Self-organized environment-sensitive inulin–doxorubicin conjugate with a selective cytotoxic effect towards cancer cells†
N. Mauroa,
S. Camporab,
C. Scialabbaa,
G. Adamob,
M. Licciardia,
G. Ghersib and
G. Giammona*a
aLaboratory of Biocompatible Polymers, Department of “Scienze e Tecnologie Biologiche, Chimiche e Farmaceutiche” (STEBICEF), University of Palermo, Via Archirafi, 32 90123 Palermo, Italy. E-mail: gaetano.giammona@unipa.it; Fax: +39 09123891928; Tel: +39 09123891928
bDepartment of “Scienze e Tecnologie Biologiche, Chimiche e Farmaceutiche” (STEBICEF), University of Palermo, Viale delle scienze Ed, 16 90128 Palermo, Italy
First published on 31st March 2015
Abstract
An inulin-based random copolymer bearing high dose doxorubicin (18.45% on a weight basis), INU-EDA-P,C-DOXO, was prepared by coupling doxorubicin with inulin though a citraconylamide bridge used as a pH sensitive spacer. A further conjugation with pentynoic acid via an amidic bond led to the hydrophobization of the copolymer which allows the acquisition of a self-assembling ability at low concentration (0.33 mg mL−1) combining both Π–Π stacking and London interactions. Drug release studies were carried out at different pH demonstrating a remarkable pH dependency, where the maximum release rate was observed at pH mimicking cancer tissue and lysosomal environments. Besides, by measuring ζ-potential variations as a function of the pH, INU-EDA-P,C-DOXO proved capable of undergoing charge reversal at acidic pH, changing its physicochemical and biological behavior. In vitro tests with cancer (MDA-MB 231) and normal (HB-2) breast cells were carried out to verify the conjugate aptitude to follow different routes to enter cells depending on the microenvironment. This finding was supported by quantitative up-take studies, which revealed that INU-EDA-P,C-DOXO released doxorubicin before entering cancer cells, as the entire copolymer diffused across normal cell membranes without relevant modifications.
1. Introduction
Up to now, the laparoscopic surgical removal of tumor mass represents the treatment of choice to efficaciously eradicate cancer.1 Even if several hundred patients take advantage of such a clinical routine, those affected by metastases are incurable and the others need to undergo invasive adjuvant treatments, with the aim of killing circulating neoplastic cells avoiding recidivisms.2 In the last decade, smart nanoscale systems for targeted drug delivery have received great attention as they are the most promising tools to overcome the limitations of conventional cancer chemotherapy, mainly due to the lack of drug accumulation in the tumor site and selectivity towards cancer cells as well as poor bioavailability.3,4 At least in principle, such systems can yield to specific drug release and accumulation into the tumor mass by means of the enhanced permeability and retention (EPR) effect5 combined with environment-sensitivity and active targeting mechanisms towards membrane molecules overexpressed by cancer cells (e.g., growth factors, peptides such as RGD or proteins). They are typically endowed with functional groups which make them sensitive to specific endogenous stimuli, such as lowered interstitial pH and a higher glutathione or enzymes concentration, bringing about massive and localized delivery of active molecules inside cancer cells.6 The higher amount of drug released inside cancer cells, rather than healthy tissues, ensure keeping the local therapeutic window into the tumor site though administrating lower dose. As consequence, toxic effects and resistance mechanisms can be significantly reduced, improving the therapeutic effect and patient compliance. Despite its exceptional promise, targeted therapy is still in its early stage, and novel selective tools are necessary to get the goal.Inulin is a natural, biocompatible, bioeliminable and biodegradable7 polysaccharide consisting of linear chains of β-(2-1) fructose units carrying a glucose unit as reducing end-chain. It is water soluble and exhibits high amount of hydroxyl functional groups available for common coupling reactions.8,9 Inulin derivatives have shown promising results in several in vitro and in vivo studies aimed for addressing drugs into a specific body district.9–11 However, they largely release drugs too slow and without responding to endogenous stimuli, implying that a very low dose of drug is available for metastatic cells eventually run away from cancer as well as a nonspecific drug release.
This paper report on the synthesis, physicochemical and biological characterization of an environment-sensitive inulin–doxorubicin conjugate which can release the linked anticancer drug in the cancer cell cytoplasm. This finding is related to the conjugate ability of releasing doxorubicin and reversing its net charge, from negative to positive, once the cancer site is reached, thus improving the preferential release of the drug at the site of action and throughout inside cells. We demonstrated that it is capable of self-assembling into organized nanosystems at low concentration, responding to variations of volume which may occur during the biodistribution at tissue and cellular level, thus protecting doxorubicin from cleavage and limiting drug release at lysosomal level in normal cells. The cytotoxicity of this polymer was systematically investigated using different cancer and physiological cell lines so as to asses any selective effect towards cancer cells instead of the controls. In addition, the cellular up-take and intracellular trafficking of the polymer was investigated on breast cancer cells as model of tumor to understand the intracellular fate of both the main polymer chain and doxorubicin once degraded by cytoplasm or organelles. Our findings shed the light on how “live” polymers, which cleverly respond to several biological stimuli (i.e., pH, volume, ionic strength and temperature changes) might be powerful, safe and chip solutions for selectively recognize and kill cancer cells minimizing by effects.
2. Results and discussion
2.1. Rational plane
Many research groups have attempted to design systems with outstanding and localized anticancer properties to avoid secondary effects onto healthy tissues. However, so far, those proved capable of reducing tumor mass in mouse model have not shown the same activity in humans, thus remarking that different and more complex approaches in terms of drug release mechanisms should be employed.12,13 This behavior might be related to retro diffusion of drugs ones quickly released inside cells, mainly assisted by the pump efflux of tumor cells, combined with a very low drug payload of the most realized systems (<5% w/w). Herein, a pH sensitive system, named INU-EDA-P,C-Doxo, was synthesized by firstly coupling pentynoic acid to an amine functionalized inulin derivative (INU-EDA) via amidic bond. In tandem, the intermediate was derivatized with citraconic anhydride to obtain pH-sensitive citraconate pendants, which were partially functionalized with doxorubicin affording to a random copolymer carrying the drug and both remaining free carboxyl and amine functions (Scheme 1). These side chains, including doxorubicin, were chosen considering that the main aim of this study was to obtain a drug–inulin conjugate combining pH-sensitivity, self-assembling ability and selective toxic effect towards cancer cells.INU-EDA is a polycation displaying good cytocompatibility but, as for others formerly studied polycations,9 probably unable to allow suitable bioavailability in vivo being accumulated into high flushed organs after intravenous administration. In this paper, we used citraconic anhydride as a multi-purpose reactant so as to introduce into the main chain free ionizabile carboxylic groups, whose ionization degree affect the physicochemical properties of the copolymer, and labile doxorubicin–polymer amidic bond which rapidly hydrolyze under acidic conditions (pH < 6.5) or in the presence of nucleophilies (amine, thiols, etc.).14 It might be expected that the free carboxylates make the system prevailingly anionic until the cancer tissue is reached (pH < 6.5), avoiding the typical toxicity profile of common polycations and ensuring that the drug payload is preferentially released into the site of action.
The self-assembling property might be expected to increase in the presence of hydrophobic moieties along the polymer chain.
Besides, the presence of moieties bearing Π bonds might provoke Π–Π structuring in water, yielding to either organized macromolecules or supramolecules. Doxorubicin itself and pentynoic pendants provided such interactions, leading to intramolecular and intermolecular structuring. Pentynoic acid was chosen taking as benchmark that known drugs bearing stable and isolated alkyne moiety, such as 17α-ethynylestradiol and linagliptin, have been shown to have no impact on either intestinal or hepatic CYP3A4 activity.15 We calculate that the volume of a late lysosome is about 105 lower than that interstitial or cytosolic one. One can deduce that amphiphiles which are present in the interstitial compartment at concentration below their CMC, after cell internalization may get to their CMC and self-assemble into nanosystem at lysosomal level. These properties led us to suppose, later on confirmed by the experiments, that the self-aggregation of the copolymer could occur inside lysosomes even at low pH thereby protecting doxorubicin from cleavage and acidic inactivation inside lysosomes.16 This mechanism was thought to discriminate between healthy and cancer cells on the basis of the status of the copolymer inside lysosomes. An unmodified copolymer should slowly release its drug payload owing to aggregation inside cells, while a partially hydrolyzed copolymer loss the self-assembling ability, being more hydrophilic and prevailingly cationic, thus keeping a proper drug dose at cytosolic level and then inside nuclei.
2.2. Synthesis and physicochemical characterization of INU-EDA-P,C-Doxo
Citraconic anhydride was then employed as heterobifunctional linker capable of easily reacting with EDA amine pendants forming acid-labile amide groups and in the other end a weakly acid carboxyl functional group (pKa > 4), made up to react with others amine functions. Under normal conditions the by-products produced in the first step of the reaction, namely isourea and the unreacted monomers, do not interfere in the nucleophilic reaction taking place between the residual amine groups of INU-EDA-Pentyne and citraconic anhydride. However, being water capable of reacting with citraconic anhydride leading to citraconic acid, a certain lowering effect on water pH was expected. Hence, this reaction was accomplished without previous purification, adding a solution of citraconic anhydride in anhydrous THF step-by-step, and in alkaline condition (8 < pH < 9) to keep the amine groups always deprotonated and available for the nucleophilic addition to citraconic anhydride. After purification by means of dialysis, the 1H NMR spectrum of the product allowed to confirm the proposed structure. Not surprisingly, the addition of INU-EDA amine function to citraconic anhydride was not regioselective, giving rise to both α- and β-methyl citraconylamide pendants. Comparing the integral of the peaks at δ 1.94 and at 1.98, relative to –OOCCH2CH3 and NHCOCH2CH3 pendants respectively, a simple relative quantification of the –OOCCH2CH3/NHCOCH2CH3 mol ratio was attained (67% vs. 33%), revealing that the primary amine preferentially react with the sterically unhindered center in a ratio equal to 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The mol% of pentynoic acid and citraconylamide were also estimated from the integrals of the peaks at δ 2.48, ascribable to the CH2CH2CCH (P), and at δ 1.94–1.98, relative to –OOCCH2CH3 and NHCOCH2CH3 (C), compared with those at δ 3.50–4.03 relative to the –CH2–OH; –CH–CH2–OH; –C–CH2–O– (INU) hydrogens, respectively. The derivation degree was found to be 4.1% for P repeating units (DDP%) and 16.1% for C repeating units (DDC%) on a molar basis.
1. The mol% of pentynoic acid and citraconylamide were also estimated from the integrals of the peaks at δ 2.48, ascribable to the CH2CH2CCH (P), and at δ 1.94–1.98, relative to –OOCCH2CH3 and NHCOCH2CH3 (C), compared with those at δ 3.50–4.03 relative to the –CH2–OH; –CH–CH2–OH; –C–CH2–O– (INU) hydrogens, respectively. The derivation degree was found to be 4.1% for P repeating units (DDP%) and 16.1% for C repeating units (DDC%) on a molar basis.
The INU-EDA-P,C intermediate had a large amount of carboxylates useful for doxorubicin conjugation. The reaction involved the daunosamine amine group of doxorubicin (Doxo) and these free carboxylates conveniently activated by a mixture of EDC and NHS as coupling agents, and employing large excess doxorubicin (moles of Doxo/moles of carboxyl pendant groups in INU-P,C = 2) to maximize the polymer functionalization. DMF was used as co-solvent to avoid precipitation of the polymer conjugate during its formation. Indeed, amide formation owing to the reaction between doxorubicin and inulin implies that very hydrophilic carboxylate groups were replaced with highly hydrophobic citraconylamide-Doxo pendants, affording to drastic changing in polymer solubility in aqueous solution.
INU-EDA-P,C-Doxo was characterized by 1H NMR spectroscopy, whose assignments were in full agreement with the proposed structure, but with qualifications (Fig. S1†). Whilst the daunosamine moiety was clearly shown from the peak at δ 1.25 attributable to the CH3CH2O hydrogens, together with the peak at 5.47 ascribable to the O–CH–O anomeric hydrogen, the doxorubicinone portion was quasi overwhelmed by the noise. The molar derivatization degree of doxorubicin (DDdoxo, 8.57%), that is the percentage of linked doxorubicin with respect to the repeating units of inulin, was calculated from the integrals of the peaks at δ 1.25 and 3.50–4.03 relative to the CH3CH2O (Doxo) and –CH2–OH; –CH–CH2–OH; –C–CH2–O– (INU) hydrogens respectively. It should be noticed that the amount of hydrophobic pendants, namely P and Doxo repeating units, was about 4.5 per macromolecule (about 12.4 mol% of the repeating units), which was sufficient for intramolecular or intermolecular self-assembling of the copolymer. Hence, the desolvation of the aromatic moiety can explain quite well this aberration in the 1H NMR spectrum, since such desolvated domains cannot be completely detected because of the Zeeman, dipolar and other effects extremely stretch out these signals almost under the threshold of detection.
The possibility that this result was biased by the hydrolysis of the doxorubicinone moiety was ruled out considering that the UV spectrum of the copolymer was nearly overlaid to that of equivalent amount of doxorubicin hydrochloride prepared in the same medium (PBS pH 7.4) (Fig. 1).
 | ||
| Fig. 1 UV spectra of INU-EDA-P,C (black), INU-EDA-P,C-Doxo (magenta) and doxorubicin hydrochloride (red). | ||
Fig. 1 clearly shows that the structure of doxorubicin pendants, characterized by n → π* band near 280 nm and a positive π → π* band near 480 nm, was preserved in INU-EDA-P,C-Doxo. In addition, the π → π* band near 480 nm seems red-shifted by ∼15 nm, confirming that doxorubicin was covalently bonded to the copolymer giving rise to strong Π–Π interactions. It is noteworthy that, the amount of doxorubicin in the copolymer evaluated spectrophotometrically endorsed that calculated by 1H NMR, which correspond to 18.45% w/w (expressed as doxorubicin hydrochloride weight equivalent).
Weight average molecular weights (Mw) and polydispersity of the synthesized copolymer and their intermediates where obtained by SEC analyses using a calibration curve of monodisperse PEG standards with Mw ranging from 0.32 to 400 kDa (Table 1).
| Sample | cMw (kDa) | cMw/Mn | Composition | Z-pot (mV) | |||
|---|---|---|---|---|---|---|---|
| aDDEDA (%) | aDDPentine (%) | aDDcitric. (%) | bDDDoxo (%) | ||||
| a Calculated by means of 1H NMR spectroscopy.b Calculate combining 1H NMR spectroscopy and UV spectrophotometry.c Obtained by SEC analysis in 0.1 M LiBr DMF solution. | |||||||
| INU-EDA | 4.0 | 1.87 | 19.0 | — | — | — | 18.8 ± 3.9 |
| INU-EDA-P,C | 4.3 | 1.70 | 19.0 | 4.1 | 16.1 | — | −24.8 ± 4.1 |
| INU-EDA-P,C-Doxo | 13.3 | 1.62 | 19.0 | 4.1 | 16.1 | 8.57 | −18.2 ± 7.0 |
In detail, although beyond our expectation, the relative molecular weight of the copolymer (Mw = 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300) displayed a remarkable increment with respect to the parent polymer (INU-EDA, Mw = 4000), proving the feasibility of the conjugation. The occurrence of supramolecular aggregation, ensuing from doxo–doxo and doxo–pentyne interactions, may explain the fictitious enormous increasing of the apparent molecular weight. Overall, further confirmation was obtained taking into account that this inconsistency was not observed for the intermediate INU-EDA-P,C (Mw = 4300), suggesting that a INU-EDA-P,C-Doxo dimer was revealed in SEC traces.
300) displayed a remarkable increment with respect to the parent polymer (INU-EDA, Mw = 4000), proving the feasibility of the conjugation. The occurrence of supramolecular aggregation, ensuing from doxo–doxo and doxo–pentyne interactions, may explain the fictitious enormous increasing of the apparent molecular weight. Overall, further confirmation was obtained taking into account that this inconsistency was not observed for the intermediate INU-EDA-P,C (Mw = 4300), suggesting that a INU-EDA-P,C-Doxo dimer was revealed in SEC traces.
 | ||
| Fig. 2 Evolution of the reduced viscosity of INU-EDA-P,C-Doxo (ηred) as function of its concentration: the critical micelle concentration (CMC) was marked with the arrow. | ||
The ability of the copolymer to lead to nano-sized aggregates was also assessed by SEM analysis. SEM micrograph (Fig. 3) shows that the copolymer assembled into homogeneous nanosystems with a quasi-spherical morphology and dimensions approximately of about 20 nm.
Being INU-EDA-P,C-Doxo prevailingly anionic and so unable to efficiently enters healthy cells, but designed to be charge reversible (from negative to positive) at the tumor site, with the aim of understanding if the citraconylamides cleavage was affected by the pH of the medium its ζ-potential was examined at different pH (Fig. 4). This investigation is motivated by the fact that after an intravenous administration, the usual body trafficking for a drug delivery system involves manifold environment variations, considering that the pH of the medium varies from 7.4 soon after administration (plasma and healthy tissues) to 6.4 in the tumor.
 | ||
| Fig. 4 ζ-potential plot of INU-EDA (solid symbol) and INU-EDA-P,C-Doxo (open symbol) as a function of the pH of the medium. | ||
For comparative purposes the same experiment was performed using INU-EDA as parent compound, whose ζ-potential value varied between 19 mV and 29 mV along the whole pH range considered (2 < pH < 7). For the INU-EDA-P,C-Doxo copolymer the observed behavior was more complicated. In particular, the ζ-potential increased from ≈−21 mV to −29 mV passing from pH 7 to pH 5.6, than, coherently decreased and reversed its charge at pH 3.9, reaching the same value of the parent INU-EDA. These results point out that in first instance citraconate-Doxo cleavage prevails over INU-EDA-citraconate one, thus increasing the amount of anionic charge. However, at pH below 5.6 this tendency flipped over and citraconylamides carrying carboxylate groups were fast cleaved providing charge inversion.
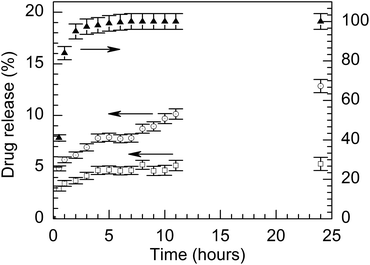 | ||
| Fig. 5 Release profile for INU-EDA-P,C-Doxo at 37 °C in PBS pH 5.5 (open circle) and PBS pH 7.4 (open square) in comparison with doxorubicin hydrochloride alone (solid symbol). | ||
The release experiments were also performed in human plasma to simulate the release after an intravenous administration (see Fig. S2†). Though an evident increment of doxorubicin release was appreciated by comparison with PBS buffer at pH 7.4, it was always lower than that observed at acidic pH. In particular, after 24 hours only 8% of the doxorubicin payload was released in plasma, providing a good circulating dose of free drug promptly available after drug administration.
2.3. Biological characterization
Preliminary cytotoxicity studies were carried out on a wide array of cancer (HCT 116, MDA-MB 231 and Sk-Hep-1) and normal (16HBE, HB-2) cell lines, so as to establish the ability of the system to preferentially kill tumor cells instead of the normal one (see ESI, Fig. S3 and S4†). Overall, this experiment showed an evident selective cytotoxicity of INU-EDA-P,C-Doxo towards cancer cells, pointing out that the system was potentially capable of focusing its toxic effect on cancer cells independently of the EPR effect.
For comparative purposes, based on the necessity to have a tumor and normal model system of the same tissue, only two cell lines were selected to further study this phenomenon from the biological standpoint. In particular, the INU-EDA-P,C-Doxo cytotoxicity was assessed on MDA-MB 231 and HB-2 cell lines, tumor and normal cells respectively, by CCK-8 assay: the absorbance values were used to metabolically quantify viable cells after the nanoparticles treatment. Fig. 6 shows the relative viability of MDA-MB 231 and HB-2 cells after 24 h of incubation in the presence of different amount of INU-EDA-P,C-Doxo or free doxorubicin (50; 25; 5; 0.5 μM) used as positive control. A dose-dependent reduction for both cell lines treated with INU-EDA-P,C-Doxo was observed, but with some outstanding difference. It might be noticed that the viability of HB-2 cells in the presence of INU-EDA-P,C-Doxo was higher than that of the others samples, even if compared with free doxorubicin treated cells. Differently, the tumor cells treated with INU-EDA-P,C-Doxo showed the lower viability following a non-obvious trend. At doxorubicin equivalent concentration lower than 25 μM the system was significantly less effective than the plain doxorubicin (75–90% vs. 45–65% cell viability respectively), whereas at higher concentration the system became about two times more effective, especially at 25 μM of doxorubicin equivalent concentration (28% vs. 42% cell viability). These results showed that the efficacy of the system was significantly higher than doxorubicin alone and somehow selective for the cancer cells, indicating that INU-EDA-P,C-Doxo preferentially target cancer cells in vitro as well.
 | ||
| Fig. 7 Quantitative uptake of INU-EDA-P,C-Doxo-FITC on MDA-MB231 (open symbol) and HB-2 (solid symbol): FITC (solid lines), Doxo (dashed lines). | ||
It is widely-know that the tumor microenvironment presents a lower pH (6.5) compared to those of normal tissues (7.4).21 This may selectively trigger a greater release of the drug from the pH-sensitive system. Perhaps doxorubicin was strongly cleaved in the tumor site to independently diffuse across cell membrane, thereby increasing the doxorubicin fluorescence inside cells. On the contrary, in the normal environment INU-EDA-P,C-Doxo-FITC was internalized without any substantial chemical modification thus leading to a parallel increasing of green and red fluorescence pathway. The enhanced uptake of free doxorubicin released in the cancer microenvironment is more evident in the graph shown in Fig. 8, in which the relative uptake is represented as a doxorubicin/FITC fluorescence ratio inside cells. According to this point of view, the uptake of the unmodified system should imply a constant Doxo/FITC ratio through time inside cells, being the fluorescence pendants covalently bonded to the main polymer backbone. In the light of this insight it is quite clear that a greater and faster release of the drug from the polymer occurred outside of tumor cells, which increases proportionally in the first 8 h of incubation (Fig. 8). After that, the fluorescence of internalized conjugate restored the normal Doxo/FITC ratio at around 3. While, with respect to the curve of the HB-2 cells, only a slight increase of the Doxo/FITC ratio was observed confirming that the system was internalized without releasing its drug payload.
3. Experimental section
3.1. Materials
Inulin-(2-aminoethyl)-carbamate (INU-EDA), used as starting copolymer, was synthesized as previously reported.9 Doxorubicin hydrochloride (DOXO-HCl), N-hydroxysuccinimide (NHS), 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide hydrochloride (EDC-HCl), citraconic anhydride, 4-pentynoic acid were purchased from Sigma Aldrich. All reagent were of analytic grade and used as received. SpectraPor dialysis tubing was purchased from Spectrum Laboratories, Inc. (Italy).Human venous plasma and uncoagulated blood were obtained from healthy voluntary donors and immediately used. These samples were collected from the first co-author with his informed consent.
1H NMR spectra were recorded using a Bruker Avance II 300 spectrometer operating at 300.12 MHz. Size exclusion chromatography (SEC) was carried out using a Phenomenex PolySep-GFC-P3000 column (California, USA) connected to a Water 2410 refractive index detector. A solution of 0.1 M LiBr in DMF was used as eluent at 50 °C with a flux of 0.6 mL min−1, and PEG standards (400–0.32 kDa, Polymer Laboratories Inc., USA) were used to set up calibration curve (R2 = 0.9958).
Human breast cancer MDA-MB 231 cells were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% v/v Fetal Bovine Serum (FBS), 2 mM L-glutamine, 100 units per mL penicillin G, 100 μg mL−1 streptomycin at 37 °C and 5% CO2. Human mammary epithelial HB-2 cells were cultured in Low-glucose Dulbecco's modified Eagle's medium (LG-DMEM), supplemented with 10% fetal bovine serum (FBS), 2 mM L-glutamine, 100 units per mL penicillin G, 100 μg mL−1 streptomycin, hydrocortisone (5 μg mL−1) and Bovine Insulin (10 μg mL−1) (Sigma Aldrich), at 37 °C and 5% CO2.
3.2. Synthesis of the polymer conjugate
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (7 mL). The pH of the reaction was adjusted to 6.5–6.8 with 0.1 M NaOH and the reaction kept under these conditions for 15 h at room temperature (Min. 25 °C, Max. 30 °C). Finally, the reaction was dialyzed through a membrane tubing with MWCO 1 kDa and the pure product retrieved as a deep red powder. Yield 76 mg. 1H NMR 400 MHz, D2O: δ 1.25 (br, 3Hdoxo, CH3CH2O sugar ring), 1.90–2.15 (br, 3Hcitraconate, –OOCCH2CH3 and NHCOCH2CH3), 2.48 (br, 4Halkyne, CH2CH2CCH), 3.00–3.50 (m, 4HEDA, NCH2CH2N), 3.50–4.50 (7 HINU, –CH2–OH; –CH–CH2–OH; –C–CH2–O–; –C–CH–OH; –CH–OH), 5.47 (1Hdoxo, O–CH–O anomeric sugar ring), 5.52 (s, 1Hcitraconate, CHCOO−), 5.83 (s, 1Hcitraconate, CHCONH), 8.48 (br, Hdoxo, aromatic ring).
4 (7 mL). The pH of the reaction was adjusted to 6.5–6.8 with 0.1 M NaOH and the reaction kept under these conditions for 15 h at room temperature (Min. 25 °C, Max. 30 °C). Finally, the reaction was dialyzed through a membrane tubing with MWCO 1 kDa and the pure product retrieved as a deep red powder. Yield 76 mg. 1H NMR 400 MHz, D2O: δ 1.25 (br, 3Hdoxo, CH3CH2O sugar ring), 1.90–2.15 (br, 3Hcitraconate, –OOCCH2CH3 and NHCOCH2CH3), 2.48 (br, 4Halkyne, CH2CH2CCH), 3.00–3.50 (m, 4HEDA, NCH2CH2N), 3.50–4.50 (7 HINU, –CH2–OH; –CH–CH2–OH; –C–CH2–O–; –C–CH–OH; –CH–OH), 5.47 (1Hdoxo, O–CH–O anomeric sugar ring), 5.52 (s, 1Hcitraconate, CHCOO−), 5.83 (s, 1Hcitraconate, CHCONH), 8.48 (br, Hdoxo, aromatic ring).3.3. Chemical and physicochemical characterization of the nanosystem
The amount of doxorubicin linked in the INU-EDA-P,C-Doxo was determined spectrophotometrically measuring the absorbance of the sample at 480 nm. A calibration curve was obtained for serially diluted concentrations of doxorubicin hydrochloride in 0.1 M phosphate buffer at pH 7.4 (R2 = 0.999). For comparative purposes, the content of drug loaded into the system was expressed as the amount of loaded doxorubicin hydrochloride per unit mass of polymer, and resulted to be 18.45 ± 0.6% (w/w).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 68 as mobile phase (flow 0.8 mL min−1). Then, the cumulative release was determined as a function of incubation time. A parallel analysis was carried out using the same procedure above described but employing PBS at pH 5.5 as medium. All release data were compared with the diffusion profile of doxorubicin hydrochloride alone (0.5 mg) obtained by using the same procedure. Data were corrected taking in account the dilution procedure. Each experiment was carried out in triplicate and the results were in agreement within ±5% standard error.
68 as mobile phase (flow 0.8 mL min−1). Then, the cumulative release was determined as a function of incubation time. A parallel analysis was carried out using the same procedure above described but employing PBS at pH 5.5 as medium. All release data were compared with the diffusion profile of doxorubicin hydrochloride alone (0.5 mg) obtained by using the same procedure. Data were corrected taking in account the dilution procedure. Each experiment was carried out in triplicate and the results were in agreement within ±5% standard error.3.4. Biological characterization of the nanosystems
After 24 h from seeding, the cells were incubated for 24 h at different concentrations (final doxorubicin concentration: 50; 25; 5; 0.5 μM) of INU-EDA-P,C-Doxo. The cells were treated also with the same concentrations of free doxorubicin as positive control; whereas the untreated cells were used as negative control. Cell viability was evaluated using Cell Counting Kit-8 (CCK-8) (Sigma-Aldrich). The water-soluble tetrazolium salt (WST-8) was added to each well (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 dilution in complete medium) followed by incubation for 2 h at 37 °C. The mitochondrial dehydrogenases of the living cells, can reduce the WST-8 into soluble fromazan dye, therefore, the amount of formazan is directly proportional to the number of living cells. The absorbance at 460 nm was determinated by a microplate reader DU-730 Life Science spectrophotometer (Beckman Coulter). The percentage of cell viability was calculated as ratio between each sample with respect to the negative control (100% of cell viability).
10 dilution in complete medium) followed by incubation for 2 h at 37 °C. The mitochondrial dehydrogenases of the living cells, can reduce the WST-8 into soluble fromazan dye, therefore, the amount of formazan is directly proportional to the number of living cells. The absorbance at 460 nm was determinated by a microplate reader DU-730 Life Science spectrophotometer (Beckman Coulter). The percentage of cell viability was calculated as ratio between each sample with respect to the negative control (100% of cell viability).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000 in PBS) for 30 minutes, at room temperature. The position of the copolymer was monitored by fluorescence microscopy analysis (Leica). Untreated cells served as a negative control for background fluorescence.
1000 in PBS) for 30 minutes, at room temperature. The position of the copolymer was monitored by fluorescence microscopy analysis (Leica). Untreated cells served as a negative control for background fluorescence.4. Conclusions
In this paper, the conjugation of inulin with doxorubicin and pentynoic acid to give an environment-sensitive polymeric amphiphile, named INU-EDA-P,C-Doxo, is reported. This copolymer was designed to be capable of self-assembling into nanosystems following volume variations during its intracellular trafficking, thus leading to controlled intracellular drug release. Doxorubicin and pentynoic pendants were introduced into the inulin backbone to confer it upon proper hydrophilic/lipophilic balance and Π–Π interactions, which were proven suitable to lead to self-aggregation varying the copolymer concentration. Doxorubicin was linked to inulin using citraconylamide spacer, which imparted pH sensitivity useful for discriminating cancer and normal microenvironments so as to selectively release doxorubicin in the site of action and buffering the known doxorubicin side effects. This polymer conjugate, besides carrying high dose of doxorubicin, is prevailingly anionic and so potentially free to circulate in blood vessel without interacting with plasmatic proteins and reticulo-endothelial system until the cancer site is reached. We demonstrate that in the cancer microenvironment (5.5 < pH < 6.4) INU-EDA-P,C-Doxo may reverse its net charge, from negative to positive, releasing free doxorubicin and, consequently, changing its conformation and biological behavior. These multiple release mechanisms reflect on the cytotoxic effect, which was preferentially expressed in cancer cells instead of the healthy cells, displaying in the meantime a local absolute cytotoxicity higher then doxorubicin alone. This work represents a proof of concept pilot insight which will pave the way for future in vivo studies on such “live polymers”.Notes and references
- M. Inomata, T. Akagi, H. Katayama, A. Kimura, J. Mizusawa, T. Etoh, S. Yamaguchi, M. Ito, Y. Kinugasa, Y. Saida, H. Hasegawa, M. Ota, Y. Kanemitsu, Y. Shimada and S. Kitano, Jpn. J. Clin. Oncol., 2014, 44, 1123–1126 CrossRef PubMed.
- S. Sakuramoto, M. Sasako, T. Yamaguchi, T. Kinoshita, M. Fujii, A. Nashimoto, H. Furukawa, T. Nakajima, Y. Ohashi, H. Imamura, M. Higashino, Y. Yamamura, A. Kurita and K. Arai, N. Engl. J. Med., 2007, 357, 1810–1820 CrossRef CAS PubMed.
- D. E. Gerber, Am. Fam. Physician, 2008, 77, 311–319 Search PubMed.
- S. M. Lee and S. B. T. Nguyen, Macromolecules, 2013, 46, 9169–9180 CrossRef CAS.
- H. Maeda, T. Sawa and T. Konno, J. Controlled Release, 2001, 74, 47–61 CrossRef CAS.
- S. Mura, J. Nicolas and P. Couvreur, Nat. Mater., 2013, 12, 991–1003 CrossRef CAS PubMed.
- M. van der Zee, J. H. Stoutjesdijk, P. A. A. W. van der Heijden and D. J. de Wit, J. Environ. Polym. Degrad., 1995, 3, 235–242 CrossRef CAS.
- S. Kolida and G. R. Gibson, J. Nutr., 2007, 137, 2503S–2506S CAS.
- M. Licciardi, C. Scialabba, C. Sardo, G. Cavallaro and G. Giammona, J. Mater. Chem., 2014, 2, 4262–4271 RSC.
- D. Mandracchia, G. Tripodo, A. Latrofa and R. Dorati, Carbohydr. Polym., 2014, 103, 46–54 CrossRef CAS PubMed.
- C. Scialabba, M. Licciardi, N. Mauro, F. Rocco, M. Ceruti and G. Giammona, Eur. J. Pharm. Biopharm., 2014, 88, 695–705 CrossRef CAS PubMed.
- L. W. Seymour, K. Ulbrich, P. S. Steyger, M. Brereton, V. Subr, J. Strohalm and R. Duncan, Br. J. Cancer, 1994, 70, 636–641 CrossRef CAS.
- K. L. Kiick, Science, 2007, 317, 1182–1183 CrossRef CAS PubMed.
- H. B. Dixon and R. N. Perham, Biochem. J., 1968, 109(2), 312–314 CAS.
- D. J. Belle, J. T. Callaghan, J. C. Gorski, J. F. Maja, O. Mousa, S. A. Wrighton and S. D. Hall, Br. J. Clin. Pharmacol., 2002, 53, 67–74 CrossRef CAS.
- F. J. Verbaan, C. Oussoren, I. M. van Dam, Y. Takakura, M. Hashida, D. J. Crommelin, W. E. Hennink and G. Storm, Int. J. Pharm., 2001, 214, 99–101 CrossRef CAS.
- K. N. Mehrotra and M. Anis, Monatshefte für Chemie, 1995, 126, 637–645 CrossRef CAS.
- D. Wolff, S. Czapla, A. G. Heyer, S. Radosta, P. Mischnick and J. Springer, Polymer, 2000, 41, 8009–8016 CrossRef CAS.
- S. Kitamura, T. Hirano, K. Takeo, M. Mimura, K. Kajiwara, B. T. Stokke and T. Harada, Int. J. Biol. Macromol., 1994, 16, 313–317 CrossRef CAS.
- A. Dan, S. Ghosh and S. P. Moulik, Biopolymers, 2009, 91, 687–699 CrossRef CAS PubMed.
- L. E. Gerweck and K. Seetharaman, Cancer Res., 1996, 55(6), 1194–1198 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra00287g |
| This journal is © The Royal Society of Chemistry 2015 |





