1H, ddd, J = 9.6, 8.0, 5.5 Hz), 2.87 (3H, s), 2.41 (1H, app dt, J = 14.2, 5.5 Hz), 2.14 (1H, ddd, J = 14.2, 9.5, 5.5 Hz); 13C NMR (101 MHz, CDCl3): δC 177.4, 173.6, 153.6, 152.2, 151.3, 136.9, 134.5, 130.9, 130.8, 129.3, 129.1, 128.9, 128.9, 128.4, 126.1, 125.8, 125.3, 123.8, 118.8, 115.7, 113.8, 69.8, 47.5, 40.0, 38.4, 27.5, 25.2; IR (neat): νmax cm−1 3462, 2969, 1699; MS (pNSI): 199.2 (16%), 387.1 (19%, (M − PTAD)+), 564.2 (59%, (M + H)+), 581.2 (100%, (M + NH4)+), 643.2 (15%), 1144.4 (39%, (2M + NH4)+); HRMS (pNSI): calcd C31H26N5O6 [M + H]+: 564.1878; observed: 564.1873.
3bb – benzyl (3aS*,5S*,10bS*)-5-(hydroxy(phenyl)amino)-1,3-dioxo-2,3,3a,4,5,10b-hexahydropyrrolo[3,4-a]carbazole-10(1H)-carboxylate
To a stirred Schlenk flask was added benzyl 3-vinyl-1H-indole-1-carboxylate (189 mg, 0.68 mmol), 1H-pyrrole-2,5-dione (66 mg, 0.68 mmol) and DCM (10 mL). The resulting solution was heated at reflux for 24 hours. The reaction was allowed to cool to room temperature and to the stirred solution nitrosobenzene (73 mg, 0.68 mmol) was added and the solution was stirred for 18 hours. The solvent was removed under reduced pressure to leave the crude product as a yellow powder. The product was purified by column chromatography (petrol (40/60)–ethyl acetate 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, column diameter = 2 cm, silica = 20 cm) to give benzyl(3aS*,5S*,10bS*)-5-(hydroxy(phenyl)amino)-1,3-dioxo-2,3,3a,4,5,10b-hexahydropyrrolo[3,4-a]carbazole-10(1H)-carboxylate (70%, 230 mg, 0.48 mmol) as a yellow powder.
1, column diameter = 2 cm, silica = 20 cm) to give benzyl(3aS*,5S*,10bS*)-5-(hydroxy(phenyl)amino)-1,3-dioxo-2,3,3a,4,5,10b-hexahydropyrrolo[3,4-a]carbazole-10(1H)-carboxylate (70%, 230 mg, 0.48 mmol) as a yellow powder.
Mp: 177.1–177.8 °C; Rf: 0.38 (Pet(40/60)–EA 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (500 MHz, CD2Cl2): δH 8.20 (1H, s), 8.11 (1H, d, J = 8.3 Hz), 7.66 (1H, d, J = 7.8 Hz), 7.50 (2H, dd, J = 7.9, 1.6 Hz), 7.43–7.36 (3H, m), 7.33 (2H, app t, J = 7.8 Hz), 7.28 (1H, app t, J = 7.8 Hz), 7.22 (2H, d, J = 8.0 Hz), 7.16 (1H, app t, J = 7.5 Hz), 7.02 (1H, app t, J = 7.3 Hz), 5.55 (1H, d, J = 11.9 Hz), 5.40 (1H, d, J = 11.9 Hz), 5.19 (1H, s), 4.96 (1H, br d, J = 8.1 Hz), 4.82 (1H, app t, J = 5.7 Hz), 3.56 (1H, br app q, J = 7.4 Hz), 2.35 (1H, br app dd, J = 13.4, 6.7 Hz), 2.00–1.92 (1H, br m); 13C NMR (101 MHz, CD2Cl2): δC 178.5, 174.5, 151.6, 150.7, 136.9, 134.9, 129.7, 129.0, 128.9, 128.9, 128.8, 127.6, 125.1, 123.3, 122.6, 119.8, 118.0, 117.3, 115.2, 69.5, 57.7, 41.4, 40.6, 23.0; IR (neat): νmax/cm−1 = 3418, 3329, 2970, 1705; MS (pNSI): 199.2 (87%), 373.1 (68%, (M − (N(OH)Ph))+), 480.2 (100%, (M − (H2) + H)+); HRMS (pNSI): calcd C28H23N3O5Na [M + Na]+: 504.1530; observed: 504.1522.
1); 1H NMR (500 MHz, CD2Cl2): δH 8.20 (1H, s), 8.11 (1H, d, J = 8.3 Hz), 7.66 (1H, d, J = 7.8 Hz), 7.50 (2H, dd, J = 7.9, 1.6 Hz), 7.43–7.36 (3H, m), 7.33 (2H, app t, J = 7.8 Hz), 7.28 (1H, app t, J = 7.8 Hz), 7.22 (2H, d, J = 8.0 Hz), 7.16 (1H, app t, J = 7.5 Hz), 7.02 (1H, app t, J = 7.3 Hz), 5.55 (1H, d, J = 11.9 Hz), 5.40 (1H, d, J = 11.9 Hz), 5.19 (1H, s), 4.96 (1H, br d, J = 8.1 Hz), 4.82 (1H, app t, J = 5.7 Hz), 3.56 (1H, br app q, J = 7.4 Hz), 2.35 (1H, br app dd, J = 13.4, 6.7 Hz), 2.00–1.92 (1H, br m); 13C NMR (101 MHz, CD2Cl2): δC 178.5, 174.5, 151.6, 150.7, 136.9, 134.9, 129.7, 129.0, 128.9, 128.9, 128.8, 127.6, 125.1, 123.3, 122.6, 119.8, 118.0, 117.3, 115.2, 69.5, 57.7, 41.4, 40.6, 23.0; IR (neat): νmax/cm−1 = 3418, 3329, 2970, 1705; MS (pNSI): 199.2 (87%), 373.1 (68%, (M − (N(OH)Ph))+), 480.2 (100%, (M − (H2) + H)+); HRMS (pNSI): calcd C28H23N3O5Na [M + Na]+: 504.1530; observed: 504.1522.
3cc – benzyl (3aS*,5S*,10bS*)-5-(hydroxy(o-tolyl)amino)-1,3-dioxo-2,3,3a,4,5,10b-hexahydropyrrolo[3,4-a]carbazole-10(1H)-carboxylate
To a stirred Schlenk flask was added benzyl 3-vinyl-1H-indole-1-carboxylate (94 mg, 0.34 mmol), DCM (5 mL) and 1H-pyrrole-2,5-dione (33 mg, 0.34 mmol). The resulting solution was heated at reflux for 24 hours. The reaction was allowed to cool to room temperature and to the stirred solution 1-methyl-2-nitrosobenzene (41 mg, 0.34 mmol) was added and the solution was stirred for 4 hours. The solvent was removed under reduced pressure to leave the crude product as a pale yellow solid. The product was purified by column chromatography (petrol (40/60)–ethyl acetate 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, column diameter = 2 cm, silica = 16 cm) to give benzyl-(3aS*,5S*,10bS*)-5-(hydroxy(o-tolyl)amino)-1,3-dioxo-2,3,3a,4,5,10b-hexahydropyrrolo[3,4-a]carbazole-10(1H)-carboxylate (83%, 140 mg, 0.28 mmol) as a yellow powder.
2, column diameter = 2 cm, silica = 16 cm) to give benzyl-(3aS*,5S*,10bS*)-5-(hydroxy(o-tolyl)amino)-1,3-dioxo-2,3,3a,4,5,10b-hexahydropyrrolo[3,4-a]carbazole-10(1H)-carboxylate (83%, 140 mg, 0.28 mmol) as a yellow powder.
Mp: 181.4–183.9 °C; Rf: 0.48 (Pet(40/60)–EA 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2); 1H NMR (400 MHz, DMSO-d6): δH 11.26 (1H, s), 8.41 (1H, s), 7.93 (1H, d, J = 8.3 Hz), 7.51 (2H, d, J = 7.0 Hz), 7.45–7.33 (4H, m), 7.18–7.08 (3H, m), 7.03–6.91 (3H, m), 5.56 (1H, d, J = 12.1 Hz), 5.32 (1H, d, J = 12.1 Hz), 4.96 (1H, d, J = 8.0 Hz), 4.34 (1H, app t, J = 3.7 Hz), 3.73 (1H, app q, J = 8.7 Hz), 2.50–2.44 (1H, m), 2.17 (3H, s), 1.74–1.64 (1H, m); 13C NMR (101 MHz, DMSO-d6): δC 180.6, 176.5, 151.6, 136.2, 135.7, 131.1, 130.8, 130.0, 129.2, 129.1, 129.1, 128.2, 126.6, 124.7, 124.5, 122.9, 122.2, 120.6, 117.5, 114.4, 69.2, 57.1, 41.8, 40.2, 26.7, 18.6; IR (neat): νmax/cm−1 3495, 3325, 2953, 1711; MS (pNSI): 373.1 (51%, (M − (N(OH)(o-Tol)))+), 494.2 (16%, (M − (H2) + H)+), 518.2 (21%, (M + Na)+); HRMS (pNSI): calcd C29H25N3O5Na [M + Na]+: 518.1686; observed: 518.1681.
2); 1H NMR (400 MHz, DMSO-d6): δH 11.26 (1H, s), 8.41 (1H, s), 7.93 (1H, d, J = 8.3 Hz), 7.51 (2H, d, J = 7.0 Hz), 7.45–7.33 (4H, m), 7.18–7.08 (3H, m), 7.03–6.91 (3H, m), 5.56 (1H, d, J = 12.1 Hz), 5.32 (1H, d, J = 12.1 Hz), 4.96 (1H, d, J = 8.0 Hz), 4.34 (1H, app t, J = 3.7 Hz), 3.73 (1H, app q, J = 8.7 Hz), 2.50–2.44 (1H, m), 2.17 (3H, s), 1.74–1.64 (1H, m); 13C NMR (101 MHz, DMSO-d6): δC 180.6, 176.5, 151.6, 136.2, 135.7, 131.1, 130.8, 130.0, 129.2, 129.1, 129.1, 128.2, 126.6, 124.7, 124.5, 122.9, 122.2, 120.6, 117.5, 114.4, 69.2, 57.1, 41.8, 40.2, 26.7, 18.6; IR (neat): νmax/cm−1 3495, 3325, 2953, 1711; MS (pNSI): 373.1 (51%, (M − (N(OH)(o-Tol)))+), 494.2 (16%, (M − (H2) + H)+), 518.2 (21%, (M + Na)+); HRMS (pNSI): calcd C29H25N3O5Na [M + Na]+: 518.1686; observed: 518.1681.
3dd – benzyl (3aS*,5S*,10bS*)-5-(3,5-dioxo-4-phenyl-1,2,4-triazolidin-1-yl)-1,3-dioxo-2,3,3a,4,5,10b-hexahydropyrrolo[3,4-a]carbazole-10(1H)-carboxylate
To a stirred Schlenk flask was added benzyl 3-vinyl-1H-indole-1-carboxylate (189 mg, 0.68 mmol), DCM (10 mL) and 1H-pyrrole-2,5-dione (66 mg, 0.68 mmol). The resulting solution was heated at reflux for 24 hours. The reaction was cooled to 0 °C and 4-phenyl-1,2,4-triazolidine-3,5-dione (120 mg, 0.68 mmol) was added. The solution was stirred at 0 °C for 1 hour. The solvent was removed under reduced pressure to leave the crude product as an off white solid. The product was purified by column chromatography (petrol (40/60)–ethyl acetate 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, column diameter = 2 cm, silica = 13 cm) to give benzyl (3aS*,5S*,10bS*)-5-(3,5-dioxo-4-phenyl-1,2,4-triazolidin-1-yl)-1,3-dioxo-2,3,3a,4,5,10b-hexahydropyrrolo[3,4-a]carbazole-10(1H)-carboxylate (58%, 216 mg, 0.39 mmol) as a pale pink powder.
1, column diameter = 2 cm, silica = 13 cm) to give benzyl (3aS*,5S*,10bS*)-5-(3,5-dioxo-4-phenyl-1,2,4-triazolidin-1-yl)-1,3-dioxo-2,3,3a,4,5,10b-hexahydropyrrolo[3,4-a]carbazole-10(1H)-carboxylate (58%, 216 mg, 0.39 mmol) as a pale pink powder.
Mp: 174.6–177.1 °C; Rf: 0.06 (Pet(40/60)–EA 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CD2Cl2): δH 8.78 (1H, s), 8.05 (1H, d, J = 8.3 Hz), 7.54 (1H, d, J = 7.7 Hz), 7.44–7.38 (6H, m), 7.38–7.30 (4H, m), 7.30–7.24 (1H, m), 7.20 (1H, app t, J = 7.5 Hz), 5.51 (1H, app t, J = 5.3 Hz), 5.45 (1H, d, J = 11.9 Hz), 5.31 (1H, d, J = 11.9 Hz), 4.98 (1H, d, J = 7.9 Hz), 3.46 (1H, app q, J = 8.1 Hz), 2.36–2.30 (1H, m), 2.18 (1H, ddd, J = 13.9, 8.6, 5.3 Hz); 13C NMR (101 MHz, CD2Cl2): δC 178.1, 173.8, 153.7, 152.4, 151.4, 136.8, 134.7, 131.1, 130.6, 129.1, 128.9, 128.8, 128.8, 128.4, 126.2, 125.7, 125.6, 123.7, 119.0, 115.5, 114.2, 69.7, 47.7, 41.0, 39.5, 26.8; IR (neat): νmax/cm−1 = 3169, 2975, 1699; MS (pNSI): 279.1 (38%), 373.1 (13%, (M − PTAD)+), 550.2 (21%, (M + H)+), 567.2 (100% (M + NH4)+), 1116.4 (39%, (2M + NH4)+), 1666.5 (6%, (3M + NH4)+); HRMS (pNSI): calcd C30H24N5O6 [M + H]+: 550.1721; observed: 550.1719.
1); 1H NMR (400 MHz, CD2Cl2): δH 8.78 (1H, s), 8.05 (1H, d, J = 8.3 Hz), 7.54 (1H, d, J = 7.7 Hz), 7.44–7.38 (6H, m), 7.38–7.30 (4H, m), 7.30–7.24 (1H, m), 7.20 (1H, app t, J = 7.5 Hz), 5.51 (1H, app t, J = 5.3 Hz), 5.45 (1H, d, J = 11.9 Hz), 5.31 (1H, d, J = 11.9 Hz), 4.98 (1H, d, J = 7.9 Hz), 3.46 (1H, app q, J = 8.1 Hz), 2.36–2.30 (1H, m), 2.18 (1H, ddd, J = 13.9, 8.6, 5.3 Hz); 13C NMR (101 MHz, CD2Cl2): δC 178.1, 173.8, 153.7, 152.4, 151.4, 136.8, 134.7, 131.1, 130.6, 129.1, 128.9, 128.8, 128.8, 128.4, 126.2, 125.7, 125.6, 123.7, 119.0, 115.5, 114.2, 69.7, 47.7, 41.0, 39.5, 26.8; IR (neat): νmax/cm−1 = 3169, 2975, 1699; MS (pNSI): 279.1 (38%), 373.1 (13%, (M − PTAD)+), 550.2 (21%, (M + H)+), 567.2 (100% (M + NH4)+), 1116.4 (39%, (2M + NH4)+), 1666.5 (6%, (3M + NH4)+); HRMS (pNSI): calcd C30H24N5O6 [M + H]+: 550.1721; observed: 550.1719.
3ee – benzyl (R*)-6-(hydroxy(phenyl)amino)-1,3-dioxo-2-phenyl-2,3,5,6-tetrahydro-1H,11H-[1,2,4]triazolo[1′,2′:1,2]pyridazino[3,4-b]indole-11-carboxylate
To a stirred Schlenk flask was added benzyl 3-vinyl-1H-indole-1-carboxylate (189 mg, 0.68 mmol) and DCM (10 mL). The solution was cooled to −78 °C and 4-phenyl-1,2,4-triazolidine-3,5-dione (120 mg, 0.68 mmol) was added. The reaction was stirred at −78 °C for 5 hours. The reaction was warmed to room temperature, nitrosobenzene (73 mg, 0.68 mmol) was added and the reaction was stirred for 3 hours. The solvent was removed under reduced pressure to leave the crude product as a pale yellow oil. The product was purified by column chromatography (petrol (40/60)–ethyl acetate 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, column diameter = 1 cm, silica = 16 cm) to give benzyl (R*)-6-(hydroxy(phenyl)amino)-1,3-dioxo-2-phenyl-2,3,5,6-tetrahydro-1H,11H-[1,2,4]triazolo[1′,2′:1,2]pyridazino[3,4-b]indole-11-carboxylate (72%, 274 mg, 0.49 mmol) as a white powder.
1, column diameter = 1 cm, silica = 16 cm) to give benzyl (R*)-6-(hydroxy(phenyl)amino)-1,3-dioxo-2-phenyl-2,3,5,6-tetrahydro-1H,11H-[1,2,4]triazolo[1′,2′:1,2]pyridazino[3,4-b]indole-11-carboxylate (72%, 274 mg, 0.49 mmol) as a white powder.
Mp: 101.2–103.1 °C; Rf: 0.53 (Pet(40/60)–EA 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CD2Cl2): δH 8.09 (1H, d, J = 8.2 Hz), 7.51–7.38 (8H, m), 7.38–7.24 (5H, m), 7.23–7.07 (4H, m), 6.87 (1H, d, J = 7.9 Hz), 6.36 (1H, s), 5.52 (1H, d, J = 12.1 Hz), 5.39 (1H, d, J = 12.1 Hz), 5.22 (1H, dd, J = 14.0, 1.7 Hz), 4.97–4.89 (1H, m), 3.66 (1H, dd, J = 14.0, 3.4 Hz); 13C NMR (101 MHz, CD2Cl2): δC 147.2, 147.0, 146.6, 145.6, 131.0, 130.1, 127.3, 126.2, 125.4, 125.2, 124.9, 124.9, 124.9, 124.8, 124.8, 122.4, 122.3, 120.7, 120.5119.7, 115.8, 114.5, 110.5, 97.2, 66.2, 55.1, 39.6; IR (neat): νmax/cm−1 = 3337, 3063, 1716; MS (pAPCI): 395.1 (100%), 451.1 (59%, (M − (N(OH)Ph))+), 542.2 (5%, (M − (H2O) + H)+), 558.2 (1%, (M − H)+), 560.2 (1%, (M + H)+); HRMS (pAPCI): calcd C32H26N5O5 [M + H]+: 560.1928; observed: 560.1913.
1); 1H NMR (400 MHz, CD2Cl2): δH 8.09 (1H, d, J = 8.2 Hz), 7.51–7.38 (8H, m), 7.38–7.24 (5H, m), 7.23–7.07 (4H, m), 6.87 (1H, d, J = 7.9 Hz), 6.36 (1H, s), 5.52 (1H, d, J = 12.1 Hz), 5.39 (1H, d, J = 12.1 Hz), 5.22 (1H, dd, J = 14.0, 1.7 Hz), 4.97–4.89 (1H, m), 3.66 (1H, dd, J = 14.0, 3.4 Hz); 13C NMR (101 MHz, CD2Cl2): δC 147.2, 147.0, 146.6, 145.6, 131.0, 130.1, 127.3, 126.2, 125.4, 125.2, 124.9, 124.9, 124.9, 124.8, 124.8, 122.4, 122.3, 120.7, 120.5119.7, 115.8, 114.5, 110.5, 97.2, 66.2, 55.1, 39.6; IR (neat): νmax/cm−1 = 3337, 3063, 1716; MS (pAPCI): 395.1 (100%), 451.1 (59%, (M − (N(OH)Ph))+), 542.2 (5%, (M − (H2O) + H)+), 558.2 (1%, (M − H)+), 560.2 (1%, (M + H)+); HRMS (pAPCI): calcd C32H26N5O5 [M + H]+: 560.1928; observed: 560.1913.
3ff – benzyl (R*)-6-(hydroxy(o-tolyl)amino)-1,3-dioxo-2-phenyl-2,3,5,6-tetrahydro-1H,11H-[1,2,4]triazolo[1′,2′:1,2]pyridazino[3,4-b]indole-11-carboxylate
To a stirred Schlenk flask was added benzyl 3-vinyl-1H-indole-1-carboxylate (94 mg, 0.34 mmol) and DCM (10 ml). The reaction mixture was cooled to −78 °C and 4-phenyl-1,2,4-triazolidine-3,5-dione (60 mg, 0.34 mmol) was added. The reaction mixture was stirred at −78 °C for 5 hours, 1-methyl-2-nitrosobenzene was added (41 mg, 0.34 mmol) and the reaction stirred at room temperature for 18 hours. The solvent was removed under reduced pressure to leave the crude product as a yellow powder. The product was purified by column chromatography (petrol (40/60)–ethyl acetate 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, column diameter = 2 cm, silica = 20 cm) to give benzyl (R*)-6-(hydroxy(o-tolyl)amino)-1,3-dioxo-2-phenyl-2,3,5,6-tetrahydro-1H,11H-[1,2,4]triazolo[1′,2′:1,2]pyridazino[3,4-b]indole-11-carboxylate (68%, 132 mg, 0.23 mmol) as an off white powder.
1, column diameter = 2 cm, silica = 20 cm) to give benzyl (R*)-6-(hydroxy(o-tolyl)amino)-1,3-dioxo-2-phenyl-2,3,5,6-tetrahydro-1H,11H-[1,2,4]triazolo[1′,2′:1,2]pyridazino[3,4-b]indole-11-carboxylate (68%, 132 mg, 0.23 mmol) as an off white powder.
Mp: 163.8–165.1 °C; Rf: 0.49 (Pet(40/60)–EA 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CD2Cl2): δH 8.01 (1H, d, J = 8.3 Hz), 7.65 (1H, dd, J = 8.1, 1.3 Hz), 7.54–7.38 (7H, m), 7.35 (3H, dd, J = 5.0, 2.1 Hz), 7.19 (2H, app dtd, J = 8.5, 7.2, 6.7, 1.4 Hz), 6.99 (2H, app tdd, J = 7.5, 3.4, 1.2 Hz), 6.90 (1H, dd, J = 7.7, 1.4 Hz), 6.75 (1H, d, J = 7.8 Hz), 5.87 (1H, s, OH), 5.48 (1H, d, J = 11.9 Hz), 5.34 (1H, d, J = 11.9 Hz), 5.19 (1H, dd, J = 14.1, 2.3 Hz), 4.63 (1H, app t, J = 2.3 Hz), 3.52 (1H, dd, J = 14.1, 2.3 Hz), 1.94 (3H, s); 13C NMR (101 MHz, CDCl3): δC 151.9, 150.3, 150.0, 148.8, 134.6, 133.6, 131.6, 131.1, 130.6, 130.0, 129.4, 128.9, 128.9, 128.8, 128.8, 127.0, 126.6, 126.0, 125.7, 124.4, 123.6, 122.7, 117.9, 114.6, 101.7, 70.1, 58.6, 43.5, 17.7; IR (neat): νmax/cm−1 = 3291, 2981, 1782, 1737, 1699; MS (pNSI): 199.2 (100%), 407.2 (79%), 451.1 (81%, (M − (N(OH)(o-Tol)))+), 572.2 (25%, (M − H)+), 596.2 (65%, (M + Na)+); HRMS (pNSI): calcd C33H27N5O5Na [M + Na]+: 596.1904; observed: 596.1898.
1); 1H NMR (400 MHz, CD2Cl2): δH 8.01 (1H, d, J = 8.3 Hz), 7.65 (1H, dd, J = 8.1, 1.3 Hz), 7.54–7.38 (7H, m), 7.35 (3H, dd, J = 5.0, 2.1 Hz), 7.19 (2H, app dtd, J = 8.5, 7.2, 6.7, 1.4 Hz), 6.99 (2H, app tdd, J = 7.5, 3.4, 1.2 Hz), 6.90 (1H, dd, J = 7.7, 1.4 Hz), 6.75 (1H, d, J = 7.8 Hz), 5.87 (1H, s, OH), 5.48 (1H, d, J = 11.9 Hz), 5.34 (1H, d, J = 11.9 Hz), 5.19 (1H, dd, J = 14.1, 2.3 Hz), 4.63 (1H, app t, J = 2.3 Hz), 3.52 (1H, dd, J = 14.1, 2.3 Hz), 1.94 (3H, s); 13C NMR (101 MHz, CDCl3): δC 151.9, 150.3, 150.0, 148.8, 134.6, 133.6, 131.6, 131.1, 130.6, 130.0, 129.4, 128.9, 128.9, 128.8, 128.8, 127.0, 126.6, 126.0, 125.7, 124.4, 123.6, 122.7, 117.9, 114.6, 101.7, 70.1, 58.6, 43.5, 17.7; IR (neat): νmax/cm−1 = 3291, 2981, 1782, 1737, 1699; MS (pNSI): 199.2 (100%), 407.2 (79%), 451.1 (81%, (M − (N(OH)(o-Tol)))+), 572.2 (25%, (M − H)+), 596.2 (65%, (M + Na)+); HRMS (pNSI): calcd C33H27N5O5Na [M + Na]+: 596.1904; observed: 596.1898.
3gg – benzyl (3aS*,5S*,10bS*)-5-(hydroxy(phenyl)amino)-7-methoxy-2-methyl-1,3-dioxo-2,3,3a,4,5,10b-hexahydropyrrolo[3,4-a]carbazole-10(1H)-carboxylate
To a stirred Schlenk flask was added benzyl 5-methoxy-3-vinyl-1H-indole-1-carboxylate (209 mg, 0.68 mmol), DCM (10 mL) and 1-methyl-1H-pyrrole-2,5-dione (76 mg, 0.68 mmol). The resulting solution was heated at reflux for 18 hours. The reaction was cooled to room temperature and nitrosobenzene (72 mg, 0.68 mmol) was added and the reaction was stirred for 1.5 hours. The solvent was removed under reduced pressure to leave the crude product as a pale orange oil. The product was purified by column chromatography (petrol (40/60)–ethyl acetate 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, column diameter = 1 cm, silica = 16 cm) to give benzyl (3aS*,5S*,10bS*)-5-(hydroxy(phenyl)amino)-7-methoxy-2-methyl-1,3-dioxo-2,3,3a,4,5,10b-hexahydropyrrolo[3,4-a]carbazole-10(1H)-carboxylate (73%, 218 mg, 0.44 mmol) as an orange powder.
1, column diameter = 1 cm, silica = 16 cm) to give benzyl (3aS*,5S*,10bS*)-5-(hydroxy(phenyl)amino)-7-methoxy-2-methyl-1,3-dioxo-2,3,3a,4,5,10b-hexahydropyrrolo[3,4-a]carbazole-10(1H)-carboxylate (73%, 218 mg, 0.44 mmol) as an orange powder.
Mp: 106.8–110.2 °C; Rf: 0.65 (Pet(40/60)–EA 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CD2Cl2): δH 7.97 (1H, br d, J = 8.3 Hz), 7.49–7.47 (2H, m), 7.41–7.37 (3H, m), 7.33–7.30 (2H, m), 7.21–7.19 (2H, m), 7.05–7.00 (2H, m), 6.85 (1H, br d, J = 8.4 Hz), 5.53 (1H, d, J = 11.9 Hz), 5.39 (1H, d, J = 11.9 Hz), 5.02 (1H, br s), 4.91–4.87 (1H, m), 4.75 (1H, br s), 3.68 (3H, s), 3.50 (1H, br s), 2.86 (3H, s), 2.36 (1H, br s), 2.01 (1H, br s); 13C NMR (101 MHz, CD2Cl2): δC 178.3, 174.5, 156.3, 151.5, 151.1, 135.0, 131.5, 130.7, 129.0, 128.9, 128.8, 128.8, 128.4, 122.4, 117.7, 117.2, 115.9, 113.4, 102.4, 69.4, 57.9, 55.6, 40.3, 39.2, 24.9, 23.4; IR (neat): νmax/cm−1 = 3408, 2969, 2890, 1699; MS (pNSI): 417.1 (100%, (M − (N(OH)Ph))+), 524.2 (68%, (M − (H2) + H)+), 548.2 (16%, (M + Na)+), 1073.4 (4%, (2M + Na)+); HRMS (pNSI): calcd C30H27N3O6Na [M + Na]+: 548.1792; observed: 548.1785.
1); 1H NMR (400 MHz, CD2Cl2): δH 7.97 (1H, br d, J = 8.3 Hz), 7.49–7.47 (2H, m), 7.41–7.37 (3H, m), 7.33–7.30 (2H, m), 7.21–7.19 (2H, m), 7.05–7.00 (2H, m), 6.85 (1H, br d, J = 8.4 Hz), 5.53 (1H, d, J = 11.9 Hz), 5.39 (1H, d, J = 11.9 Hz), 5.02 (1H, br s), 4.91–4.87 (1H, m), 4.75 (1H, br s), 3.68 (3H, s), 3.50 (1H, br s), 2.86 (3H, s), 2.36 (1H, br s), 2.01 (1H, br s); 13C NMR (101 MHz, CD2Cl2): δC 178.3, 174.5, 156.3, 151.5, 151.1, 135.0, 131.5, 130.7, 129.0, 128.9, 128.8, 128.8, 128.4, 122.4, 117.7, 117.2, 115.9, 113.4, 102.4, 69.4, 57.9, 55.6, 40.3, 39.2, 24.9, 23.4; IR (neat): νmax/cm−1 = 3408, 2969, 2890, 1699; MS (pNSI): 417.1 (100%, (M − (N(OH)Ph))+), 524.2 (68%, (M − (H2) + H)+), 548.2 (16%, (M + Na)+), 1073.4 (4%, (2M + Na)+); HRMS (pNSI): calcd C30H27N3O6Na [M + Na]+: 548.1792; observed: 548.1785.
3hh – benzyl (3aS*,5S*,10bS*)-5-(hydroxy(o-tolyl)amino)-7-methoxy-2-methyl-1,3-dioxo-2,3,3a,4,5,10b-hexahydropyrrolo[3,4-a]carbazole-10(1H)-carboxylate
To a stirred Schlenk flask was added benzyl 5-methoxy-3-vinyl-1H-indole-1-carboxylate (209 mg, 0.68 mmol), DCM (10 mL) and 1-methyl-1H-pyrrole-2,5-dione (76 mg, 0.68 mmol). The resulting solution was heated at reflux for 18 hours. The reaction was cooled to room temperature and 1-methyl-2-nitrosobenzene (82 mg, 0.68 mmol) was added. The solution was stirred at room temperature for 3 hours. The solvent was removed under reduced pressure to leave the crude product as a pale yellow solid. The product was purified by column chromatography (petrol (40/60)–ethyl acetate 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, column diameter = 2 cm, silica = 15 cm) to give benzyl (3aS*,5S*,10bS*)-5-(hydroxy(o-tolyl)amino)-7-methoxy-2-methyl-1,3-dioxo-2,3,3a,4,5,10b-hexahydropyrrolo[3,4-a]carbazole-10(1H)-carboxylate (74%, 279 mg, 0.50 mmol) as a pale yellow powder.
1, column diameter = 2 cm, silica = 15 cm) to give benzyl (3aS*,5S*,10bS*)-5-(hydroxy(o-tolyl)amino)-7-methoxy-2-methyl-1,3-dioxo-2,3,3a,4,5,10b-hexahydropyrrolo[3,4-a]carbazole-10(1H)-carboxylate (74%, 279 mg, 0.50 mmol) as a pale yellow powder.
Mp: 107.6–110.1 °C; Rf: 0.29 (Pet(40/60)–EA 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CD2Cl2): δH 7.93 (1H, d, J = 9.1 Hz), 7.54 (1H, dd, J = 8.1, 1.3 Hz), 7.49–7.45 (2H, m), 7.43–7.35 (3H, m), 7.19 (1H, ddd, J = 7.6, 6.9, 1.9 Hz), 7.10–7.01 (2H, m), 6.88 (1H, d, J = 2.6 Hz), 6.80 (1H, dd, J = 9.1, 2.6 Hz), 5.52 (1H, d, J = 11.9 Hz), 5.37 (1H, d, J = 11.9 Hz), 5.18 (1H, s), 4.87 (1H, d, J = 8.1 Hz), 4.33–4.29 (1H, m), 3.65 (3H, s), 3.67–3.61 (1H, m), 2.86 (3H, s), 2.61 (1H, app dt, J = 13.4, 5.9 Hz), 2.22 (3H, s), 1.86 (1H, ddd, J = 13.4, 8.5, 4.3 Hz); 13C NMR (101 MHz, CD2Cl2): δC 178.4, 174.4, 156.1, 151.6, 149.8, 135.0, 134.9, 131.2, 130.8, 130.6, 130.4, 128.8, 128.8, 128.5, 126.4, 125.3, 121.7, 117.5, 115.7, 113.6, 102.0, 69.3, 57.8, 55.4, 40.5, 38.9, 25.0, 24.9, 18.2; IR (neat): νmax/cm−1 = 3370, 2965, 2887, 1699; MS (pNSI): 207.1 (39%), 417.1 (34%, (M − (N(OH)(o-Tol)))+), 438.2 (100%, (M − (H2) + H)+), 1075.4 (15%, (2(M − (H2) + H)+)); HRMS (pNSI): calcd C31H28N3O6 [M − (H2) + H]+: 538.1976; observed: 538.1973.
1); 1H NMR (400 MHz, CD2Cl2): δH 7.93 (1H, d, J = 9.1 Hz), 7.54 (1H, dd, J = 8.1, 1.3 Hz), 7.49–7.45 (2H, m), 7.43–7.35 (3H, m), 7.19 (1H, ddd, J = 7.6, 6.9, 1.9 Hz), 7.10–7.01 (2H, m), 6.88 (1H, d, J = 2.6 Hz), 6.80 (1H, dd, J = 9.1, 2.6 Hz), 5.52 (1H, d, J = 11.9 Hz), 5.37 (1H, d, J = 11.9 Hz), 5.18 (1H, s), 4.87 (1H, d, J = 8.1 Hz), 4.33–4.29 (1H, m), 3.65 (3H, s), 3.67–3.61 (1H, m), 2.86 (3H, s), 2.61 (1H, app dt, J = 13.4, 5.9 Hz), 2.22 (3H, s), 1.86 (1H, ddd, J = 13.4, 8.5, 4.3 Hz); 13C NMR (101 MHz, CD2Cl2): δC 178.4, 174.4, 156.1, 151.6, 149.8, 135.0, 134.9, 131.2, 130.8, 130.6, 130.4, 128.8, 128.8, 128.5, 126.4, 125.3, 121.7, 117.5, 115.7, 113.6, 102.0, 69.3, 57.8, 55.4, 40.5, 38.9, 25.0, 24.9, 18.2; IR (neat): νmax/cm−1 = 3370, 2965, 2887, 1699; MS (pNSI): 207.1 (39%), 417.1 (34%, (M − (N(OH)(o-Tol)))+), 438.2 (100%, (M − (H2) + H)+), 1075.4 (15%, (2(M − (H2) + H)+)); HRMS (pNSI): calcd C31H28N3O6 [M − (H2) + H]+: 538.1976; observed: 538.1973.
3ii – benzyl (3aS*,5S*,10bS*)-5-(hydroxy(phenyl)amino)-7-methoxy-1,3-dioxo-2,3,3a,4,5,10b-hexahydropyrrolo[3,4-a]carbazole-10(1H)-carboxylate
To a stirred Schlenk flask was added benzyl 5-methoxy-3-vinyl-1H-indole-1-carboxylate (209 mg, 0.68 mmol), DCM (10 mL) and 1H-pyrrole-2,5-dione (66 mg, 0.68 mmol). The resulting solution was heated at reflux for 18 hours. The reaction was cooled to room temperature and nitrosobenzene (72 mg, 0.68 mmol) was added and the reaction was stirred for 2.5 hours. The solvent was removed under reduced pressure to leave the crude product as a pale orange oil. The product was purified by column chromatography (petrol (40/60)–ethyl acetate 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, column diameter = 1 cm, silica = 16 cm) to give benzyl (3aS*,5S*,10bS*)-5-(hydroxy(phenyl)amino)-7-methoxy-1,3-dioxo-2,3,3a,4,5,10b-hexahydropyrrolo[3,4-a]carbazole-10(1H)-carboxylate (79%, 317 mg, 0.54 mmol) as a pale orange powder.
1, column diameter = 1 cm, silica = 16 cm) to give benzyl (3aS*,5S*,10bS*)-5-(hydroxy(phenyl)amino)-7-methoxy-1,3-dioxo-2,3,3a,4,5,10b-hexahydropyrrolo[3,4-a]carbazole-10(1H)-carboxylate (79%, 317 mg, 0.54 mmol) as a pale orange powder.
Mp: 134.2–136.9 °C; Rf: 0.34 (Pet(40/60)–EA 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2); 1H NMR (400 MHz, CD2Cl2): δH 8.42 (1H, br s), 7.94 (1H, d, J = 9.0 Hz), 7.47–7.44 (2H, m), 7.40–7.34 (3H, m), 7.30–7.27 (2H, m), 7.19–7.17 (2H, m), 7.01–6.98 (1H, m), 6.93 (1H, br s), 6.81 (1H, d, J = 9.0 Hz), 5.49 (1H, d, J = 11.9 Hz), 5.39 (1H, br s), 5.34 (1H, d, J = 11.9 Hz), 4.92 (1H, br d, J = 6.2 Hz), 4.74 (1H, br s), 3.64 (3H, s), 3.57–3.51 (1H, br m), 2.39–2.35 (1H, br m), 1.91–1.89 (1H, br m); 13C NMR (101 MHz, CD2Cl2): δC 178.6, 174.4, 156.2, 151.5, 151.0, 135.0, 131.4, 130.3, 129.0, 128.9, 128.8, 128.8, 128.4, 122.6, 117.5, 117.4, 116.0, 113.5, 102.3, 69.4, 57.9, 55.6, 41.5, 40.5, 24.0; IR (neat): νmax/cm−1 = 3233, 2952, 1708; MS (pNSI): 403.1 (37%, (M − (N(OH)Ph))+), 510.2 (100%, (M − (H2) + H)+), 532.1 (26%, (M − (H2) + Na)+); HRMS (pNSI): calcd C29H24N3O6 [M − (H2) + H]+: 510.1654; observed: 510.1660.
2); 1H NMR (400 MHz, CD2Cl2): δH 8.42 (1H, br s), 7.94 (1H, d, J = 9.0 Hz), 7.47–7.44 (2H, m), 7.40–7.34 (3H, m), 7.30–7.27 (2H, m), 7.19–7.17 (2H, m), 7.01–6.98 (1H, m), 6.93 (1H, br s), 6.81 (1H, d, J = 9.0 Hz), 5.49 (1H, d, J = 11.9 Hz), 5.39 (1H, br s), 5.34 (1H, d, J = 11.9 Hz), 4.92 (1H, br d, J = 6.2 Hz), 4.74 (1H, br s), 3.64 (3H, s), 3.57–3.51 (1H, br m), 2.39–2.35 (1H, br m), 1.91–1.89 (1H, br m); 13C NMR (101 MHz, CD2Cl2): δC 178.6, 174.4, 156.2, 151.5, 151.0, 135.0, 131.4, 130.3, 129.0, 128.9, 128.8, 128.8, 128.4, 122.6, 117.5, 117.4, 116.0, 113.5, 102.3, 69.4, 57.9, 55.6, 41.5, 40.5, 24.0; IR (neat): νmax/cm−1 = 3233, 2952, 1708; MS (pNSI): 403.1 (37%, (M − (N(OH)Ph))+), 510.2 (100%, (M − (H2) + H)+), 532.1 (26%, (M − (H2) + Na)+); HRMS (pNSI): calcd C29H24N3O6 [M − (H2) + H]+: 510.1654; observed: 510.1660.
3jj – benzyl (3aS*,5S*,10bS*)-5-(hydroxy(o-tolyl)amino)-7-methoxy-1,3-dioxo-2,3,3a,4,5,10b-hexahydropyrrolo[3,4-a]carbazole-10(1H)-carboxylate
To a stirred Schlenk flask was added benzyl 5-methoxy-3-vinyl-1H-indole-1-carboxylate (209 mg, 0.68 mmol), DCM (10 mL) and 1H-pyrrole-2,5-dione (66 mg, 0.68 mmol). The resulting solution was heated at reflux for 18 hours. The reaction was cooled to room temperature and 1-methyl-2-nitrosobenzene (82 mg, 0.68 mmol) was added. The solution was stirred at room temperature for 3.5 hours. The solvent was removed under reduced pressure to leave the crude product as a pale yellow solid. The product was purified by column chromatography (petrol (40/60)–ethyl acetate 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, column diameter = 2 cm, silica = 14 cm) to give benzyl (3aS*,5S*,10bS*)-5-(hydroxy(o-tolyl)amino)-7-methoxy-1,3-dioxo-2,3,3a,4,5,10b-hexahydropyrrolo[3,4-a]carbazole-10(1H)-carboxylate (76%, 255 mg, 0.52 mmol) as a pale yellow powder.
1, column diameter = 2 cm, silica = 14 cm) to give benzyl (3aS*,5S*,10bS*)-5-(hydroxy(o-tolyl)amino)-7-methoxy-1,3-dioxo-2,3,3a,4,5,10b-hexahydropyrrolo[3,4-a]carbazole-10(1H)-carboxylate (76%, 255 mg, 0.52 mmol) as a pale yellow powder.
Mp: 193.0–195.6 °C; Rf: 0.20 (Pet(40/60)–EA 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CD2Cl2): δH 7.92 (1H, d, J = 9.7 Hz), 7.81 (1H, s), 7.53 (1H, d, J = 7.8 Hz), 7.46 (2H, dd, J = 7.8, 1.6 Hz), 7.42–7.33 (3H, m), 7.21–7.15 (1H, m), 7.09–7.00 (2H, m), 6.84–6.75 (2H, m), 5.50 (1H, d, J = 11.9 Hz), 5.35 (1H, d, J = 11.9 Hz), 5.16 (1H, s), 4.96 (1H, d, J = 7.6 Hz), 4.37 (1H, app t, J = 4.9 Hz), 3.71 (1H, app td, J = 8.7, 5.6 Hz), 3.64 (3H, s), 2.64 (1H, app dt, J = 13.6, 5.6 Hz), 2.21 (3H, s), 1.88 (1H, ddd, J = 13.6, 9.3, 4.3 Hz); 13C NMR (101 MHz, DMSO-d6): δC 180.6, 176.4, 155.6, 151.8, 151.7, 135.8, 131.6, 130.8, 130.7, 130.2, 129.1, 129.1, 129.0, 126.7, 124.8, 122.3, 117.2, 115.2, 113.5, 102.7, 69.1, 57.3, 55.5, 41.9, 40.3, 27.2, 18.5; IR (neat): νmax/cm−1 = 3457, 3367, 2981, 2886, 1712; MS (pNSI): 403.1 (100%, (M − (N(OH)(o-Tol)))+), 524.1 (75%, (M − (H2) + H)+), 548.2 (16%, (M + Na)+), 1073.4 (5%, (2M + Na)+); HRMS (pNSI): calcd C30H27N3O6Na [M + Na]+: 548.1792; observed: 548.1785.
1); 1H NMR (400 MHz, CD2Cl2): δH 7.92 (1H, d, J = 9.7 Hz), 7.81 (1H, s), 7.53 (1H, d, J = 7.8 Hz), 7.46 (2H, dd, J = 7.8, 1.6 Hz), 7.42–7.33 (3H, m), 7.21–7.15 (1H, m), 7.09–7.00 (2H, m), 6.84–6.75 (2H, m), 5.50 (1H, d, J = 11.9 Hz), 5.35 (1H, d, J = 11.9 Hz), 5.16 (1H, s), 4.96 (1H, d, J = 7.6 Hz), 4.37 (1H, app t, J = 4.9 Hz), 3.71 (1H, app td, J = 8.7, 5.6 Hz), 3.64 (3H, s), 2.64 (1H, app dt, J = 13.6, 5.6 Hz), 2.21 (3H, s), 1.88 (1H, ddd, J = 13.6, 9.3, 4.3 Hz); 13C NMR (101 MHz, DMSO-d6): δC 180.6, 176.4, 155.6, 151.8, 151.7, 135.8, 131.6, 130.8, 130.7, 130.2, 129.1, 129.1, 129.0, 126.7, 124.8, 122.3, 117.2, 115.2, 113.5, 102.7, 69.1, 57.3, 55.5, 41.9, 40.3, 27.2, 18.5; IR (neat): νmax/cm−1 = 3457, 3367, 2981, 2886, 1712; MS (pNSI): 403.1 (100%, (M − (N(OH)(o-Tol)))+), 524.1 (75%, (M − (H2) + H)+), 548.2 (16%, (M + Na)+), 1073.4 (5%, (2M + Na)+); HRMS (pNSI): calcd C30H27N3O6Na [M + Na]+: 548.1792; observed: 548.1785.
3kk – benzyl (R*)-6-(hydroxy(phenyl)amino)-8-methoxy-1,3-dioxo-2-phenyl-2,3,5,6-tetrahydro-1H,11H-[1,2,4]triazolo[1′,2′:1,2]pyridazino[3,4-b]indole-11-carboxylate
To a stirred Schlenk flask was added benzyl 5-methoxy-3-vinyl-1H-indole-1-carboxylate (209 mg, 0.68 mmol) and DCM (10 mL). The solution was cooled to −78 °C and 4-phenyl-1,2,4-triazolidine-3,5-dione (120 mg, 0.68 mmol) was added. The reaction was stirred at −78 °C for 1.5 hours. The reaction was warmed to room temperature, nitrosobenzene (73 mg, 0.68 mmol) was added and the reaction was stirred for 20 hours. The solvent was removed under reduced pressure to leave the crude product as a pale yellow oil. The product was purified by column chromatography (petrol (40/60)–ethyl acetate 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, column diameter = 1 cm, silica = 16 cm) to give benzyl (R*)-6-(hydroxy(phenyl)amino)-8-methoxy-1,3-dioxo-2-phenyl-2,3,5,6-tetrahydro-1H,11H-[1,2,4]triazolo[1′,2′:1,2]pyridazino[3,4-b]indole-11-carboxylate (78%, 312 mg, 0.53 mmol) as a white powder.
1, column diameter = 1 cm, silica = 16 cm) to give benzyl (R*)-6-(hydroxy(phenyl)amino)-8-methoxy-1,3-dioxo-2-phenyl-2,3,5,6-tetrahydro-1H,11H-[1,2,4]triazolo[1′,2′:1,2]pyridazino[3,4-b]indole-11-carboxylate (78%, 312 mg, 0.53 mmol) as a white powder.
Mp: 110.4–113.2 °C; Rf: 0.20 (Pet(40/60)–EA 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CD2Cl2): δH 7.88 (1H, d, J = 9.1 Hz), 7.42–7.38 (5H, m), 7.36–7.32 (3H, m), 7.29–7.26 (2H, m), 7.24–7.20 (2H, m), 7.13 (2H, d, J = 8.1 Hz), 7.05 (1H, app t, J = 7.3 Hz). 6.76 (1H, dd, J = 9.1, 2.6 Hz), 6.24 (1H, s), 6.10 (1H, d, J = 2.5 Hz), 5.43 (1H, d, J = 12.0 Hz), 5.32–5.29 (1H, m), 5.25–5.18 (1H, m), 4.84–4.80 (1H, m), 3.64 (1H, dd, J = 14.0, 3.3 Hz), 3.55 (3H, s); 13C NMR (101 MHz, CD2Cl2): δC 156.5, 151.1, 150.3, 149.4, 134.8, 131.2, 130.3, 129.2, 129.0, 129.0, 128.70, 128.7, 128.7, 128.6, 128.2, 126.9, 126.2, 124.6, 119.7, 115.3, 113.2, 101.0, 100.5, 69.9, 59.1, 55.5, 44.0; IR (neat): νmax/cm−1 = 3336, 2935, 1716; MS (pNSI): 481.1 (17%, (M − (N(OH)Ph))+), 588.2 (100%, (M − (H2) + H)+), 612.2 (15%, (M + Na)+); HRMS (pNSI): calcd C33H27N5O6Na [M + Na]+: 612.1854; observed: 612.1838.
1); 1H NMR (400 MHz, CD2Cl2): δH 7.88 (1H, d, J = 9.1 Hz), 7.42–7.38 (5H, m), 7.36–7.32 (3H, m), 7.29–7.26 (2H, m), 7.24–7.20 (2H, m), 7.13 (2H, d, J = 8.1 Hz), 7.05 (1H, app t, J = 7.3 Hz). 6.76 (1H, dd, J = 9.1, 2.6 Hz), 6.24 (1H, s), 6.10 (1H, d, J = 2.5 Hz), 5.43 (1H, d, J = 12.0 Hz), 5.32–5.29 (1H, m), 5.25–5.18 (1H, m), 4.84–4.80 (1H, m), 3.64 (1H, dd, J = 14.0, 3.3 Hz), 3.55 (3H, s); 13C NMR (101 MHz, CD2Cl2): δC 156.5, 151.1, 150.3, 149.4, 134.8, 131.2, 130.3, 129.2, 129.0, 129.0, 128.70, 128.7, 128.7, 128.6, 128.2, 126.9, 126.2, 124.6, 119.7, 115.3, 113.2, 101.0, 100.5, 69.9, 59.1, 55.5, 44.0; IR (neat): νmax/cm−1 = 3336, 2935, 1716; MS (pNSI): 481.1 (17%, (M − (N(OH)Ph))+), 588.2 (100%, (M − (H2) + H)+), 612.2 (15%, (M + Na)+); HRMS (pNSI): calcd C33H27N5O6Na [M + Na]+: 612.1854; observed: 612.1838.
3ll – benzyl (R*)-6-(hydroxy(o-tolyl)amino)-8-methoxy-1,3-dioxo-2-phenyl-2,3,5,6-tetrahydro-1H,11H-[1,2,4]triazolo[1′,2′:1,2]pyridazino[3,4-b]indole-11-carboxylate
To a stirred Schlenk flask was added benzyl 5-methoxy-3-vinyl-1H-indole-1-carboxylate (209 mg, 0.68 mmol) and DCM (10 mL). The solution was cooled to −78 °C and 4-phenyl-1,2,4-triazolidine-3,5-dione (120 mg, 0.68 mmol) was added. The reaction was stirred at −78 °C for 1.5 hours. The reaction was warmed to room temperature, 1-methyl-2-nitrosobenzene (73 mg, 0.68 mmol) was added and the reaction was stirred for 24 hours. The solvent was removed under reduced pressure to leave the crude product as a pale orange oil. The product was purified by column chromatography (petrol (40/60)–ethyl acetate 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, column diameter = 2 cm, silica = 17 cm) to give benzyl (R*)-6-(hydroxy(o-tolyl)amino)-8-methoxy-1,3-dioxo-2-phenyl-2,3,5,6-tetrahydro-1H,11H-[1,2,4]triazolo[1′,2′:1,2]pyridazino[3,4-b]indole-11-carboxylate (82%, 338 mg, 0.56 mmol) as an off white powder.
1, column diameter = 2 cm, silica = 17 cm) to give benzyl (R*)-6-(hydroxy(o-tolyl)amino)-8-methoxy-1,3-dioxo-2-phenyl-2,3,5,6-tetrahydro-1H,11H-[1,2,4]triazolo[1′,2′:1,2]pyridazino[3,4-b]indole-11-carboxylate (82%, 338 mg, 0.56 mmol) as an off white powder.
Mp: 181.1–183.0 °C; Rf: 0.55 (Pet(40/60)–EA 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, DMSO-d6): δH 8.70 (1H, s), 7.84 (1H, d, J = 9.0 Hz), 7.55–7.48 (2H, m), 7.46–7.41 (3H, m), 7.41–7.33 (4H, m), 7.33–7.29 (2H, m), 7.13–7.03 (2H, m), 6.97 (1H, app td, J = 7.4, 1.3 Hz), 6.83 (1H, dd, J = 9.1, 2.6 Hz). 6.38 (1H, s), 5.43 (1H, d, J = 12.1 Hz), 5.33 (1H, d, J = 12.1 Hz), 4.80 (1H, dd, J = 13.7, 1.8 Hz), 4.71 (1H, dd, J = 3.3, 1.8 Hz), 3.68–3.61 (1H, dd, J = 13.7, 3.3 Hz), 3.58 (s, 3H), 2.30 (s, 3H); 13C NMR (101 MHz, CD2Cl2): δC 156.3, 151.6, 150.8, 150.3, 149.8, 135.3, 131.6, 130.9, 130.3, 130.0, 129.7, 129.2, 129.1, 129.1, 128.9, 128.0, 127.4, 127.1, 126.8, 125.2, 121.8, 115.3, 113.2, 103.1, 101.9, 69.9, 55.9, 55.6, 43.9, 18.2; IR (neat): νmax/cm−1 = 3212, 2939, 1720; MS (pNSI): 481.2 (100%, (M − N(OH)(o-Tol))+), 602.2 (34%, (M − (H2) + H)+), 626.2 (100%, (M + Na)+); HRMS (pNSI): calcd C34H29N5O6Na [M + Na]+: 626.2010; observed: 626.2006.
1); 1H NMR (400 MHz, DMSO-d6): δH 8.70 (1H, s), 7.84 (1H, d, J = 9.0 Hz), 7.55–7.48 (2H, m), 7.46–7.41 (3H, m), 7.41–7.33 (4H, m), 7.33–7.29 (2H, m), 7.13–7.03 (2H, m), 6.97 (1H, app td, J = 7.4, 1.3 Hz), 6.83 (1H, dd, J = 9.1, 2.6 Hz). 6.38 (1H, s), 5.43 (1H, d, J = 12.1 Hz), 5.33 (1H, d, J = 12.1 Hz), 4.80 (1H, dd, J = 13.7, 1.8 Hz), 4.71 (1H, dd, J = 3.3, 1.8 Hz), 3.68–3.61 (1H, dd, J = 13.7, 3.3 Hz), 3.58 (s, 3H), 2.30 (s, 3H); 13C NMR (101 MHz, CD2Cl2): δC 156.3, 151.6, 150.8, 150.3, 149.8, 135.3, 131.6, 130.9, 130.3, 130.0, 129.7, 129.2, 129.1, 129.1, 128.9, 128.0, 127.4, 127.1, 126.8, 125.2, 121.8, 115.3, 113.2, 103.1, 101.9, 69.9, 55.9, 55.6, 43.9, 18.2; IR (neat): νmax/cm−1 = 3212, 2939, 1720; MS (pNSI): 481.2 (100%, (M − N(OH)(o-Tol))+), 602.2 (34%, (M − (H2) + H)+), 626.2 (100%, (M + Na)+); HRMS (pNSI): calcd C34H29N5O6Na [M + Na]+: 626.2010; observed: 626.2006.
4a – (3aS*,5S*,10bS*)-5-methoxy-2-methyl-4,5,10,10b-tetrahydropyrrolo[3,4-a]carbazole-1,3(2H,3aH)-dione
To a stirred Schlenk flask was added benzyl (3aS*,5S*,10bS*)-5-(hydroxy(o-tolyl)amino)-2-methyl-1,3-dioxo-2,3,3a,4,5,10b-hexahydropyrrolo[3,4-a]carbazole-10(1H)-carboxylate (120 mg, 0.24 mmol), platinum(IV) oxide (53 mg, 0.24 mmol) and methanol (5 mL). The resulting suspension was placed under an atmosphere of H2 and stirred at room temperature for 18 hours. The suspension was filtered through celite and the solvent was removed under reduced pressure to leave the crude product as an orange solid, The crude product was purified by column chromatography (petrol (40/60)–ethyl acetate 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, column diameter = 2 cm, silica = 13 cm) to give (3aS*,5S*,10bS*)-5-methoxy-2-methyl-4,5,10,10b-tetrahydropyrrolo[3,4-a]carbazole-1,3(2H,3aH)-dione (60%, 41 mg, 0.14 mmol) as a pale yellow powder.
1, column diameter = 2 cm, silica = 13 cm) to give (3aS*,5S*,10bS*)-5-methoxy-2-methyl-4,5,10,10b-tetrahydropyrrolo[3,4-a]carbazole-1,3(2H,3aH)-dione (60%, 41 mg, 0.14 mmol) as a pale yellow powder.
Mp: 139.4–142.7 °C; Rf: 0.25 (Pet(40/60)–EA 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CD2Cl2): δH 8.91 (1H, s), 7.56 (1H, d, J = 7.8 Hz), 7.34 (1H, d, J = 7.7 Hz), 7.14 (1H, app t, J = 7.5 Hz), 7.09 (1H, app t, J = 7.4 Hz), 4.73 (1H, app t, J = 2.7 Hz), 4.14 (1H, d, J = 8.8 Hz), 3.31–3.25 (1H, m), 3.22 (3H, s), 2.94 (1H, app t, J = 2.4 Hz), 2.90 (3H, s), 1.88 (1H, ddd, J = 14.2, 6.9, 2.2 Hz); 13C NMR (101 MHz, CD2Cl2): δC 178.9, 176.1, 136.4, 128.8, 126.6, 122.3, 120.1, 118.2, 111.9, 111.3, 69.4, 56.1, 39.5, 37.3, 27.4, 25.1; IR (neat): νmax/cm−1 3398, 2931, 2870, 1689; MS (pNSI): 285.0 (29%), 355.1 (100%), 371.1 (57%), 560.0 (21%); HRMS (pNSI): calcd C15H13N2O2 [M − OMe]+: 253.0972; observed: 253.0974.
1); 1H NMR (400 MHz, CD2Cl2): δH 8.91 (1H, s), 7.56 (1H, d, J = 7.8 Hz), 7.34 (1H, d, J = 7.7 Hz), 7.14 (1H, app t, J = 7.5 Hz), 7.09 (1H, app t, J = 7.4 Hz), 4.73 (1H, app t, J = 2.7 Hz), 4.14 (1H, d, J = 8.8 Hz), 3.31–3.25 (1H, m), 3.22 (3H, s), 2.94 (1H, app t, J = 2.4 Hz), 2.90 (3H, s), 1.88 (1H, ddd, J = 14.2, 6.9, 2.2 Hz); 13C NMR (101 MHz, CD2Cl2): δC 178.9, 176.1, 136.4, 128.8, 126.6, 122.3, 120.1, 118.2, 111.9, 111.3, 69.4, 56.1, 39.5, 37.3, 27.4, 25.1; IR (neat): νmax/cm−1 3398, 2931, 2870, 1689; MS (pNSI): 285.0 (29%), 355.1 (100%), 371.1 (57%), 560.0 (21%); HRMS (pNSI): calcd C15H13N2O2 [M − OMe]+: 253.0972; observed: 253.0974.
4b – (3aS*,5S*,10bS*)-5-ethoxy-2-methyl-4,5,10,10b-tetrahydropyrrolo[3,4-a]carbazole-1,3(2H,3aH)-dione
To a stirred Schlenk flask was added benzyl (3aS*,5S*,10bS*)-5-(hydroxy(o-tolyl)amino)-2-methyl-1,3-dioxo-2,3,3a,4,5,10b-hexahydropyrrolo[3,4-a]carbazole-10(1H)-carboxylate (100 mg, 0.20 mmol), platinum(IV) oxide (45 mg, 0.20 mmol) and ethanol (5 mL). The resulting suspension was placed under an atmosphere of H2 and stirred at room temperature for 18 hours. The suspension was filtered through celite and the solvent was removed under reduced pressure to leave the crude product as an yellow solid, The crude product was purified by column chromatography (petrol (40/60)–ethyl acetate 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, column diameter = 2 cm, silica = 15 cm) to give (3aS*,5S*,10bS*)-5-ethoxy-2-methyl-4,5,10,10b-tetrahydropyrrolo[3,4-a]carbazole-1,3(2H,3aH)-dione (24%, 20 mg, 0.04 mmol) as a brown powder.
1, column diameter = 2 cm, silica = 15 cm) to give (3aS*,5S*,10bS*)-5-ethoxy-2-methyl-4,5,10,10b-tetrahydropyrrolo[3,4-a]carbazole-1,3(2H,3aH)-dione (24%, 20 mg, 0.04 mmol) as a brown powder.
Mp: 100.6–102.8 °C; Rf: 0.16 (Pet(40/60)–EA 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CD2Cl2): δH 8.78 (1H, s), 7.54 (1H, d, J = 7.7 Hz), 7.35 (1H, d, J = 7.7 Hz), 7.17–7.12 (1H, m), 7.09 (1H, app t, J = 7.0 Hz), 4.83 (1H, app t, J = 2.8 Hz), 4.16 (1H, d, J = 8.8 Hz), 3.55 (1H, app td, J = 6.9, 1.8 Hz), 3.32–3.26 (2H, m), 2.93–2.91 (1H, m), 2.90 (3H, s), 1.88 (1H, ddd, J = 14.3, 7.1, 2.7 Hz), 0.97 (1H, t, J = 7.0 Hz); 13C NMR (101 MHz, CD2Cl2): δC 178.9, 176.0, 136.3, 128.8, 126.5, 122.3, 120.1, 118.1, 112.5, 111.3, 67.4, 63.5, 39.5, 37.3, 27.9, 25.0, 15.2; IR (neat): νmax/cm−1 3300, 2969, 1690; MS (pAPCI): 108.1 (24%), 298.1 (6%, (M)+), 299.1 (5%, (M + H)+); HRMS (pAPCI): calcd C17H19N2O3 [M + H]+: 299.1390; observed: 299.1387.
1); 1H NMR (400 MHz, CD2Cl2): δH 8.78 (1H, s), 7.54 (1H, d, J = 7.7 Hz), 7.35 (1H, d, J = 7.7 Hz), 7.17–7.12 (1H, m), 7.09 (1H, app t, J = 7.0 Hz), 4.83 (1H, app t, J = 2.8 Hz), 4.16 (1H, d, J = 8.8 Hz), 3.55 (1H, app td, J = 6.9, 1.8 Hz), 3.32–3.26 (2H, m), 2.93–2.91 (1H, m), 2.90 (3H, s), 1.88 (1H, ddd, J = 14.3, 7.1, 2.7 Hz), 0.97 (1H, t, J = 7.0 Hz); 13C NMR (101 MHz, CD2Cl2): δC 178.9, 176.0, 136.3, 128.8, 126.5, 122.3, 120.1, 118.1, 112.5, 111.3, 67.4, 63.5, 39.5, 37.3, 27.9, 25.0, 15.2; IR (neat): νmax/cm−1 3300, 2969, 1690; MS (pAPCI): 108.1 (24%), 298.1 (6%, (M)+), 299.1 (5%, (M + H)+); HRMS (pAPCI): calcd C17H19N2O3 [M + H]+: 299.1390; observed: 299.1387.
4c – (3aS*,5S*,10bS*)-5-(hydroxy(phenyl)amino)-2-methyl-4,5,10,10b-tetrahydropyrrolo[3,4-a]carbazole-1,3(2H,3aH)-dione
To a stirred Schlenk flask was added benzyl (3aS*,5S*,10bS*)-5-(hydroxy(phenyl)amino)-2-methyl-1,3-dioxo-2,3,3a,4,5,10b-hexahydropyrrolo[3,4-a]carbazole-10(1H)-carboxylate (120 mg, 0.25 mmol), platinum(IV) oxide (57 mg, 0.25 mmol) and THF (5 mL). The resulting suspension was placed under an atmosphere of H2 and stirred at room temperature for 5 hours. The suspension was filtered through celite and the solvent was removed under reduced pressure to leave the crude product as a yellow solid, The crude product was purified by trituration from DCM to give (3aS*,5S*,10bS*)-5-(hydroxy(phenyl)amino)-2-methyl-4,5,10,10b-tetrahydropyrrolo[3,4-a]carbazole-1,3(2H,3aH)-dione (75%, 68 mg, 0.19 mmol) as a pale yellow powder.Mp: 157.2–161.9 °C; Rf: 0.17 (Pet(40/60)–EA 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, DMSO-d6): δH 11.06 (1H, s), 8.20 (1H, s), 7.35 (1H, d, J = 8.0 Hz), 7.26 (1H, d, J = 8.0 Hz), 7.24–7.19 (2H, m), 7.17–7.08 (2H, m), 6.98 (1H, app ddd, J = 8.2, 7.0, 1.2 Hz), 6.91–6.81 (1H, m), 6.81 (1H, app ddd, J = 8.0, 6.9, 1.0 Hz), 4.88 (1H app t, J = 4.9 Hz), 4.27 (1H, d, J = 8.8 Hz), 3.64 (1H, app td, J = 8.8, 6.1 Hz), 2.81 (3H, s), 2.33 (1H, app dt, J = 13.7, 6.1 Hz), 1.84 (1H, ddd, J = 13.7, 8.8, 4.9 Hz); 13C NMR (101 MHz, DMSO-d6): δC 179.8, 176.3, 153.3, 137.0, 130.0, 129.0, 126.3, 121.5, 121.2, 119.9, 119.0, 117.2, 111.7, 110.0, 57.4, 39.6, 39.3, 25.8, 25.1; IR (neat): νmax/cm−1 3374, 3306, 2919, 1683; MS (pAPCI): 108.0 (28%), 251.1 (100%), 253.1 (68%, (M − (N(OH)Ph) + H)+), 344.1 (13%, (M − (OH) + H)+), 361.1 (3%, (M + H)+); HRMS (pAPCI): calcd C21H20N3O3 [M + H]+: 362.1499; observed: 362.1501.
1); 1H NMR (400 MHz, DMSO-d6): δH 11.06 (1H, s), 8.20 (1H, s), 7.35 (1H, d, J = 8.0 Hz), 7.26 (1H, d, J = 8.0 Hz), 7.24–7.19 (2H, m), 7.17–7.08 (2H, m), 6.98 (1H, app ddd, J = 8.2, 7.0, 1.2 Hz), 6.91–6.81 (1H, m), 6.81 (1H, app ddd, J = 8.0, 6.9, 1.0 Hz), 4.88 (1H app t, J = 4.9 Hz), 4.27 (1H, d, J = 8.8 Hz), 3.64 (1H, app td, J = 8.8, 6.1 Hz), 2.81 (3H, s), 2.33 (1H, app dt, J = 13.7, 6.1 Hz), 1.84 (1H, ddd, J = 13.7, 8.8, 4.9 Hz); 13C NMR (101 MHz, DMSO-d6): δC 179.8, 176.3, 153.3, 137.0, 130.0, 129.0, 126.3, 121.5, 121.2, 119.9, 119.0, 117.2, 111.7, 110.0, 57.4, 39.6, 39.3, 25.8, 25.1; IR (neat): νmax/cm−1 3374, 3306, 2919, 1683; MS (pAPCI): 108.0 (28%), 251.1 (100%), 253.1 (68%, (M − (N(OH)Ph) + H)+), 344.1 (13%, (M − (OH) + H)+), 361.1 (3%, (M + H)+); HRMS (pAPCI): calcd C21H20N3O3 [M + H]+: 362.1499; observed: 362.1501.
Note: H1 NMR ran at 40 °C.
4d – (3aS*,5S*,10bS*)-5-(hydroxy(o-tolyl)amino)-2-methyl-4,5,10,10b-tetrahydropyrrolo[3,4-a]carbazole-1,3(2H,3aH)-dione
To a stirred Schlenk flask was added benzyl (3aS*,5S*,10bS*)-5-(hydroxy(o-tolyl)amino)-2-methyl-1,3-dioxo-2,3,3a,4,5,10b-hexahydropyrrolo[3,4-a]carbazole-10(1H)-carboxylate (100 mg, 0.20 mmol), platinum(IV) oxide (45 mg, 0.20 mmol) and THF (5 mL). The resulting suspension was placed under an atmosphere of H2 and stirred at room temperature for 7 hours. The suspension was filtered through celite and the solvent was removed under reduced pressure to leave the crude product as a yellow solid. The crude product was purified by column chromatography (petrol (40/60)–ethyl acetate 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, column diameter = 2 cm, silica = 16 cm) to give (3aS*,5S*,10bS*)-5-(hydroxy(o-tolyl)amino)-2-methyl-4,5,10,10b-tetrahydropyrrolo[3,4-a]carbazole-1,3(2H,3aH)-dione (87%, 65 mg, 0.17 mmol) as a pale orange powder.
1, column diameter = 2 cm, silica = 16 cm) to give (3aS*,5S*,10bS*)-5-(hydroxy(o-tolyl)amino)-2-methyl-4,5,10,10b-tetrahydropyrrolo[3,4-a]carbazole-1,3(2H,3aH)-dione (87%, 65 mg, 0.17 mmol) as a pale orange powder.
Mp: 179.3–181.0 °C; Rf: 0.60 (Pet(40/60)–EA 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, DMSO-d6): δH 11.08 (1H, s), 8.28 (1H, s), 7.50 (1H, d, J = 8.0 Hz), 7.29 (1H, d, J = 8.1 Hz), 7.14–7.10 (1H, m), 6.91–6.87 (4H, m), 6.67 (1H, app t, J = 7.4 Hz), 4.29 (1H, d, J = 8.2 Hz), 4.27 (1H, app t, J = 3.9 Hz), 3.83–3.74 (1H, m), 2.81 (3H, s), 2.65–2.56 (1H, m), 1.98 (3H, s), 1.67 (1H, ddd, J = 13.6, 10.5, 3.9 Hz); 13C NMR (101 MHz, DMSO-d6): δC 180.0, 176.3, 152.2, 136.7, 130.7, 130.5, 130.3, 126.5, 126.5, 124.5, 122.4, 121.2, 119.3, 118.9, 111.7, 109.7, 58.3, 40.0, 38.6, 27.3, 25.1, 18.3; IR (neat): νmax/cm−1 3379, 2955, 2873, 1691; MS (pAPCI): 108.1 (98%), 251.1 (100%), 253.1 (61%, (M − (N(OH)(o-Tol)) + H)+), 271.1 (6%, (M − (N(OH)(o-Tol)) + OH2)+), 358.2 (13%, (M − OH)+); HRMS (pAPCI): calcd C22H22N3O3 [M + H]+: 376.1656; observed: 376.1658.
1); 1H NMR (400 MHz, DMSO-d6): δH 11.08 (1H, s), 8.28 (1H, s), 7.50 (1H, d, J = 8.0 Hz), 7.29 (1H, d, J = 8.1 Hz), 7.14–7.10 (1H, m), 6.91–6.87 (4H, m), 6.67 (1H, app t, J = 7.4 Hz), 4.29 (1H, d, J = 8.2 Hz), 4.27 (1H, app t, J = 3.9 Hz), 3.83–3.74 (1H, m), 2.81 (3H, s), 2.65–2.56 (1H, m), 1.98 (3H, s), 1.67 (1H, ddd, J = 13.6, 10.5, 3.9 Hz); 13C NMR (101 MHz, DMSO-d6): δC 180.0, 176.3, 152.2, 136.7, 130.7, 130.5, 130.3, 126.5, 126.5, 124.5, 122.4, 121.2, 119.3, 118.9, 111.7, 109.7, 58.3, 40.0, 38.6, 27.3, 25.1, 18.3; IR (neat): νmax/cm−1 3379, 2955, 2873, 1691; MS (pAPCI): 108.1 (98%), 251.1 (100%), 253.1 (61%, (M − (N(OH)(o-Tol)) + H)+), 271.1 (6%, (M − (N(OH)(o-Tol)) + OH2)+), 358.2 (13%, (M − OH)+); HRMS (pAPCI): calcd C22H22N3O3 [M + H]+: 376.1656; observed: 376.1658.
4e – (3aS*,5S*,10bS*)-5-(3,5-dioxo-4-phenyl-1,2,4-triazolidin-1-yl)-2-methyl-4,5,10,10b-tetrahydropyrrolo[3,4-a]carbazole-1,3(2H,3aH)-dione
To a stirred Schlenk flask was added benzyl (3aS*,5S*,10bS*)-5-(3,5-dioxo-4-phenyl-1,2,4-triazolidin-1-yl)-2-methyl-1,3-dioxo-2,3,3a,4,5,10b-hexahydropyrrolo[3,4-a]carbazole-10(1H)-carboxylate (110 mg, 0.20 mmol), platinum(IV) oxide (46 mg, 0.20 mmol) and THF (5 mL). The resulting suspension was placed under an atmosphere of H2 and stirred at room temperature for 5 hours. The suspension was filtered through celite and the solvent was removed under reduced pressure to leave the crude product as a yellow solid. The product was purified by trituration from DCM to give (3aS*,5S*,10bS*)-5-(3,5-dioxo-4-phenyl-1,2,4-triazolidin-1-yl)-2-methyl-4,5,10,10b-tetrahydropyrrolo[3,4-a]carbazole-1,3(2H,3aH)-dione (91%, 79 mg, 0.18 mmol) as an off-white solid.Mp: 262.4–264.0 °C; 1H NMR (400 MHz, DMSO-d6): δH 11.43 (1H, s), 10.67 (1H, br s), 7.52–7.42 (4H, m), 7.39–7.37 (2H, m), 7.21 (1H, d, J = 7.9 Hz), 7.06 (1H, app t, J = 7.6 Hz), 6.94 (1H, app t, J = 7.5 Hz), 5.39 (1H, app t, J = 6.1 Hz), 4.33 (1H, d, J = 8.0 Hz), 3.72 (1H, app q, J = 6.8 Hz), 2.81 (3H, s), 2.35–2.31 (2H, m); 13C NMR (101 MHz, DMSO-d6): δC 178.9, 175.8, 152.7, 152.7, 137.2, 132.3, 130.7, 129.5, 128.5, 126.7, 125.3, 122.2, 119.9, 118.4, 112.3, 107.3, 48.1, 39.5, 38.3, 27.7, 25.3; IR (neat): νmax/cm−1 3229, 1693; MS (pAPCI): 178.1 (35%), 253.1 (100%, (M − (PTAD) + H)+); HRMS (ASAP) calcd C15H13N2O2 [M − PTAD + H]+: 253.0972; observed: 253.0969.
4f – (3aS*,5S*,10bS*)-5-(hydroxy(phenyl)amino)-4,5,10,10b-tetrahydropyrrolo[3,4-a]carbazole-1,3(2H,3aH)-dione
To a stirred Schlenk flask was added benzyl (3aS*,5S*,10bS*)-5-(hydroxy(phenyl)amino)-1,3-dioxo-2,3,3a,4,5,10b-hexahydropyrrolo[3,4-a]carbazole-10(1H)-carboxylate (90 mg, 0.18 mmol), platinum(IV) oxide (41 mg, 0.18 mmol) and THF (5 mL). The resulting suspension was placed under an atmosphere of H2 and stirred at room temperature for 10 hours. The suspension was filtered through celite and the solvent was removed under reduced pressure to leave the crude product as an yellow solid, The crude product was purified by column chromatography (petrol (40/60)–ethyl acetate 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, column diameter = 2 cm, silica = 14 cm) to give (3aS*,5S*,10bS*)-5-(hydroxy(phenyl)amino)-4,5,10,10b-tetrahydropyrrolo[3,4-a]carbazole-1,3(2H,3aH)-dione (41%, 26 mg, 0.07 mmol) as a yellow powder.
1, column diameter = 2 cm, silica = 14 cm) to give (3aS*,5S*,10bS*)-5-(hydroxy(phenyl)amino)-4,5,10,10b-tetrahydropyrrolo[3,4-a]carbazole-1,3(2H,3aH)-dione (41%, 26 mg, 0.07 mmol) as a yellow powder.
Mp: 150.0–153.1 °C; Rf: 0.33 (Pet(40/60)–EA 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, DMSO-d6): δH 11.22 (1H, s), 11.09 (1H, s), 8.24 (1H, s), 7.33 (1H, d, J = 8.1 Hz), 7.27–7.23 (1H, m), 7.20 (2H, app d, J = 7.3 Hz), 7.12 (2H, d, J = 7.7 Hz), 7.00–6.95 (1H, m), 6.85 (1H, app t, J = 7.2 Hz), 6.80 (1H app t, J = 7.3 Hz), 4.89 (1H, app t, J = 4.9 Hz), 4.25 (1H, d, J = 8.1 Hz), 3.63–3.52 (1H, m), 2.27 (1H, app dt, J = 13.6, 5.6 Hz), 1.80 (1H, ddd, J = 13.6, 9.2, 4.8 Hz); 13C NMR (101 MHz, DMSO-d6): δC 181.2, 177.6, 153.3, 137.0, 130.3, 129.0, 126.3, 121.4, 121.1, 119.9, 118.9, 117.2, 111.7, 109.9, 57.4, 40.9, 40.6, 25.7; IR (neat): νmax/cm−1 3302, 2924, 1706; MS (pAPCI): 108.1 (18%), 237.1 (100), 239.1 (46%, (M − (N(OH)Ph) + H)+); HRMS (pAPCI): calcd C14H11N2O2 [M − (N(OH)Ph) + H]+: 239.0815; observed: 239.0810.
1); 1H NMR (400 MHz, DMSO-d6): δH 11.22 (1H, s), 11.09 (1H, s), 8.24 (1H, s), 7.33 (1H, d, J = 8.1 Hz), 7.27–7.23 (1H, m), 7.20 (2H, app d, J = 7.3 Hz), 7.12 (2H, d, J = 7.7 Hz), 7.00–6.95 (1H, m), 6.85 (1H, app t, J = 7.2 Hz), 6.80 (1H app t, J = 7.3 Hz), 4.89 (1H, app t, J = 4.9 Hz), 4.25 (1H, d, J = 8.1 Hz), 3.63–3.52 (1H, m), 2.27 (1H, app dt, J = 13.6, 5.6 Hz), 1.80 (1H, ddd, J = 13.6, 9.2, 4.8 Hz); 13C NMR (101 MHz, DMSO-d6): δC 181.2, 177.6, 153.3, 137.0, 130.3, 129.0, 126.3, 121.4, 121.1, 119.9, 118.9, 117.2, 111.7, 109.9, 57.4, 40.9, 40.6, 25.7; IR (neat): νmax/cm−1 3302, 2924, 1706; MS (pAPCI): 108.1 (18%), 237.1 (100), 239.1 (46%, (M − (N(OH)Ph) + H)+); HRMS (pAPCI): calcd C14H11N2O2 [M − (N(OH)Ph) + H]+: 239.0815; observed: 239.0810.
4g – (3aS*,5S*,10bS*)-5-(hydroxy(o-tolyl)amino)-4,5,10,10b-tetrahydropyrrolo[3,4-a]carbazole-1,3(2H,3aH)-dione
To a stirred Schlenk flask was added benzyl (3aS*,5S*,10bS*)-5-(hydroxy(o-tolyl)amino)-1,3-dioxo-2,3,3a,4,5,10b-hexahydropyrrolo[3,4-a]carbazole-10(1H)-carboxylate (120 mg, 0.24 mmol), platinum(IV) oxide (54 mg, 0.24 mmol) and THF (5 mL). The resulting suspension was placed under an atmosphere of H2 and stirred at room temperature for 6 hours. The suspension was filtered through celite and the solvent was removed under reduced pressure to leave the crude product as a yellow solid, The crude product was purified by column chromatography (petrol (40/60)–ethyl acetate 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, column diameter = 2 cm, silica = 17 cm) to give (3aS*,5S*,10bS*)-5-(hydroxy(o-tolyl)amino)-4,5,10,10b-tetrahydropyrrolo[3,4-a]carbazole-1,3(2H,3aH)-dione (70%, 61 mg, 0.17 mmol) as an off white solid.
3, column diameter = 2 cm, silica = 17 cm) to give (3aS*,5S*,10bS*)-5-(hydroxy(o-tolyl)amino)-4,5,10,10b-tetrahydropyrrolo[3,4-a]carbazole-1,3(2H,3aH)-dione (70%, 61 mg, 0.17 mmol) as an off white solid.
Mp: 149.9–153.2 °C; Rf: 0.52 (Pet(40/60)–EA 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3); 1H NMR (400 MHz, CD2Cl2): δH 8.81 (1H, s), 8.21 (1H, s), 7.56 (1H, d, J = 8.0 Hz), 7.30 (1H, d, J = 7.9 Hz), 7.18 (2H, d, J = 6.9 Hz), 7.06 (1H, app t, J = 7.6 Hz), 7.03–6.99 (2H, m), 6.91–6.85 (1H, m), 5.36 (1H, s), 4.51 (1H, app t, J = 4.5 Hz), 4.19 (1H, d, J = 8.6 Hz), 3.80 (1H, app td, J = 9.3, 6.4 Hz), 2.74 (1H, app dt, J = 13.6, 5.5 Hz), 2.15 (3H, s), 1.83 (1H, ddd, J = 13.6, 10.1, 4.1 Hz); 13C NMR (101 MHz, DMSO-d6): δC 181.5, 177.6, 152.2, 136.7, 130.6, 130.5, 130.5, 126.5, 126.5, 124.5, 122.4, 121.1, 119.3, 118.9, 111.6, 109.5, 58.3, 55.5, 40.9, 27.3, 18.3; IR (neat): νmax/cm−1 3372, 3298, 1683; MS (nNSI) = 186.0 (100%), 237.1 (97%, (M − (N(OH)(o-Tol)) − H)−), 358.1 (35%, (M − H2)−), 394.1 (23%); HRMS (nNSI): calcd C21H18N3O3 [M − H]−: 360.1354; observed: 360.1348.
3); 1H NMR (400 MHz, CD2Cl2): δH 8.81 (1H, s), 8.21 (1H, s), 7.56 (1H, d, J = 8.0 Hz), 7.30 (1H, d, J = 7.9 Hz), 7.18 (2H, d, J = 6.9 Hz), 7.06 (1H, app t, J = 7.6 Hz), 7.03–6.99 (2H, m), 6.91–6.85 (1H, m), 5.36 (1H, s), 4.51 (1H, app t, J = 4.5 Hz), 4.19 (1H, d, J = 8.6 Hz), 3.80 (1H, app td, J = 9.3, 6.4 Hz), 2.74 (1H, app dt, J = 13.6, 5.5 Hz), 2.15 (3H, s), 1.83 (1H, ddd, J = 13.6, 10.1, 4.1 Hz); 13C NMR (101 MHz, DMSO-d6): δC 181.5, 177.6, 152.2, 136.7, 130.6, 130.5, 130.5, 126.5, 126.5, 124.5, 122.4, 121.1, 119.3, 118.9, 111.6, 109.5, 58.3, 55.5, 40.9, 27.3, 18.3; IR (neat): νmax/cm−1 3372, 3298, 1683; MS (nNSI) = 186.0 (100%), 237.1 (97%, (M − (N(OH)(o-Tol)) − H)−), 358.1 (35%, (M − H2)−), 394.1 (23%); HRMS (nNSI): calcd C21H18N3O3 [M − H]−: 360.1354; observed: 360.1348.
4h – (3aS*,5S*,10bS*)-5-(3,5-dioxo-4-phenyl-1,2,4-triazolidin-1-yl)-4,5,10,10b-tetrahydropyrrolo[3,4-a]carbazole-1,3(2H,3aH)-dione
To a stirred Schlenk flask was added benzyl (3aS*,5S*,10bS*)-5-(3,5-dioxo-4-phenyl-1,2,4-triazolidin-1-yl)-1,3-dioxo-2,3,3a,4,5,10b-hexahydropyrrolo[3,4-a]carbazole-10(1H)-carboxylate (110 mg, 0.20 mmol), platinum(IV) oxide (46 mg, 0.20 mmol) and THF (5 mL). The resulting suspension was placed under an atmosphere of H2 and stirred at room temperature for 5 hours. The suspension was filtered through celite and the solvent was removed under reduced pressure to leave the crude product as a white solid. The crude product was purified by trituration from DCM to give (3aS*,5S*,10bS*)-5-(3,5-dioxo-4-phenyl-1,2,4-triazolidin-1-yl)-4,5,10,10b-tetrahydropyrrolo[3,4-a]carbazole-1,3(2H,3aH)-dione (64%, 53 mg, 0.13 mmol) as a white powder.Mp: 212.6–213.9 °C; 1H NMR (400 MHz, DMSO-d6): δH 11.39 (1H, s), 11.36 (1H, s), 10.66 (1H, s), 7.51–7.44 (4H, m), 7.41–7.37 (2H, m), 7.23 (1H, d, J = 7.8 Hz), 7.07 (1H, app t, J = 7.5 Hz), 6.96 (1H, app t, J = 7.5 Hz), 5.42 (1H, app t, J = 6.0 Hz), 4.29 (1H, d, J = 8.0 Hz), 3.69 (1H, app q, J = 6.8 Hz), 2.37–2.21 (2H, m); 13C NMR (101 MHz, DMSO-d6): δC 180.3, 177.1, 152.7, 152.6, 137.2, 132.3, 131.0, 129.5, 128.5, 126.7, 125.3, 122.2, 119.9, 118.4, 112.3, 107.1, 55.5, 48.1, 40.9, 27.5; IR (neat): νmax/cm−1 = 3310, 3155, 3077, 1719, 1674; MS (pAPCI): 239.1 (100%, (M − (PTAD) + H)+), 414.1 (2%, (M − H)+); HRMS (pAPCI): calcd C22H16N5O4 [M − H]+: 414.1197; observed: 414.1185.
4i – (R*)-6-(hydroxy(phenyl)amino)-2-phenyl-6,11-dihydro-1H,5H-[1,2,4]triazolo[1′,2′:1,2]pyridazino[3,4-b]indole-1,3(2H)-dione
To a stirred Schlenk flask was added benzyl (R*)-6-(hydroxy(phenyl)amino)-1,3-dioxo-2-phenyl-2,3,5,6-tetrahydro-1H,11H-[1,2,4]triazolo[1′,2′:1,2]pyridazino[3,4-b]indole-11-carboxylate (110 mg, 0.20 mmol), platinum(IV) oxide (46 mg, 0.20 mmol) and THF (5 mL). The resulting suspension was placed under an atmosphere of H2 and stirred at room temperature for 5 hours. The suspension was filtered through celite and the solvent was removed under reduced pressure to leave the crude product as a yellow solid. The product was purified by trituration from DCM to give (R*)-6-(hydroxy(phenyl)amino)-2-phenyl-6,11-dihydro-1H,5H-[1,2,4]triazolo[1′,2′:1,2]pyridazino[3,4-b]indole-1,3(2H)-dione (65%, 55 mg, 0.13 mmol) as an off-white solid.Mp: 174.3–175.2 °C; 1H NMR (400 MHz, DMSO-d6): δH 11.63 (1H, s), 8.61 (1H, s), 7.54–7.38 (6H, m), 7.18 (2H, app t, J = 7.8 Hz), 7.10 (2H, app d, J = 7.8 Hz), 7.00–6.96 (2H, m), 6.91–6.83 (2H, m), 5.18–5.15 (1H, m), 4.48 (1H, dd, J = 13.0, 2.0 Hz), 3.77 (1H, dd, J = 13.0, 4.2 Hz); 13C NMR (101 MHz, DMSO-d6): δC 152.6, 149.6, 146.6, 134.2, 131.7, 129.8, 129.6, 128.9, 128.9, 127.0, 125.8, 122.3, 121.1, 120.2, 118.5, 118.3, 112.3, 92.4, 57.4, 43.4; IR (neat): νmax/cm−1 3431, 3054, 1698; MS (pAPCI): 317.1 (100%, (M − (N(OH)Ph) + H)+), 407.1 (5%, (M − H2O)+); HRMS (pAPCI) calcd C24H19N5O3 [M − H]+: 424.1404; observed: 424.1398.
4j – (R*)-6-(hydroxy(o-tolyl)amino)-2-phenyl-6,11-dihydro-1H,5H-[1,2,4]triazolo[1′,2′:1,2]pyridazino[3,4-b]indole-1,3(2H)-dione
To a stirred Schlenk flask was added benzyl (R*)-6-(hydroxy(o-tolyl)amino)-1,3-dioxo-2-phenyl-2,3,5,6-tetrahydro-1H,11H-[1,2,4]triazolo[1′,2′:1,2]pyridazino[3,4-b]indole-11-carboxylate (140 mg, 0.24 mmol), platinum(IV) oxide (55 mg, 0.24 mmol) and THF (5 mL). The resulting suspension was placed under an atmosphere of H2 and stirred at room temperature for 5 hours. The suspension was filtered through celite and the solvent was removed under reduced pressure to leave the crude product as a white solid. The crude product was purified by trituration from DCM to give (R*)-6-(hydroxy(o-tolyl)amino)-2-phenyl-6,11-dihydro-1H,5H-[1,2,4]triazolo[1′,2′:1,2]pyridazino[3,4-b]indole-1,3(2H)-dione (44%, 46 mg, 0.11 mmol) as a white powder.Mp: 188.1–189.6 °C; 1H NMR (400 MHz, DMSO-d6): δH 11.65 (1H, s), 8.58 (1H, s), 7.59–7.51 (5H, m), 7.47–7.42 (1H, m), 7.36 (1H, d, J = 8.0 Hz), 7.17–7.13 (1H, m), 6.96–6.91 (3H, m), 6.79–6.69 (2H, m), 4.69 (1H, d, J = 12.8 Hz), 4.58 (1H, br s), 3.62 (1H, dd, J = 12.8, 3.3 Hz), 2.06 (3H, s); 13C NMR (101 MHz, DMSO-d6): δC 151.4, 150.4, 146.8, 134.0, 131.8, 131.2, 130.6, 129.8, 129.7, 128.9, 127.0, 126.7, 126.0, 125.1, 122.6, 121.0, 120.1, 118.1, 112.2, 92.3, 57.8, 43.5, 18.2; IR (neat): νmax/cm−1 = 3426, 3380, 2950, 1712; MS (pAPCI): 108.1 (100%), 317.1 (37%, (M − (N(OH)(o-Tol)) + H)+), 422.2 (4%, (M − (H2O) + H)+), 438.2 (6%, (M − H)+); HRMS (pAPCI): calcd C25H20N5O3 [M − H]+: 438.1561; observed: 438.1553.
4k – (3aS*,5S*,10bS*)-5-(hydroxy(phenyl)amino)-7-methoxy-2-methyl-4,5,10,10b-tetrahydropyrrolo[3,4-a]carbazole-1,3(2H,3aH)-dione
To a stirred Schlenk flask was added benzyl (3aS*,5S*,10bS*)-5-(hydroxy(phenyl)amino)-7-methoxy-2-methyl-1,3-dioxo-2,3,3a,4,5,10b-hexahydropyrrolo[3,4-a]carbazole-10(1H)-carboxylate (130 mg, 0.25 mmol), platinum(IV) oxide (57 mg, 0.25 mmol) and THF (5 mL). The resulting suspension was placed under an atmosphere of H2 and stirred at room temperature for 5 hours. The suspension was filtered through celite and the solvent was removed under reduced pressure to leave the crude product as an orange solid. The crude product was purified by column chromatography (petrol (40/60)–ethyl acetate 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, column diameter = 2 cm, silica = 14 cm) to give (3aS*,5S*,10bS*)-5-(hydroxy(phenyl)amino)-7-methoxy-2-methyl-4,5,10,10b-tetrahydropyrrolo[3,4-a]carbazole-1,3(2H,3aH)-dione (70%, 69 mg, 0.18 mmol) as a pale yellow powder.
2, column diameter = 2 cm, silica = 14 cm) to give (3aS*,5S*,10bS*)-5-(hydroxy(phenyl)amino)-7-methoxy-2-methyl-4,5,10,10b-tetrahydropyrrolo[3,4-a]carbazole-1,3(2H,3aH)-dione (70%, 69 mg, 0.18 mmol) as a pale yellow powder.
Mp: 149.1–151.2 °C; Rf: 0.19 (Pet(40/60)–EA 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2); 1H NMR (400 MHz, DMSO-d6): δH 10.95 (1H, s), 8.29 (1H, s), 7.23–7.19 (2H, m), 7.18 (1H, d, J = 2.8 Hz), 7.14–7.10 (2H, m), 6.87–6.83 (1H, m), 6.57 (1H, dd, J = 8.7, 2.5 Hz), 6.51 (1H, d, J = 2.5 Hz), 4.83 (1H, app t, J = 4.8 Hz), 4.24 (1H, d, J = 8.2 Hz), 3.64 (1H, app td, J = 9.1, 6.1 Hz), 3.48 (3H, s), 2.80 (3H, s), 2.35 (1H, ddd, J = 13.7, 6.1, 4.8 Hz), 1.83 (1H, ddd, J = 13.7, 9.1, 4.8 Hz); 13C NMR (101 MHz, DMSO-d6): δC 179.9, 176.3, 153.6C1, 153.4, 132.0, 130.6, 129.0, 126.6, 121.2, 117.4, 112.3, 111.5, 109.5, 101.7, 57.7, 55.5, 39.5, 39.1, 27.1, 25.1; IR (neat): νmax/cm−1 = 3394, 2937, 2833, 1690; MS (pAPCI): 283.1 (100%, (M − (N(OH)Ph) + H)+), 374.1 (18%, (M − (H2O) + H)+), 390.1 (2%, (M − H)+), 392.2 (1%, (M + H)+); HRMS (pAPCI): calcd C22H22N3O4 [M + H]+: 392.1605; observed: 392.1597.
2); 1H NMR (400 MHz, DMSO-d6): δH 10.95 (1H, s), 8.29 (1H, s), 7.23–7.19 (2H, m), 7.18 (1H, d, J = 2.8 Hz), 7.14–7.10 (2H, m), 6.87–6.83 (1H, m), 6.57 (1H, dd, J = 8.7, 2.5 Hz), 6.51 (1H, d, J = 2.5 Hz), 4.83 (1H, app t, J = 4.8 Hz), 4.24 (1H, d, J = 8.2 Hz), 3.64 (1H, app td, J = 9.1, 6.1 Hz), 3.48 (3H, s), 2.80 (3H, s), 2.35 (1H, ddd, J = 13.7, 6.1, 4.8 Hz), 1.83 (1H, ddd, J = 13.7, 9.1, 4.8 Hz); 13C NMR (101 MHz, DMSO-d6): δC 179.9, 176.3, 153.6C1, 153.4, 132.0, 130.6, 129.0, 126.6, 121.2, 117.4, 112.3, 111.5, 109.5, 101.7, 57.7, 55.5, 39.5, 39.1, 27.1, 25.1; IR (neat): νmax/cm−1 = 3394, 2937, 2833, 1690; MS (pAPCI): 283.1 (100%, (M − (N(OH)Ph) + H)+), 374.1 (18%, (M − (H2O) + H)+), 390.1 (2%, (M − H)+), 392.2 (1%, (M + H)+); HRMS (pAPCI): calcd C22H22N3O4 [M + H]+: 392.1605; observed: 392.1597.
4l – (3aS*,5S*,10bS*)-5-(hydroxy(o-tolyl)amino)-7-methoxy-2-methyl-4,5,10,10b-tetrahydropyrrolo[3,4-a]carbazole-1,3(2H,3aH)-dione
To a stirred Schlenk flask was added benzyl (3aS*,5S*,10bS*)-5-(hydroxy(o-tolyl)amino)-7-methoxy-2-methyl-1,3-dioxo-2,3,3a,4,5,10b-hexahydropyrrolo[3,4-a]carbazole-10(1H)-carboxylate (108 mg, 0.20 mmol), platinum(IV) oxide (46 mg, 0.20 mmol) and THF (5 mL). The resulting suspension was placed under an atmosphere of H2 and stirred at room temperature for 5 hours. The suspension was filtered through celite and the solvent was removed under reduced pressure to leave the crude product as a white solid. The crude product was purified by column chromatography (petrol (40/60)–ethyl acetate 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, column diameter = 2 cm, silica = 15 cm) to give (3aS*,5S*,10bS*)-5-(hydroxy(o-tolyl)amino)-7-methoxy-2-methyl-4,5,10,10b-tetrahydropyrrolo[3,4-a]carbazole-1,3(2H,3aH)-dione (70%, 57 mg, 0.14 mmol) as an off white powder.
1, column diameter = 2 cm, silica = 15 cm) to give (3aS*,5S*,10bS*)-5-(hydroxy(o-tolyl)amino)-7-methoxy-2-methyl-4,5,10,10b-tetrahydropyrrolo[3,4-a]carbazole-1,3(2H,3aH)-dione (70%, 57 mg, 0.14 mmol) as an off white powder.
Mp: 139.7–142.5 °C; Rf: 0.36 (Pet(40/60)–EA 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, DMSO-d6): δH 10.89 (1H, s), 8.36 (1H, s), 7.52 (1H, d, J = 8.0 Hz), 7.15–7.11 (2H, d, J = 8.7 Hz), 6.93–6.85 (2H, m), 6.49 (1H, dd, J = 8.7, 2.4 Hz), 6.23–6.17 (1H, m), 4.28 (1H, d, J = 8.2 Hz), 4.22 (1H, app t, J = 3.8 Hz), 3.78 (1H, ddd, J = 10.8, 8.2, 6.1 Hz), 3.45 (3H, s), 2.81 (3H, s), 2.70–2.63 (1H, m), 1.89 (3H, s), 1.66 (1H, ddd, J = 14.1, 10.8, 3.8 Hz); 13C NMR (101 MHz, DMSO-d6): δC 180.1, 176.3, 153.2, 152.5, 131.6, 131.1, 130.7, 130.4, 126.8, 126.5, 124.7, 122.7, 112.2, 111.4, 109.2, 100.6, 58.8, 55.3, 39.6, 38.4, 28.3, 25.1, 18.1; IR (neat): νmax/cm−1 = 3384, 2954, 2866, 1693; MS (pAPCI): 283.1 (100%, (M − (N(OH)(o-Tol)) + H)+), 388.2 (34%, (M − (H2O) + H)+), 404.2 (13%, (M − H)+), 406.2 (11%, (M + H)+); HRMS (pAPCI): calcd C23H24N3O4 [M + H]+: 406.1761; observed: 406.1750.
1); 1H NMR (400 MHz, DMSO-d6): δH 10.89 (1H, s), 8.36 (1H, s), 7.52 (1H, d, J = 8.0 Hz), 7.15–7.11 (2H, d, J = 8.7 Hz), 6.93–6.85 (2H, m), 6.49 (1H, dd, J = 8.7, 2.4 Hz), 6.23–6.17 (1H, m), 4.28 (1H, d, J = 8.2 Hz), 4.22 (1H, app t, J = 3.8 Hz), 3.78 (1H, ddd, J = 10.8, 8.2, 6.1 Hz), 3.45 (3H, s), 2.81 (3H, s), 2.70–2.63 (1H, m), 1.89 (3H, s), 1.66 (1H, ddd, J = 14.1, 10.8, 3.8 Hz); 13C NMR (101 MHz, DMSO-d6): δC 180.1, 176.3, 153.2, 152.5, 131.6, 131.1, 130.7, 130.4, 126.8, 126.5, 124.7, 122.7, 112.2, 111.4, 109.2, 100.6, 58.8, 55.3, 39.6, 38.4, 28.3, 25.1, 18.1; IR (neat): νmax/cm−1 = 3384, 2954, 2866, 1693; MS (pAPCI): 283.1 (100%, (M − (N(OH)(o-Tol)) + H)+), 388.2 (34%, (M − (H2O) + H)+), 404.2 (13%, (M − H)+), 406.2 (11%, (M + H)+); HRMS (pAPCI): calcd C23H24N3O4 [M + H]+: 406.1761; observed: 406.1750.
4m – (3aS*,5S*,10bS*)-5-(hydroxy(phenyl)amino)-4,5,10,10b-tetrahydropyrrolo[3,4-a]carbazole-1,3(2H,3aH)-dione
To a stirred Schlenk flask was added benzyl (3aS*,5S*,10bS*)-5-(hydroxy(phenyl)amino)-1,3-dioxo-2,3,3a,4,5,10b-hexahydropyrrolo[3,4-a]carbazole-10(1H)-carboxylate (90 mg, 0.18 mmol), platinum(IV) oxide (41 mg, 0.18 mmol) and THF (5 mL). The resulting suspension was placed under an atmosphere of H2 and stirred at room temperature for 10 hours. The suspension was filtered through celite and the solvent was removed under reduced pressure to leave the crude product as an yellow solid, The crude product was purified by column chromatography (petrol (40/60)–ethyl acetate 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, column diameter = 2 cm, silica = 14 cm) to give (3aS*,5S*,10bS*)-5-(hydroxy(phenyl)amino)-4,5,10,10b-tetrahydropyrrolo[3,4-a]carbazole-1,3(2H,3aH)-dione (41%, 26 mg, 0.07 mmol) as a yellow powder.
1, column diameter = 2 cm, silica = 14 cm) to give (3aS*,5S*,10bS*)-5-(hydroxy(phenyl)amino)-4,5,10,10b-tetrahydropyrrolo[3,4-a]carbazole-1,3(2H,3aH)-dione (41%, 26 mg, 0.07 mmol) as a yellow powder.
Mp: 150.0–153.1 °C; Rf: 0.33 (Pet(40/60)–EA 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, DMSO-d6): δH 11.22 (1H, s), 11.09 (1H, s), 8.24 (1H, s), 7.33 (1H, d, J = 8.1 Hz), 7.27–7.23 (1H, m), 7.20 (2H, app d, J = 7.3 Hz), 7.12 (2H, d, J = 7.7 Hz), 7.00–6.95 (1H, m), 6.85 (1H, app t, J = 7.2 Hz), 6.80 (1H app t, J = 7.3 Hz), 4.89 (1H, app t, J = 4.9 Hz), 4.25 (1H, d, J = 8.1 Hz), 3.63–3.52 (1H, m), 2.27 (1H, app dt, J = 13.6, 5.6 Hz), 1.80 (1H, ddd, J = 13.6, 9.2, 4.8 Hz); 13C NMR (101 MHz, DMSO-d6): δC 181.2, 177.6, 153.3, 137.0, 130.3, 129.0, 126.3, 121.4, 121.1, 119.9, 118.9, 117.2, 111.7, 109.9, 57.4, 40.9, 40.6, 25.7; IR (neat): νmax/cm−1 3302, 2924, 1706; MS (pAPCI): 108.1 (18%), 237.1 (100), 239.1 (46%, (M − (N(OH)Ph) + H)+); HRMS (pAPCI): calcd C14H11N2O2 [M − (N(OH)Ph) + H]+: 239.0815; observed: 239.0810.
1); 1H NMR (400 MHz, DMSO-d6): δH 11.22 (1H, s), 11.09 (1H, s), 8.24 (1H, s), 7.33 (1H, d, J = 8.1 Hz), 7.27–7.23 (1H, m), 7.20 (2H, app d, J = 7.3 Hz), 7.12 (2H, d, J = 7.7 Hz), 7.00–6.95 (1H, m), 6.85 (1H, app t, J = 7.2 Hz), 6.80 (1H app t, J = 7.3 Hz), 4.89 (1H, app t, J = 4.9 Hz), 4.25 (1H, d, J = 8.1 Hz), 3.63–3.52 (1H, m), 2.27 (1H, app dt, J = 13.6, 5.6 Hz), 1.80 (1H, ddd, J = 13.6, 9.2, 4.8 Hz); 13C NMR (101 MHz, DMSO-d6): δC 181.2, 177.6, 153.3, 137.0, 130.3, 129.0, 126.3, 121.4, 121.1, 119.9, 118.9, 117.2, 111.7, 109.9, 57.4, 40.9, 40.6, 25.7; IR (neat): νmax/cm−1 3302, 2924, 1706; MS (pAPCI): 108.1 (18%), 237.1 (100), 239.1 (46%, (M − (N(OH)Ph) + H)+); HRMS (pAPCI): calcd C14H11N2O2 [M − (N(OH)Ph) + H]+: 239.0815; observed: 239.0810.
4n – (3aS*,5S*,10bS*)-5-(hydroxy(o-tolyl)amino)-4,5,10,10b-tetrahydropyrrolo[3,4-a]carbazole-1,3(2H,3aH)-dione
To a stirred Schlenk flask was added benzyl (3aS*,5S*,10bS*)-5-(hydroxy(o-tolyl)amino)-1,3-dioxo-2,3,3a,4,5,10b-hexahydropyrrolo[3,4-a]carbazole-10(1H)-carboxylate (120 mg, 0.24 mmol), platinum(IV) oxide (54 mg, 0.24 mmol) and THF (5 mL). The resulting suspension was placed under an atmosphere of H2 and stirred at room temperature for 6 hours. The suspension was filtered through celite and the solvent was removed under reduced pressure to leave the crude product as a yellow solid, The crude product was purified by column chromatography (petrol (40/60)–ethyl acetate 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, column diameter = 2 cm, silica = 17 cm) to give (3aS*,5S*,10bS*)-5-(hydroxy(o-tolyl)amino)-4,5,10,10b-tetrahydropyrrolo[3,4-a]carbazole-1,3(2H,3aH)-dione (70%, 61 mg, 0.17 mmol) as an off white solid.
3, column diameter = 2 cm, silica = 17 cm) to give (3aS*,5S*,10bS*)-5-(hydroxy(o-tolyl)amino)-4,5,10,10b-tetrahydropyrrolo[3,4-a]carbazole-1,3(2H,3aH)-dione (70%, 61 mg, 0.17 mmol) as an off white solid.
Mp: 149.9–153.2 °C; Rf: 0.52 (Pet(40/60)–EA 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3); 1H NMR (400 MHz, CD2Cl2): δH 8.81 (1H, s), 8.21 (1H, s), 7.56 (1H, d, J = 8.0 Hz), 7.30 (1H, d, J = 7.9 Hz), 7.18 (2H, d, J = 6.9 Hz), 7.06 (1H, app t, J = 7.6 Hz), 7.03–6.99 (2H, m), 6.91–6.85 (1H, m), 5.36 (1H, s), 4.51 (1H, app t, J = 4.5 Hz), 4.19 (1H, d, J = 8.6 Hz), 3.80 (1H, app td, J = 9.3, 6.4 Hz), 2.74 (1H, app dt, J = 13.6, 5.5 Hz), 2.15 (3H, s), 1.83 (1H, ddd, J = 13.6, 10.1, 4.1 Hz); 13C NMR (101 MHz, DMSO-d6): δC 181.5, 177.6, 152.2, 136.7, 130.6, 130.5, 130.5, 126.5, 126.5, 124.5, 122.4, 121.1, 119.3, 118.9, 111.6, 109.5, 58.3, 55.5, 40.9, 27.3, 18.3; IR (neat): νmax/cm−1 3372, 3298, 1683; MS (nNSI) = 186.0 (100%), 237.1 (97%, (M − (N(OH)(o-Tol)) − H)−), 358.1 (35%, (M − H2)−), 394.1 (23%); HRMS (nNSI): calcd C21H18N3O3 [M − H]−: 360.1354; observed: 360.1348.
3); 1H NMR (400 MHz, CD2Cl2): δH 8.81 (1H, s), 8.21 (1H, s), 7.56 (1H, d, J = 8.0 Hz), 7.30 (1H, d, J = 7.9 Hz), 7.18 (2H, d, J = 6.9 Hz), 7.06 (1H, app t, J = 7.6 Hz), 7.03–6.99 (2H, m), 6.91–6.85 (1H, m), 5.36 (1H, s), 4.51 (1H, app t, J = 4.5 Hz), 4.19 (1H, d, J = 8.6 Hz), 3.80 (1H, app td, J = 9.3, 6.4 Hz), 2.74 (1H, app dt, J = 13.6, 5.5 Hz), 2.15 (3H, s), 1.83 (1H, ddd, J = 13.6, 10.1, 4.1 Hz); 13C NMR (101 MHz, DMSO-d6): δC 181.5, 177.6, 152.2, 136.7, 130.6, 130.5, 130.5, 126.5, 126.5, 124.5, 122.4, 121.1, 119.3, 118.9, 111.6, 109.5, 58.3, 55.5, 40.9, 27.3, 18.3; IR (neat): νmax/cm−1 3372, 3298, 1683; MS (nNSI) = 186.0 (100%), 237.1 (97%, (M − (N(OH)(o-Tol)) − H)−), 358.1 (35%, (M − H2)−), 394.1 (23%); HRMS (nNSI): calcd C21H18N3O3 [M − H]−: 360.1354; observed: 360.1348.
4o – (R*)-6-(hydroxy(phenyl)amino)-8-methoxy-2-phenyl-6,11-dihydro-1H,5H-[1,2,4]triazolo[1′,2′:1,2]pyridazino[3,4-b]indole-1,3(2H)-dione
To a stirred Schlenk flask was added benzyl (R*)-6-(hydroxy(phenyl)amino)-8-methoxy-1,3-dioxo-2-phenyl-2,3,5,6-tetrahydro-1H,11H-[1,2,4]triazolo[1′,2′:1,2]pyridazino[3,4-b]indole-11-carboxylate (118 mg, 0.20 mmol), platinum(IV) oxide (46 mg, 0.20 mmol) and THF (5 mL). The resulting suspension was placed under an atmosphere of H2 and stirred at room temperature for 5 hours. The suspension was filtered through celite and the solvent was removed under reduced pressure to leave the crude product as a yellow solid. The product was purified by trituration from DCM to give (R*)-6-(hydroxy(phenyl)amino)-8-methoxy-2-phenyl-6,11-dihydro-1H,5H-[1,2,4]triazolo[1′,2′:1,2]pyridazino[3,4-b]indole-1,3(2H)-dione (38%, 35 mg, 0.08 mmol) as an off-white solid.Mp: 173.7–176.4 °C; Rf: 0.18 (Pet–EA 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, DMSO-d6): δH 11.44 (1H, s) 8.62 (1H, s), 7.54–7.47 (4H, m), 7.44–7.41 (1H, m) 7.25–7.18 (3H, m), 7.12 (2H, d, J = 7.7 Hz), 6.90 (1H, app t, J = 6.8 Hz), 6.56 (1H, d, J = 8.7 Hz), 6.26 (1H, s), 5.12 (1H, br s), 4.51 (1H, d, J = 13.0 Hz), 3.81 (1H, dd, J = 13.0, 3.3 Hz), 3.49 (3H, s); 13C NMR (101 MHz, DMSO-d6): δC 154.3, 152.9, 149.7, 146.4, 131.8, 130.1, 129.6, 129.6, 128.9, 128.9, 127.0, 126.3, 122.3, 118.4, 112.9, 110.7, 100.8, 92.3, 57.7, 55.5, 44.3; IR (neat): νmax/cm−1 3362, 3000, 1758, 1700; MS (pAPCI): 213.1 (70%), 347.1 (100%, (M − (N(OH)Ph) + H)+); HRMS (pAPCI): calcd C25H20N5O4 [M − H]+: 454.1510; observed: 454.1502.
1); 1H NMR (400 MHz, DMSO-d6): δH 11.44 (1H, s) 8.62 (1H, s), 7.54–7.47 (4H, m), 7.44–7.41 (1H, m) 7.25–7.18 (3H, m), 7.12 (2H, d, J = 7.7 Hz), 6.90 (1H, app t, J = 6.8 Hz), 6.56 (1H, d, J = 8.7 Hz), 6.26 (1H, s), 5.12 (1H, br s), 4.51 (1H, d, J = 13.0 Hz), 3.81 (1H, dd, J = 13.0, 3.3 Hz), 3.49 (3H, s); 13C NMR (101 MHz, DMSO-d6): δC 154.3, 152.9, 149.7, 146.4, 131.8, 130.1, 129.6, 129.6, 128.9, 128.9, 127.0, 126.3, 122.3, 118.4, 112.9, 110.7, 100.8, 92.3, 57.7, 55.5, 44.3; IR (neat): νmax/cm−1 3362, 3000, 1758, 1700; MS (pAPCI): 213.1 (70%), 347.1 (100%, (M − (N(OH)Ph) + H)+); HRMS (pAPCI): calcd C25H20N5O4 [M − H]+: 454.1510; observed: 454.1502.
4p – (R*)-6-(hydroxy(o-tolyl)amino)-8-methoxy-2-phenyl-6,11-dihydro-1H,5H-[1,2,4]triazolo[1′,2′:1,2]pyridazino[3,4-b]indole-1,3(2H)-dione
To a stirred Schlenk flask was added benzyl (R*)-6-(hydroxy(o-tolyl)amino)-8-methoxy-1,3-dioxo-2-phenyl-2,3,5,6-tetrahydro-1H,11H-[1,2,4]triazolo[1′,2′:1,2]pyridazino[3,4 b]indole-11-carboxylate (120 mg, 0.20 mmol), platinum(IV) oxide (46 mg, 0.20 mmol) and THF (5 mL). The resulting suspension was placed under an atmosphere of H2 and stirred at room temperature for 5 hours. The suspension was filtered through celite and the solvent was removed under reduced pressure to leave the crude product as a white solid. The crude product was purified by trituration from DCM to (R*)-6-(hydroxy(o-tolyl)amino)-8-methoxy-2-phenyl-6,11-dihydro-1H,5H-[1,2,4]triazolo[1′,2′:1,2]pyridazino[3,4 b]indole-1,3(2H)-dione (64%, 53 mg, 0.13 mmol) as a white powder.Mp: 171.9–173.8 °C; 1H NMR (400 MHz, DMSO-d6): δH 11.44 (1H, s), 8.64 (1H, s), 7.59 (1H, d, J = 8.1 Hz), 7.54–7.50 (4H, m), 7.46–7.43 (1H, m), 7.19 (1H, d, J = 8.5 Hz), 7.15 (1H, d, J = 7.5 Hz), 6.97–6.91 (2H, m), 6.50 (1H, dd, J = 8.7, 2.2 Hz), 6.01 (1H, s), 4.73 (1H, d, J = 12.8 Hz), 4.52 (1H, s), 3.67–3.61 (1H, m), 3.47 (3H, s), 1.96 (3H, s); 13C NMR (101 MHz, DMSO-d6): δC 154.1, 151.8, 150.5, 146.4, 131.8, 131.6, 130.6, 130.0, 129.7, 128.9, 128.7, 127.0, 126.7, 126.5, 125.3, 122.8, 112.7, 110.7, 100.0, 91.8, 58.3, 55.4, 44.3, 18.0; IR (neat): νmax/cm−1 = 3442, 3394, 2939, 1699; MS (pAPCI): 347.1 (68%, (M − (N(OH)(o-Tol) + H)+)), 391.1 (100%), 452.2 (4%, (M − (H2O) + H)+), 468.2 (2%, (M − H)+); HRMS (pAPCI): calcd C26H22N5O4 [M − H]+: 468.1666; observed: 468.1658.
Acknowledgements
The authors thank Newcastle University and EPSRC (EP/I033959/1) for funding, MA thanks the Ministry of Higher Education of Saudi Arabia for a PhD scholarship, EPSRC for X-ray crystallography facilities at Newcastle (EP/F03637X/1), Prof. W. McFarlane and Dr C. Wills (Newcastle) for NMR support, L. Watson and S. Thompson (Newcastle) for preliminary studies. Mass spectrometry data was acquired at the EPSRC UK National Mass Spectrometry Facility at Swansea University.References
- S. Omura, Y. Iwai, A. Hirano, A. Nakagawa, J. Awaya, H. Tsuchiya, Y. Takahashi and R. Asuma, J. Antibiot., 1977, 30, 275–282 CrossRef CAS.
- N. Funato, H. Takayanagi, Y. Konda, Y. Toda, Y. Harigaya, Y. Iwai and S. Ōmura, Tetrahedron Lett., 1994, 35, 1251–1254 CrossRef CAS.
- O. A. B. S. M. Gani and R. A. Engh, Nat. Prod. Rep., 2010, 27, 489–498 RSC.
- M. S. Lopez, J. W. Choy, U. Peters, M. L. Sos, D. O. Morgan and K. M. Shokat, J. Am. Chem. Soc., 2013, 135, 18153–18159 CrossRef CAS PubMed.
- E. Conchon, F. Anizon, R. M. Golsteyn, S. Léonce, B. Pfeiffer and M. Prudhomme, Tetrahedron, 2006, 62, 11136–11144 CrossRef CAS PubMed.
- A. Voldoire, M. Sancelme, M. Prudhomme, P. Colson, C. Houssier, C. Bailly, S. Léonce and S. Lambel, Bioorg. Med. Chem., 2001, 9, 357–365 CrossRef CAS.
- N. Ty, G. Dupeyre, G. G. Chabot, J. Seguin, L. Quentin, A. Chiaroni, F. Tillequin, D. Scherman, S. Michel and X. Cachet, Eur. J. Med. Chem., 2010, 45, 3726–3739 CrossRef CAS PubMed.
- E. Conchon, F. Anizon, B. Aboab, R. M. Golsteyn, S. Léonce, B. Pfeiffer and M. Prudhomme, Eur. J. Med. Chem., 2008, 43, 282–292 CrossRef CAS PubMed.
- E. Pereira, A. Youssef, M. El-Ghozzi, D. Avignant, J. Bain, M. Prudhomme, F. Anizon and P. Moreau, Tetrahedron Lett., 2014, 55, 834–837 CrossRef CAS PubMed.
- M. Eitel and U. Pindur, J. Org. Chem., 1990, 55, 5368–5374 CrossRef CAS.
- W. E. Noland, W. C. Kuryla and R. F. Lange, J. Am. Chem. Soc., 1959, 81, 6010–6017 CrossRef CAS.
- W. E. Noland and S. R. Wann, J. Org. Chem., 1979, 44, 4402–4410 CrossRef CAS.
- M. Bleile, T. Wagner and H.-H. Otto, Helv. Chim. Acta, 2005, 88, 2879–2891 CrossRef CAS.
- U. Pindur and M.-H. Kim, Tetrahedron Lett., 1988, 29, 3927–3928 CrossRef CAS.
- M. Medion-Simon and U. Pindur, Helv. Chim. Acta, 1991, 74, 430–437 CrossRef CAS.
- U. Pindur and C. Otto, Tetrahedron, 1992, 48, 3515–3526 CrossRef CAS.
- R. Bergamasco, Q. N. Porter and C. Yap, Aust. J. Chem., 1977, 30, 1531–1544 CrossRef CAS.
- J. D. Lambert and Q. N. Porter, Aust. J. Chem., 1981, 34, 1483–1490 CrossRef CAS.
- C. Gioia, A. Hauville, L. Bernardi, F. Fini and A. Ricci, Angew. Chem., Int. Ed., 2008, 47, 9236–9239 CrossRef CAS PubMed.
- C. Gioia, L. Bernardi and A. Ricci, Synthesis, 2010, 2010, 161–170 CrossRef PubMed.
- B. Tan, G. Hernández-Torres and C. F. Barbas, J. Am. Chem. Soc., 2011, 133, 12354–12357 CrossRef CAS PubMed.
- U. Pindur, M. H. Kim, M. Rogge, W. Massa and M. Molinier, J. Org. Chem., 1992, 57, 910–915 CrossRef CAS.
- L. J. Watson, R. W. Harrington, W. Clegg and M. J. Hall, Org. Biomol. Chem., 2012, 10, 6649–6655 CAS.
- L. J. Cotterill, R. W. Harrington, W. Clegg and M. J. Hall, J. Org. Chem., 2010, 75, 4604–4607 CrossRef CAS PubMed.
- L. Pfeuffer and U. Pindur, Helv. Chim. Acta, 1988, 71, 467–471 CrossRef CAS.
- K. Suzuki, K. Inomata and Y. Endo, Org. Lett., 2004, 6, 409–411 CrossRef CAS PubMed.
- G. A. Kraus and J. Kim, Org. Lett., 2004, 6, 3115–3117 CrossRef CAS PubMed.
- R. B. Ruggeri, M. M. Hansen and C. H. Heathcock, J. Am. Chem. Soc., 1988, 110, 8734–8736 CrossRef CAS.
- D. Li, Y. Cao, A. Shi and Z. Xi, Chem.–Asian J., 2011, 6, 392–395 CrossRef CAS PubMed.
- D. G. Hingane, S. K. Goswami, V. Puranik and R. S. Kusurkar, Synth. Commun., 2011, 42, 1786–1795 CrossRef.
- B. t. Joseph, M. Facompré, H. Da Costa, S. Routier, J.-Y. Mérour, P. Colson, C. Houssier and C. Bailly, Bioorg. Med. Chem., 2001, 9, 1533–1541 CrossRef CAS.
- B. Saroja and P. C. Srinivasan, Synthesis, 1986, 1986, 748–749 CrossRef PubMed.
- X. Zhang, S. I. Khan and C. S. Foote, J. Org. Chem., 1993, 58, 7839–7847 CrossRef CAS.
- P. R. Schreiner, Chem. Soc. Rev., 2003, 32, 289–296 RSC.
- J. Robertson, M. J. Hall, P. M. Stafford and S. P. Green, Org. Biomol. Chem., 2003, 1, 3758–3767 CAS.
- L. F. Tietze, Chem. Rev., 1996, 96, 115–136 CrossRef CAS PubMed.
- C. J. Lovely, H. Du, Y. He and H. V. Rasika Dias, Org. Lett., 2004, 6, 735–738 CrossRef CAS PubMed.
- N. Uludag and S. Patir, J. Heterocycl. Chem., 2007, 44, 1317–1322 CrossRef CAS.
- P. Magnus, N. L. Sear, C. S. Kim and N. Vicker, J. Org. Chem., 1992, 57, 70–78 CrossRef CAS.
- R. Yang and F. G. Qiu, Angew. Chem., Int. Ed., 2013, 52, 6015–6018 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: 1H and 13C spectra for all new compounds, crystal data and structure refinement tables for compounds 2a, 2b, 2d, 3l, 3r, 3u and 3v. The crystallographic coordinates of 2a, 2b, 2d, 3l, 3r, 3u and 3v have been deposited with the Cambridge Crystallographic Data Centre, deposition nos. CCDC 952356, 1040305, 1040306, 952229, 1040307, 1040308 and 952357. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ra00499c |
| ‡ Authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2015 |