Multichannel detection of Cu2+ based on a rhodamine–ethynylferrocene conjugate†
Abstract
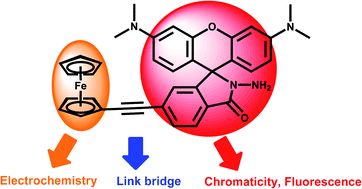
A novel multichannel chemosensor DR3 juxtaposed with a rhodamine chromophore and the electrochemical characterization of an ethynylferrocene group was developed. This chemosensor could selectively recognized Cu2+ in the presence of other competing ions in a wide pH range, which exhibits multiple responses for UV/vis absorption, fluorescence emission, and electrochemical parameters.

 Please wait while we load your content...
Please wait while we load your content...