Catalytic oxidation of formaldehyde in water by calcium phosphate-based Pt composites†
Abstract
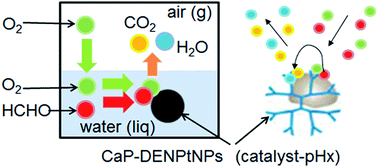
Platinum nanoparticles (PtNPs) protected by dendrimer were incorporated in calcium phosphate particles, and the degradation of a pollutant, formaldehyde (HCHO), in water was investigated by using the resultant composite powders as a catalyst. The reaction was performed with dissolved oxygen from air as an oxidant. The analytical results revealed that high PtNP and HCHO concentrations and a high temperature effectively accelerated the oxidation of HCHO. The results were also kinetically analyzed using the Elovich equation. Additionally, the complete oxidation process of HCHO could be concluded to depend on the adsorption process of HCHO on the catalyst. The present system is a valuable catalyst for use with atmospheric oxygen at atmospheric pressure and at mild temperatures, and more importantly this catalytic system can easily be removed from the reaction solution.

 Please wait while we load your content...
Please wait while we load your content...