Resistance investigation of wheat bran polyphenols extracts on HEK293 cells against oxidative damage
Yuan-Yuan Bian,
Jia Guo,
Ke-Xue Zhu*,
Xiao-Na Guo,
Wei Peng and
Hui-Ming Zhou*
State Key Laboratory of Food Science and Technology, School of Food Science and Technology, Collaborative Innovation Center for Modern Grain Circulation and Safety, Jiangnan University, 1800 Lihu Avenue, Wuxi-214122, Jiangsu Province, PR China. E-mail: kxzhu@jiangnan.edu.cn; hmzhou@jiangnan.edu.cn; Fax: +86 510 85329037, +86 510 85809610; Tel: +86 510 85329037, +86 510 85809610
First published on 19th January 2015
Abstract
Oxidative stress has been considered as a major cause of cellular injury in a variety of clinical abnormalities. One of popular methods to inhibit reactive-oxygen-species (ROS)-induced cellular injury is dietary or pharmaceutical augmentation of the endogenous antioxidant defense capacity. In this study, the resistance effects of wheat bran polyphenols extracts (WBPE) against H2O2-induced cytotoxicity in HEK293 cells were investigated. The phenolic components of WBPE were analysed using UPLC/TQD, and the presence of ferulic acid, p-coumaric acid, o-coumaric acid and gallic acid components were confirmed. The cytotoxicity of such contents and their effects on cell morphology were also evaluated. The results demonstrated that the incubation of WBPE-N9-4 with cells prior to H2O2 exposure could significantly improve cell viability corresponding with increased catalase (CAT), superoxide dismutase (SOD), and glutathione peroxidase (GSH-Px) levels. Moreover, reduction in the levels of lactate dehydrogenase (LDH), malondialdehyde (MDA), and ROS generation were observed. The presence of WBPE-N9-4 inhibited H2O2-induced apoptosis in HEK293 cells, which was confirmed by the flow cytometry of sub-G1 DNA content and an Annexin V assay. It is reasonable to assume that WBPE-N9-4 has an excellent ability to prevent HEK293 cells from oxidative-damage. Our studies, for the first time, reveal that WBPE-N9-4 has resistance effects against H2O2-induced cytotoxicity in HEK293 cells.
1. Introduction
Cereal bran are outer grain layers, which are separated from the milling process during the production of refined flours.1,2 Wheat bran, a by-product generated abundantly during wheat processing, is regarded as a good dietary source of wheat antioxidants.3 The antioxidant components in wheat are mainly phenolic compounds,4,5 including ferulic acid6 and protocatechuic, sinapic, vanillic, p-hydroxybenzoic and p-coumaric acids,7–9 which are distributed in the bran fractions.10,11 Wheat bran extracts (WBEs) have potential anti-proliferative activity, and the phenolic compounds derived from WBEs possess various physiological activities including antioxidative activity and anti-radiation activity,12 but the exact mechanism is not yet fully understood. The relationships between dietary antioxidant potential and tumor multiplicity have been revealed after ingesting whole wheat as well as wheat bran diets, and after ingesting a wheat bran diet with tumor load.13 The results indicate that the antioxidant ability of wheat in the diets is associated with their anti-tumor activity. These studies demonstrated that WBEs may serve as an excellent dietary source for disease prevention and health promotion.Although the antioxidant activities of WBEs have been demonstrated in previous reports,3,14,15 deep and comprehensive understanding about their possible resistance effects against radical-initiated oxidative damage is still in its infancy. Therefore, it is necessary to develop a cell model for exposure to specific components to investigate how these components affect the physiological processes of biological systems.16–20 In a cellular system, the generation and elimination of reactive oxygen species (ROS) are regulated by the antioxidant system. In a normal physiological process, the mitochondria converts 1–2% of the consumed oxygen to ROS, while ROS levels are dramatically increased under environmental stress (e.g. exposure to ultraviolet light or heat).21 The excessive ROS can damage vital cellular structures, such as lipids, DNA, RNA and proteins,22,23 via the oxidation of relevant small molecular components, leading to a severe biological response such as mutation and cell death.24,25 These damages induced by ROS can be attributed to the pathogenesis of inflammatory disease, cardiovascular disease, cancer, diabetes, Alzheimer's disease, cataracts, autism and aging.24,26 Human embryonic kidney (HEK) 293 cells are a specific cell line originating from HEK cells grown in tissue cultures; such cell lines have been widely used in cell biology as well as toxicology studies.27,28 In particular, hydrogen peroxide (H2O2) is one of the major contributors to oxidative stress. Exogenous treatment with H2O2 in HEK293 cells could serve as an in vitro mimic model for investigating oxidative stress-induced injury.29 It has been illustrated that natural antioxidants may increase cell resistance against H2O2-induced oxidative stress.30 Some studies have reported that the ferulic acid ester of oligosaccharides (FQs) released either from microorganisms in the colon or from the enzymatic hydrolysis of arabinoxylans present in wheat bran has a strong ability against oxidative DNA damage in normal human peripheral blood lymphocytes induced by H2O2.31 To the best of our knowledge, very few studies involve the evaluation of the effects of wheat bran polyphenols extracts (WBPE) on cell resistance towards radical-initiated oxidative damage. In addition, the preparative enrichment and separation of polyphenol acid from WBEs with NKA-9 macroporous resin was performed before the subsequent experiments. The products of WBEs in NKA-9 with 40% ethanol elution were named WBPE-N9-4. To have a deep and comprehensive understanding of the effects of WBPE on cell antioxidation ability, HEK293 cells were chosen as an oxidative stress model for studying the effects of WBPE-N9-4 against H2O2-induced apoptosis as well as the related mechanisms.
2. Materials and methods
2.1 Chemicals, reagents and equipment
Raw wheat bran was provided by Yihai Kerry Food Industry Co., Ltd. (Kunshan, China). HEK293 cells were purchased from American Type Culture Collection; chemical reagents, including ferulic acid, o-coumaric acid, p-coumaric acid, gallic acid, and olivetol (Sigma-Aldrich, St. Louis, USA), high-glucose Dulbecco's modified Eagle's medium (HG-DMEM) (Gibco BRL, Life Technologies, USA), trypsin-ethylene diamine tetraacetic acid (EDTA) (Beyotime, Jiangsu, China), fetal bovine serum (FBS) (Sijiqing, Zhejiang, China), and methyl thiazolyl tetrazolium (MTT) (Sigma, St. Louis, USA) were also purchased from commercial suppliers. Lactate dehydrogenase (LDH) and glutathione peroxidase (GSH-Px) assay kits were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Malondialdehyde (MDA), superoxide dismutase (SOD), catalase (CAT), Annexin V/FITC kit, Hochest33258 kit, cell cycle and ROS assay kit, and mitochondrial membrane potential assay kit with JC-1 were all purchased from Beyotime Institute of Biotechnology (Haimen, China).2.2 Preparation of WBPE
Initially, wheat bran (1 kg) was washed 3 times with 5–6 volumes (w/v) of water to remove starch and then heated at 50 °C for about 12 h. Then, the dried wheat bran was ground to powder using a hammer mill and passed through a 100 mesh sieve. WBEs were prepared according to a previous report.32 The bran (30 g) was then extracted twice with 80% ethanol at a ratio of 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/w) at room temperature for 15 h. The mixture was centrifuged at 9000×g for 20 min and dried at 40 °C using a rotary evaporator. The ethanol extract (4 g) was freeze-dried and stored in a sealed container at 4 °C in a dark environment for further use and analysis. The freeze-dried dispersed product was denoted as WBEs.
1 (v/w) at room temperature for 15 h. The mixture was centrifuged at 9000×g for 20 min and dried at 40 °C using a rotary evaporator. The ethanol extract (4 g) was freeze-dried and stored in a sealed container at 4 °C in a dark environment for further use and analysis. The freeze-dried dispersed product was denoted as WBEs.
In addition, the preparative enrichment and separation of polyphenol acid from WBEs with 4 types of macroporous resins were performed before the subsequent experiments. The WBEs were then subjected to NKA-9 macroporous resin column chromatography eluting with a gradient of ethanol–water (40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60). The fractions were collected, concentrated, and lyophilized for the subsequent experiments. The freeze-dried dispersed products were defined as WBPE-N9-4.
60). The fractions were collected, concentrated, and lyophilized for the subsequent experiments. The freeze-dried dispersed products were defined as WBPE-N9-4.
2.3 Chromatographic system and conditions
The products were analyzed using an Acquity UPLC/TQD system (Waters, Milford, MA), including an autosampler, photodiode array detector and an MS pump, equipped with an electrospray ionization (ESI) probe as the interface. The samples were separated using ultra-high performance liquid chromatography on an Acquity UPLC BEH C18 column (2.1 × 50 mm2, 1.7 μm) with a mobile phase consisting of acetonitrile solution (A) and 0.1% (v/v) formic acid water solution (B) at a flow rate of 0.3 mL min−1 (Table 1). The detection of the phenolic compounds was conducted in the multiple reaction monitoring (MRM) mode. Individual compounds were first identified by MRM using a specific precursor-production transition: m/z 163.08 > 119.64 for o-coumaric acid; m/z 163.08 > 119.15 for p-coumaric acid; m/z 193.00 > 134.10 for ferulic acid; m/z 169.05 > 125.00 for gallic acid and m/z 181.08 > 43.03 for olivetol. The detected phenolic compounds were quantified against standard curves generated with phenolic standards. Results were expressed as milligrams of compound per gram of extract (mg compound/g extract).| Time (min) | Flow (mL min−1) | Eluent A (%) | Eluent B (%) |
|---|---|---|---|
| 0.00 | 0.30 | 5.0 | 95.0 |
| 0.80 | 0.30 | 5.0 | 95.0 |
| 1.20 | 0.30 | 10.0 | 90.0 |
| 1.90 | 0.30 | 10.0 | 90.0 |
| 2.40 | 0.30 | 15.0 | 85.0 |
| 3.70 | 0.30 | 15.0 | 85.0 |
| 4.00 | 0.30 | 21.0 | 79.0 |
| 5.20 | 0.30 | 21.0 | 79.0 |
| 5.50 | 0.30 | 27.0 | 73.0 |
| 7.80 | 0.30 | 50.0 | 50.0 |
| 8.80 | 0.30 | 100.0 | 0.0 |
| 9.30 | 0.30 | 5.0 | 95.0 |
2.4 Cell culture and treatment
HEK293 cells were cultured in high-glucose Dulbecco's modified Eagle's medium (HG-DMEM) supplemented with 10% fetal bovine serum (FBS) maintained at 37 °C in an incubator with 5% CO2. The medium was changed every two days. All the experiments were performed for 12 h after the cells were seeded in microplates. WBPE-N9-4 were freshly prepared as a stock solution in ethanol and diluted with HG-DMEM. A control experiment was performed in the presence of 1% (v/v) ethanol33 under the same culture conditions, while ferulic acid was used as a positive control.2.5 Cell viability and LDH release assay
Cell viability was determined using the MTT assay.34 The MTT cytotoxicity assay was performed as previously described35,36 with minor modifications. Briefly, HEK293 cells were seeded at a density of 3 × 104 cells per well in 96-well plates (Costar 3599; Corning, NY) for 12 h. WBPE-N9-4 and ferulic acid were dissolved separately in ethanol and diluted with HG-DMEM. The cells were incubated first with WBPE-N9-4 (0.1–1 mg mL−1) or ferulic acid (1 mM) for 1 h, then with 1 mM H2O2 for 2 h.37 After being replaced with fresh medium, 20 μL of MTT (5 mg mL−1 in phosphate buffer solution, PBS) was added and incubated for 4 h at 37 °C in a humidified incubator in the presence of 5% CO2. The cells were incubated in a dark incubator to avoid unexpected phototoxicity.34 The medium was then carefully removed and stained using formazan dissolved in 150 μL of dimethyl sulfoxide (DMSO). The plate was shaken for 10 min and the absorbance of the samples was measured at 570 nm using a SH-1000 microplate reader. The results were expressed as the percentage of viability (%) = [(optical density of treated cells/optical density of control cells)]. All the assays were repeated as three independent experiments, each experiment containing at least six replicates.When the cells were damaged by H2O2, the cell viability decreased and a change in the LDH value occurred. The release of intracellular LDH in the culture medium is an indicator of irreversible cell death due to membrane damage.38 LDH activity can be achieved in cytotoxicity quantitative analysis through the detection of its release from the plasma membrane to rupture the cell culture medium. LDH leakage was detected using an assay kit. First, HEK293 cells were seeded at a density of 9 × 105 cells per well in 6-well plates. First, the cells were incubated with WBPE-N9-4 or ferulic acid for 1 h, and then exposed to 1 mM H2O2 for 2 h. Then, the cells were washed twice with PBS, and then lysed using cell lysis buffer to release the LDH inside the living cells into the new supernatant. After the reaction, the absorbance of the samples was recorded at 450 nm. The results were presented as a ratio of the control value.
2.6 Observation of morphological changes
HEK293 cells were seeded in 96-well plates at a density of 3 × 104 cells per well. After treatment with WBPE-N9-4 (1 mg mL−1) or ferulic acid (1 mM) for 1 h and H2O2 for 2 h, the cells were examined using inverted phase contrast microscopy (BX41 Olympus Optical Co. Ltd., Japan). The cells were observed with fluorescence microscopy39 (BX41 Olympus Optical Co. Ltd., Japan) after being stained with Hochest33258 dye and seeded into 6-well plates at a density of 9 × 105 cells per well. Then, the cells were observed and cell images were recorded.40,412.7 ROS assay
HEK293 cells were seeded at 3 × 104 cells per well in clear-bottom, black-walled 96-well plates (Costar 3606; Corning, NY) for 12 h. Then, the cells were incubated with a carboxy-2′,7′-dichloro-dihydro-fluorescein diacetate probe for 20 min and washed twice with PBS. The cells were then exposed to WBPE-N9-4 (1 mg mL−1) or ferulic acid (1 mM) for 1 h and H2O2 for 2 h. The fluorescence of the samples was measured at 488 nm (excitation) and 525 nm (emission) wavelengths using a SpectraMax M5 microplate reader. All the assays were repeated for three independent experiments, each experiment containing at least six replicates.2.8 Measurements of CAT, SOD, GSH-Px and MDA
The activities of catalase (CAT), superoxide dismutase (SOD), and the content of malondialdehyde (MDA) were measured using assay kits (Beyotime Institute of Biotechnology, Haimen, China). GSH-Px activity was determined using an assay kit (Jiancheng Bioengineering). The assay for GSH-Px activity analysis was performed by quantifying the rate of oxidation of reduced glutathione to oxidized glutathione with H2O2 catalyzed by GSH-Px. Initially, the HEK293 cells were washed with PBS after treatment (1 mg mL−1 WBPE-N9-4, 1 mM ferulic acid and 1 mM H2O2), and then scraped in ice-cold PBS and homogenized with sonication. The homogenate was centrifuged at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 10 min at 4 °C, and the supernatant used to measure the CAT, SOD, and GSH-Px activities. Protein content was measured by the Coomassie blue protein-binding method with bovine serum albumin as the standard.42 The concentration of MDA can be determined at a wavelength of 532 nm by subjecting it to form a stable chromophoric production via reaction with thiobarbituric acid (TBA). The level of MDA was expressed as nmol per mg protein.
000g for 10 min at 4 °C, and the supernatant used to measure the CAT, SOD, and GSH-Px activities. Protein content was measured by the Coomassie blue protein-binding method with bovine serum albumin as the standard.42 The concentration of MDA can be determined at a wavelength of 532 nm by subjecting it to form a stable chromophoric production via reaction with thiobarbituric acid (TBA). The level of MDA was expressed as nmol per mg protein.
2.9 Cell cycle analysis and sub-G1 DNA content assay
The growing cells were seeded at 9 × 105 cells per well in six-well plates for 12 h. After incubation with WBPE-N9-4 (1 mg mL−1), ferulic acid (1 mM) and H2O2 (1 mM), the cells were washed with PBS, trypsinized, fixed in 70% (v/v) ice-cold ethanol and stored at 4 °C for 24 h. Then, cell pellets were washed with ice-cold PBS, centrifuged, resuspended in 1 mL of PBS containing 1 mg mL−1 RNase and 50 μg mL−1 propidium iodide (PI), and incubated in the dark at 37 °C for 30 min and then on ice. Samples were filtered through a nylon mesh and the cell cycle was analyzed using flow cytometry with the software package Cell Quest Pro (Becton Dickinson, CA, USA).2.10 Cell apoptosis test
HEK293 cells were seeded at 9 × 105 cells per well in 6-well plates for 12 h attachment. Cell apoptosis was detected by Annexin V-FITC kit after 3 h treatment with WBPE-N9-4 (1 mg mL−1), ferulic acid (1 mM) and H2O2 (1 mM). Briefly, the cells were gently trypsinized, washed with PBS, suspended in binding buffer, and incubated with Annexin V-FITC and PI at room temperature in the dark for 10 min. The cells were analyzed immediately using flow cytometry. In particular, cells were delivered at a high flow rate of 200–300 cells per s. Digital signals of forward scatter (FS), sideward scatter (SS), and green and red fluorescence (FL1, FL2) were recorded for further analysis. The cells stained positively with Annexin V-FITC but remaining impermeable to PI (AV+/PI−) were regarded as early apoptotic cells.432.11 Measurement of mitochondrial membrane potential (Δψm)
Mitochondrial membrane potential (Δψm) was measured using a dual-emission potential-sensitive probe JC-1 in the monomeric form with excitation at 490 nm and emission at 527 nm.44 The treated HEK293 cells (1 mg mL−1 WBPE-N9-4 and 1 mM ferulic acid for 1 h, and then 1 mM H2O2 for 2 h) were harvested and incubated with 10 μg mL−1 JC-1 at 37 °C in the dark for 10 min. The cells were then washed twice with PBS, resuspended in 0.5 mL of PBS and analyzed using flow cytometry for green and orange fluorescence with a 525 and 575 nm filter, respectively, for changes in Δψm. The values of fluorescence intensity were averaged.2.12 Statistical analysis
All the experiments were performed in triplicate and data were expressed as the mean ± standard deviation (SD). Statistical analysis was performed according to Student's t-test and one-way analysis45 on SPSS 18.0 (SPSS Inc. US Chicago), p values < 0.05 were considered to be significant.3. Results and discussion
3.1 UPLC/TDQ analysis
The UPLC-MS/MS analysis of WBPE-N9-4 was conducted to quantify the phenolic compounds present, which may be responsible for its antioxidant activity. The total ion chromatogram (TIC) of a standard solution containing five phenolic compounds is shown in Fig. 1. The presence of the standard phenolic compounds using UPLC/TQD was confirmed in Table 2. These analysis results confirmed the existence of ferulic acid (35.49 ± 2.7 mg g−1), p-coumaric acid (16.79 ± 0.68 mg g−1), o-coumaric acid (0.92 ± 0.05 mg g−1) and gallic acid (2.08 ± 0.09 mg g−1). However, olivetol was not detected. Ferulic acid was the most abundant phenolic compound in WBEs. In a previous study, similar results were obtained from phenolic acid concentrations in spring and winter wheat.9 The results showed that the content of ferulic acid was higher than the other phenolic acids in WBPE-N9-4, which may be associated with the antioxidative activity of WBPE-N9-4. Moreover, in the ROS assay, the antioxidant activity of WBPE-N9-4 was very close to that of the positive control-ferulic acid (Fig. 3).| Compound | Retention time (min) | Molecular weight (g mol−1) | MS (m/z) | MS/MS MRM (m/z) | Cone voltage (V) | Collision energy (V) |
|---|---|---|---|---|---|---|
| o-Coumaric acid | 6.68 | 164.16 | 163.08 | 163.08 > 93.00 | 22 | 24 |
| 163.08 > 119.64 | 22 | 26 | ||||
| p-Coumaric acid | 5.12 | 164.16 | 163.08 | 163.08 > 93.03 | 24 | 26 |
| 163.08 > 119.15 | 24 | 30 | ||||
| Ferulic acid | 5.51 | 194.1 | 193.00 | 193.00 > 134.10 | 28 | 13 |
| 193.00 > 149.20 | 28 | 10 | ||||
| 193.00 > 178.20 | 28 | 12 | ||||
| Gallic acid | 1.57 | 170.0 | 169.05 | 169.05 > 79.01 | 28 | 22 |
| 169.05 > 97.02 | 28 | 16 | ||||
| 169.05 > 125.00 | 28 | 13 | ||||
| Olivetol | 8.96 | 180.24 | 181.08 | 181.08 > 43.03 | 26 | 18 |
| 181.08 > 71.03 | 26 | 12 | ||||
| 181.08 > 110.97 | 26 | 12 |
3.2 Effects of WBPE-N9-4 on H2O2-injured growth and viability in HEK293 cells
Cell viability was determined using an MTT assay; the cell count after exposure to H2O2 decreased in a dose- and time-dependent manner. In particular, cell viability decreased significantly after exposure to H2O2 for 2 h, suggesting that HEK293 cells were injured at an IC50 value of 0.98 ± 0.02 mM. Therefore, H2O2 at a concentration of 1 mM was used for further experiments. Moreover, no cytotoxic and proliferative effects of WBEs or WBPE-N9-4 were observed when normal HEK293 cells were treated for 12 or 24 h. As shown in Table 3, cell viability decreased to 50.90% ± 2.20% after exposure to 1 mM H2O2 only. However, cell viability significantly increased to 66.65% ± 2.39% and 72.78% ± 2.34%, respectively, when treated with WBPE-N9-4 (0.50 & 1.00 mg mL−1) prior to H2O2 exposure for 1 h. LDH, which is a stable cytoplasmic enzyme in cells, is an important indicator of cytotoxicity.46 LDH will be released into the culture supernatant when the cell membrane is damaged.47 Therefore, the percentage of LDH leakage could also be employed to investigate the resistance effect of WBPE-N9-4 (Table 3). The results indicated that when treated with WBPE-N9-4, LDH decreased from 13.09 to 7.64, which is in agreement with the cell viability results.| Cell viability (%) | LDH leakage (%) | |
|---|---|---|
| a Cells were incubated with 1 mM H2O2 for 2 h for the MTT assay and LDH assay. WBPE-N9-4 were added to the culture 1 h prior to H2O2 addition. Ferulic acid (1 mM) served as a positive control. Results are reported as the mean ± SD (n = 6). Numbers followed by different letters are significantly different at the level of p < 0.05 according to the Duncan test. | ||
| Control | 100.52 ± 3.04f | 2.91 ± 0.52a |
| H2O2 (1 mM) | 50.90 ± 2.20a | 13.09 ± 1.14g |
| WBPE-N9-4 (0.10 mg mL−1) + H2O2 (1 mM) | 53.59 ± 3.47a | 11.86 ± 1.05f |
| WBPE-N9-4 (0.25 mg mL−1) + H2O2 (1 mM) | 61.64 ± 2.75b | 10.14 ± 0.09e |
| WBPE-N9-4 (0.50 mg mL−1) + H2O2 (1 mM) | 66.65 ± 2.39c | 9.15 ± 0.71d |
| WBPE-N9-4 (1.00 mg mL−1) + H2O2 (1 mM) | 72.78 ± 2.34d | 7.64 ± 0.40c |
| Ferulic acid (1 mM) + H2O2 (1 mM) | 78.58 ± 1.81e | 5.75 ± 0.48b |
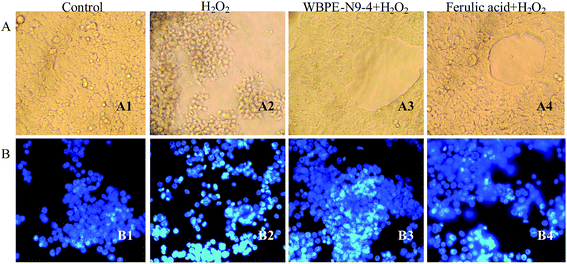
3.3 Effects of WBPE-N9-4 on the morphology of H2O2-treated HEK293 cells
The morphological alteration was observed in cells exposed to WBPE-N9-4 using a phase-contrast microscope. The HEK293 cells in the control group grew efficiently and maintained a normal, round morphology (Fig. 2A1). However, cells exposed to H2O2 for 2 h displayed an obvious reduction in the viable cell counts as well as cell body shrinkage (Fig. 2A2). In contrast, after treatment with WBPE-N9-4 (1 mg mL−1) and ferulic acid (1 mM) for 1 h before exposure to H2O2, the majority of the cells maintained a good status without a remarkable reduction in cell number and cells congregation (Fig. 2A3 and A4). These results revealed that WBPE-N9-4 protected against H2O2-induced oxidative damage in HEK293 cells.To further investigate the effects of different treatments on DNA and the nuclear structure in HEK293 cells, the DNA fluorescent dye Hoechst 33258 was used. Upon H2O2 treatment, the shrinkage of the cell nuclei and delineation of condensed chromatin material adjacent to plasma membrane was observed. Nuclei broke into small fragments and cellular debris was extruded out via budding from the plasma membrane to form apoptotic bodies (Fig. 2B2). Compared to the control group, cells incubated with H2O2 alone contained many small white dots, representing chromatin condensation and/or nuclear fragmentation. Similar morphological characteristics were detected in PC12 cells upon treatment with 500 μM H2O2 for 24 h.48 However, these morphological changes were inhibited by the prior addition of WBPE-N9-4 and ferulic acid (Fig. 2B3 and B4). The majority of cell nuclei maintained normal shape and size.
3.4 Effect of WBPE-N9-4 on H2O2 intracellular ROS accumulation
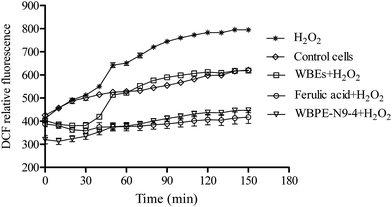
It is well-known that treatment with H2O2 can cause nuclear damage, loss of Δψm and higher ROS levels. In a normal physiological process, mitochondria convert 1–2% of the consumed oxygen to ROS, while ROS levels dramatically increase under environmental stress.21 The curve for control cells demonstrated ROS levels under the normal state of the cells (Fig. 3). After adding WBPE-N9-4 and ferulic acid to cells and allowing them to stand for 20 min, the plates were placed in a microplate reader and the value of (dichlorofluorescein) DCF relative fluorescence was measured. Then, after 30 min, H2O2 was added to the cell samples. The results showed that the curves for control cells and H2O2 were coincident in 30 min. However, the curves for WBPE-N9-4 and ferulic acid obey a similar tendency to that found in 30 min, and the ROS levels were significantly lower compared with the control cells. These results clearly illustrate that WBEs can reduce the level of ROS. The decrease of ROS levels was the same as that of ferulic acid when the H2O2 was absent. When H2O2 was added, the WBPE-N9-4 curve exhibited an increase and also maintained the ROS levels found in the control cells, which was significantly lower than the curve for H2O2. These results suggested that WBPE-N9-4 had the ability to reduce intracellular ROS levels in HEK293 cells and prevent cell death induced in the presence of H2O2. It is reasonable to conclude that WBPE-N9-4 has a strong ability to decrease ROS levels (Fig. 3).3.5 Effects of WBPE-N9-4 on the activities of antioxidant enzymes and lipid peroxide levels in H2O2-treated HEK293 cells
The accumulation of highly-reactive oxygen radicals causes damage to the biomolecules in cells as well as disrupts the cell membrane, alters enzyme activities, and eventually leads to DNA damage. However, several enzymes, including CAT, SOD and GSH-Px, forming part of the cellular antioxidant system can play crucial roles in scavenging ROS and reducing oxidative damage.49 The treatment of HEK293 cells with 1 mM H2O2 caused an increase in intracellular MDA levels by 139.08%, while the pre-incubation of cells with WBPE-N9-4 or ferulic acid significantly attenuated the increase (Table 4). In addition, HEK293 cells treated with H2O2 exhibited a decrease in the activity of SOD, CAT, and GSH-Px by 41.96%, 66.44%, and 77.33%, respectively. However, pretreatment with WBPE-N9-4 significantly inhibited the change in SOD, CAT, and GSH-Px activities in a dose-dependent manner (Table 4).| Treatment | MDA (nmol mgprot−1) | SOD (U mgprot−1) | CAT (U mgprot−1) | GSH-Px (U mgprot−1) |
|---|---|---|---|---|
| a Cells were incubated with 1 mM H2O2 for 2 h for the assays of MDA, SOD, CAT and GSH-Px. WBPE-N9-4 was added to the culture 1 h prior to H2O2 addition. Ferulic acid (1 mM) served as a positive control. Results are reported as the mean ± SD (n = 6). Numbers followed by different letters are significantly different at the level of p < 0.05 according to the Duncan test. | ||||
| Control | 1.74 ± 0.06a | 110.64 ± 1.85g | 12.01 ± 1.05e | 22.36 ± 1.16f |
| H2O2 (1 mM) | 4.16 ± 0.13g | 64.22 ± 0.94a | 4.03 ± 0.64a | 5.07 ± 1.06a |
| WBPE-N9-4 (0.10 mg mL−1) + H2O2 (1 mM) | 3.91 ± 0.03f | 71.15 ± 1.06b | 5.18 ± 0.06 ab | 7.01 ± 0.63b |
| WBPE-N9-4 (0.25 mg mL−1) + H2O2 (1 mM) | 3.75 ± 0.05e | 75.29 ± 1.10c | 5.85 ± 0.08b | 8.21 ± 0.45bc |
| WBPE-N9-4 (0.50 mg mL−1) + H2O2 (1 mM) | 3.11 ± 0.12d | 81.71 ± 0.53d | 7.66 ± 0.61c | 9.28 ± 0.57c |
| WBPE-N9-4 (1.00 mg mL−1) + H2O2 (1 mM) | 2.79 ± 0.09c | 91.34 ± 1.51e | 8.73 ± 0.96 cd | 11.27 ± 0.87d |
| Ferulic acid (1 mM) + H2O2 (1 mM) | 2.09 ± 0.06b | 102.43 ± 1.24f | 9.43 ± 1.04d | 13.52 ± 1.13e |
3.6 Effect of WBPE-N9-4 on the apoptosis of H2O2-treated HEK293 cells
Flow cytometry was used to monitor the changes in DNA content of PI-stained and H2O2-treated HEK293 cells in the presence of WBPE-N9-4. Compared to the control group, the percentage of the sub-G1 peak, indicative of necrosis and reduced DNA content,50 was increased after exposure to H2O2. However, it decreased from 15.2% to 12.8% and 11.7% after pretreatment with WBPE-N9-4 and ferulic acid, respectively. An Annexin V-FITC/PI assay based on flow cytometry was used to confirm cell apoptosis in the presence of WBPE-N9-4. As shown in Fig. 4B, control cells were AV−/PI− and appeared in the lower left quadrant. The cells in the lower right quadrant were AV+/PI−, indicating early apoptosis. The majority of normal cells were AV−/PI−, and H2O2-injured cells increased the AV+ population from 0.1% to 1.69%, the PI+ population increased from 0.00% to 40.04%, AV−/PI− in H2O2-injured cells were only 50.11%. However, pretreatment with WBPE-N9-4 and ferulic acid could increase the AV−/PI− cell population to 86.78% and 89.86%, respectively. Similarly, a decrease in apoptotic cell numbers were observed in PC12 cells pretreated with WGPIH5.51 In conclusion, our results demonstrated that H2O2 suppressed the proliferation of HEK293 cells and induced cell death. Furthermore, WBPE-N9-4 could protect H2O2-injured HEK293 cells against the induction of necrosis.The Δψm in HEK293 cells was also examined using flow cytometry with JC-1. The effects of various treatments on changes in Δψm are shown in Fig. 4C. The ratio of red to green arithmetic mean (FL2-H and FL1-H) was used to demonstrate cell damage. The ratio was 1.578 in control cells and 0.203 in H2O2-injured cells. However, the ratio was increased after pretreatment with WBPE-N9-4 (0.979) or ferulic acid (1.218). A decreased ratio (from 1.587 to 0.203) indicated cells were damaged by H2O2. The ratio increased after pretreatment with WBPE-N9-4 (from 0.203 to 0.979), suggesting that WBPE-N9-4 can prevent the HEK293 cells from oxidative damage.
4. Conclusions
Incubation of HEK293 cells with WBPE-N9-4 prior to H2O2 induced stress was successful in enhancing cell resistance to oxidative damage. The WBPE-N9-4 exhibited antioxidant activity through restoring cell viability and diminishing ROS content and LDH release, which also displayed DNA protective effects in HEK293 cells exposed to H2O2-induced oxidative stress. The improved resistance impacts of WBPE-N9-4 on cells might be due to phenolic acids, which can neutralize radicals and ROS. Further investigations to determine the presence of phenolic compounds using UPLC/TQD analysis revealed that WBPE-N9-4 with high antioxidant activity contained large amounts of ferulic acid. Further work is being carried out to isolate and purify the bioactive phenolic acids from WBPE-N9-4 and to clarify their structure–function relationship for elucidating the specific antioxidant pathway for cell protection.Acknowledgements
This work was sponsored by Qing Lan Project, China Postdoctoral Science Foundation (Grant no. 2014M560396), Jiangsu Planned Projects for Postdoctoral Research Funds (Grant no.1402072C), and the National Key Technology R&D Program (Grant no. 2013AA102201).References
- S. P. Zou, J. J. Zhou, I. Kaleem, L. P. Xie, G. Y. Liu and C. Li, Sep. Sci. Technol., 2012, 47, 1055–1062 CrossRef CAS.
- O. K. Santala, E. A. Nordlund and K. S. Poutanen, Food Bioprocess Technol., 2013, 6, 3102–3112 CrossRef CAS.
- L. L. Yu, K. Q. Zhou and J. W. Parry, LWT–Food Sci. Technol., 2005, 38, 463–470 CrossRef CAS PubMed.
- M. Martinez-Tome, M. A. Murcia, N. Frega, S. Ruggieri, A. M. Jimenez, F. Roses and P. Parras, J. Agric. Food Chem., 2004, 52, 4690–4699 CrossRef CAS PubMed.
- A. Jayadeep, V. Singh, B. V. S. Rao, A. Srinivas and S. Z. Ali, Food Bioprocess Technol., 2009, 2, 57–67 CrossRef CAS PubMed.
- K. K. Adom, M. E. Sorrells and R. H. Liu, J. Agric. Food Chem., 2003, 51, 7825–7834 CrossRef CAS PubMed.
- M. N. Maillard and C. Berset, J. Agric. Food Chem., 1995, 43, 1789–1793 CrossRef CAS.
- S. N. Onyeneho and N. S. Hettiarachchy, J. Agric. Food Chem., 1992, 40, 1496–1500 CrossRef CAS.
- J. Zuchowski, K. Jonczyk, L. Pecio and W. Oleszek, J. Sci. Food Agric., 2011, 91, 1089–1095 CrossRef CAS PubMed.
- K. Q. Zhou and L. L. Yu, LWT–Food Sci. Technol., 2004, 37, 717–721 CrossRef CAS PubMed.
- K. Q. Zhou and L. L. Yu, J. Agric. Food Chem., 2004, 52, 1112–1117 CrossRef CAS PubMed.
- L. Liu, K. M. Winter, L. Stevenson, C. Morris and D. N. Leach, Food Chem., 2012, 130, 156–164 CrossRef CAS PubMed.
- J. W. Carter, R. Madl and F. Padula, Nutr. Res., 2006, 26, 33–38 CrossRef CAS PubMed.
- L. L. Yu, S. Haley, J. Perret, M. Harris, J. Wilson and M. Qian, J. Agric. Food Chem., 2002, 50, 1619–1624 CrossRef CAS PubMed.
- L. L. Yu, S. Haley, J. Perret and M. Harris, Food Chem., 2002, 78, 457–461 CrossRef CAS.
- F. Cheli and A. Baldi, J. Food Sci., 2011, 76, R197–R205 CrossRef CAS PubMed.
- C. Dong, R. EIdawud, L. M. Sargent, M. L. Kashon, D. Lowry, Y. Rojanasakul and C. Z. Dinu, Environ. Sci.: Nano, 2014, 6, 595–603 RSC.
- K. J. Siegrist, S. H. Reynolds, M. L. Kashon, D. T. Lowry, C. B. Dong, A. F. Hubbs, S. H. Young, J. L. Salisbury, D. W. Porter, S. A. Benkovic, M. McCawley, M. J. Keane, J. T. Mastovich, K. L. Bunker, L. G. Cena, M. C. Sparrow, J. L. Sturgeon, C. Z. Dinu and L. M. Sargent, Part. Fibre Toxicol., 2014, 11, 1–15 CrossRef PubMed.
- C. B. Dong, M. L. Kashon, D. Lowry, J. S. Dordick, S. H. Reynolds, Y. Rojanasakul, L. M. Sargent and C. Z. Dinu, Adv. Healthcare Mater., 2013, 2, 945–951 CrossRef CAS PubMed.
- C. B. Dong, A. S. Campell, R. Eldawud, G. Perhinschi, Y. Rojanasakul and C. Z. Dinu, Appl. Surf. Sci., 2013, 264, 261–268 CrossRef CAS PubMed.
- T. Devasagayam, J. Tilak, K. Boloor, K. Sane, S. Ghaskadbi and R. Lele, JAPI, 2004, 52, 794–804 CAS.
- B. Halliwell and J. Gutteridge, Biochem. J., 1984, 219, 1 CAS.
- L. I. Leichert, F. Gehrke, H. V. Gudiseva, T. Blackwell, M. Ilbert, A. K. Walker, J. R. Strahler, P. C. Andrews and U. Jakob, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 8197–8202 CrossRef CAS PubMed.
- S. Bhattacharjee, L. J. Deterding, S. Chatterjee, J. Jiang, M. Ehrenshaft, O. Lardinois, D. C. Ramirez, K. B. Tomer and R. P. Mason, Free Radical Biol. Med., 2011, 50, 1536–1545 CrossRef CAS PubMed.
- A. Manke, S. Luanpitpong, C. B. Dong, L. Y. Wang, X. Q. He, L. Battelli, R. Derk, T. A. Stueckle, D. W. Porter, T. Sager, H. L. Gou, C. Z. Dinu, N. Q. Wu, R. R. Mercer and Y. Rojanasakul, Int. J. Mol. Sci., 2014, 15, 7444–7461 CrossRef PubMed.
- E. Sedlák, M. Fabian, N. C. Robinson and A. Musatov, Free Radical Biol. Med., 2010, 49, 1574–1581 CrossRef PubMed.
- M. Zhang, E. Mahoney, T. Zuo, P. K. Manchanda and R. V. Davuluri, PLoS One, 2014, 9, e109523 Search PubMed.
- M. Zhang, P. K. Manchanda, D. Y. Wu, Q. B. Wang and L. S. Kirschner, Mol. Endocrinol., 2014, 28, 295–307 CrossRef CAS PubMed.
- Y. Chen, E. McMillan-Ward, J. Kong, S. J. Israels and S. B. Gibson, Cell Death Differ., 2008, 15, 171–182 CrossRef CAS PubMed.
- J. J. Xiong, Y. Zhang, J. L. Wang, G. D. Bao and Z. Y. Zhu, Biosci., Biotechnol., Biochem., 2008, 72, 3206–3210 CrossRef CAS PubMed.
- J. Wang, B. Sun, Y. Cao, H. Song and Y. Tian, Food Chem., 2008, 109, 129–136 CrossRef CAS PubMed.
- L. L. Yu, J. Perret, M. Harris, J. Wilson and S. Haley, J. Agric. Food Chem., 2003, 51, 1566–1570 CrossRef CAS PubMed.
- X. Luo, R. Wang, L. Wang, Y. Li, Y. Bian and Z. Chen, Food Control, 2014, 37, 171–176 CrossRef CAS PubMed.
- S.-B. Wu, J.-J. Su, L.-H. Sun, W.-X. Wang, Y. Zhao, H. Li, S.-P. Zhang, G.-H. Dai, C.-G. Wang and J.-F. Hu, J. Nat. Prod., 2010, 73, 1898–1906 CrossRef CAS PubMed.
- Y. Liu, J. Fang, Y. J. Kim, M. K. Wong and P. Wang, Mol. Pharm., 2014, 11, 1651–1661 CrossRef CAS PubMed.
- B. R. Schroeder, M. I. Ghare, C. Bhattacharya, R. Paul, Z. Yu, P. A. Zaleski, T. C. Bozeman, M. J. Rishel and S. M. Hecht, J. Am. Chem. Soc., 2014, 136, 13641–13656 CrossRef CAS PubMed.
- X. Gong, Phys. Chem. Chem. Phys., 2013, 15, 10459–10465 RSC.
- G. Fotakis and J. A. Timbrell, Toxicol. Lett., 2006, 160, 171–177 CrossRef CAS PubMed.
- X. Gong, RSC Adv., 2014, 4, 54494–54499 RSC.
- Z. Yu, R. M. Schmaltz, T. C. Bozeman, R. Paul, M. J. Rishel, K. S. Tsosie and S. M. Hecht, J. Am. Chem. Soc., 2013, 135, 2883–2886 CrossRef CAS PubMed.
- Y. Liu, M. Ji, M. K. Wong, K.-I. Joo and P. Wang, BioMed Res. Int., 2013, 2013, 378380 Search PubMed.
- X. Gao, C. Y. Zheng, G. W. Qin, X. C. Tang and H. Y. Zhang, J. Neurosci. Res., 2012, 90, 1981–1988 CrossRef CAS PubMed.
- S.-B. Wu, F. Pang, Y. Wen, H.-F. Zhang, Z. Zhao and J.-F. Hu, Planta Med., 2010, 76, 82–85 CrossRef CAS PubMed.
- W. Cao, X. Li, X. Wang, H. Fan, X. Zhang, Y. Hou, S. Liu and Q. Mei, Phytomedicine, 2010, 17, 598–605 CrossRef CAS PubMed.
- Z. Qiao, C. Xia, S. Shen, F. D. Corwin, M. Liu, R. Guan, J. R. Grider and L. Y. Qiao, PLoS One, 2014, 9, e114536 Search PubMed.
- S. Hussain, K. Hess, J. Gearhart, K. Geiss and J. Schlager, Toxicol. In Vitro, 2005, 19, 975–983 CrossRef CAS PubMed.
- H. O. Jauregui, N. T. Hayner, J. L. Driscoll, R. Williams-Holland, M. H. Lipsky and P. M. Galletti, In Vitro, 1981, 17, 1100–1110 CrossRef CAS.
- Y. Gao, H. W. Zhang, H. L. Qiao, W. Wang and J. B. Chang, Brain Res., 2010, 1358, 239–247 CrossRef CAS PubMed.
- G. S. Liu, Z. S. Zhang, B. Yang and W. He, Life Sci., 2012, 91, 872–877 CrossRef CAS PubMed.
- S. K. Bhutia, S. K. Mallick, S. Maiti, D. Mishra and T. K. Maiti, Cell Biol. Int., 2009, 33, 720–727 CrossRef CAS PubMed.
- K. X. Zhu, X. Guo, X. N. Guo, W. Peng and H. M. Zhou, Food Res. Int., 2013, 53, 297–303 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |