A supramolecular hydrogel self-assembled from pentafluorobenzyl-dipeptide†
Abstract
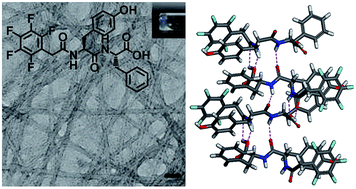
We report a new aromatic-capped peptide amphiphile which is able to form a supramolecular hydrogel under neutral pH. This hydrogel can also be obtained by enzymatic transformation from the hydrogelator precursor. The newly discovered hydrogel has excellent biocompatibility for four different cell lines, thus making it a potentially useful scaffolding material for biomedical applications.

 Please wait while we load your content...
Please wait while we load your content...