DOI:
10.1039/C5RA00118H
(Communication)
RSC Adv., 2015,
5, 34769-34776
Reversible chain transfer catalyzed polymerization (RTCP) in nitrogen-based solvents without additional catalysts
Received
4th January 2015
, Accepted 9th April 2015
First published on 9th April 2015
Abstract
Nitrogen-based solvents (N,N-dimethylformamide (DMF) or N-methyl-2-pyrrolidone (NMP)) were used for reversible chain transfer catalyzed polymerizations (RTCPs) without additional catalysts. The polymerizations of typical monomers (i.e. methyl methacrylate, styrene, acrylonitrile, glycidyl methacrylate) showed features of RTCP, and well-defined polymers with designable molecular weights and narrow molecular weight distributions were obtained. The frequent activation of Polymer-I as a deactivator leads to narrow molecular weight distributions. Importantly, RTCPs using nitrogen-based solvents as novel catalysts not only could be conducted in the presence of a limited amount of air but also would be applicable as one of the most robust and powerful techniques for polymer synthesis.
Introduction
Over the past 30 years, reversible-deactivation radical polymerization (RDRP)1 methods were developed and allow the preparation of a multitude of previously unattainable well-defined polymeric materials. The basic concept behind the various RDRP procedures is the reversible activation of a dormant species to form a propagating radical.2 A dynamic and rapid equilibrium between the dormant and the active species not only minimizes the probability of bimolecular radical termination reactions but also provides an equal opportunity for propagation of all polymer (or dormant) chains. The most widely used RDRP methods are atom transfer radical polymerization (ATRP),3 stable free radical (most often nitroxide-mediated) polymerization,4 and degenerative transfer polymerization (DT)5 such as reversible addition-fragmentation chain transfer (RAFT) polymerization,6 reversible chain transfer catalyzed polymerization (RTCP)7 and tellurium, stibine, bismuth compounds-mediated polymerization.8
Recently, Goto, Tsujii and Fukuda et al. reported a novel class of RDRP strategy using a tin (Sn),7,9 germanium (Ge),7,9 phosphorus (P),7,10 nitrogen (N)11 or oxygen (O)12 and carbon (C)12 compound as a catalyst for the reversible activation and have named this kind of polymerization as reversible chain transfer catalyzed polymerization (RTCP)7 for its new reversible activation mechanism. RTCP (Scheme 1) includes a conventional radical initiator, an alkyl iodide as a dormant species (I), and a catalyst such as GeI4 (ref. 7) as a deactivator (A–I). In these systems, the conventional radical initiator gives Polymer˙. It reacts with GeI4 (A–I), in situ producing the Ge-centered catalyst radical (GeI˙3). GeI˙3 works as an activator (A˙) of Polymer-I, producing Polymer˙ and GeI˙3 again. This reversible process allows a frequent activation of Polymer-I, attaining low molecular weight distributions.13 Mechanistically, this process is a reversible chain transfer (RT) of Polymer˙ with GeI4, and GeI4 works like a catalyst, and thus they termed the related polymerization RTCP and GeI4 was called catalyst.
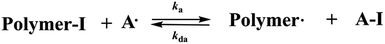 |
| | Scheme 1 The mechanism of reversible chain transfer catalyzed polymerization (RTCP). | |
In RTCP, catalysts (A) as requisites, (1) the activator radical A˙ should undergo no or little initiation (addition to monomer) but still be active enough to abstract iodine from Polymer-I and (2) A˙ should be relatively stable and easily be formed.7 However, these systems provide a high cost RTCP catalysts using tin (Sn), germanium (Ge), phosphorus (P), nitrogen (N) or oxygen (O) and carbon (C) compound. And most of these reactions were carried out in bulk monomer. Recently, a novel class of RDRP using amines as organic catalysts was also developed by Atsushi Goto et al., which was named as reversible complexation mediated polymerization (RCMP).14 The catalysts include such simple amines as triethylamine and tetramethylethylenediamine (TMEDA), which abstracts an iodine from Polymer-I to generate Polymer˙ and a complex of the iodine radical and amine. The molecular weights and its distribution were well controlled in the polymerizations of some traditional monomers. Based on this, the cheapness, good environmental safety, and ease of handling nitrogen-based solvents could also form a complex with the iodine molecule. Herein, we present results of RTCP of methyl methacrylate (MMA) in traditional nitrogen-based solvents (DMF and NMP) without any additional catalysts. It is important that the RTCP in nitrogen-based solvents can be carried out in the presence of a limited amount of air but still with a “living” fashion, which simplified the polymerization process and thereafter facilitated RTCP to be highly attractive for practical applications.
Experimental section
Materials
Methyl methacrylate (MMA) (>99%), styrene (St), acrylonitrile (AN), glycidyl methacrylate (GMA), vinyl acetate (VAc), pyridine, triethylamine, ethylenediamine, 1-aminobutane, and ammonium hydroxide were purchased from Shanghai Chemical Reagents Co. Ltd (Shanghai, China). The monomers were purified by passing through a column filled with neutral alumina before use. Azobis(isobutyronitrile) (AIBN, 98%, Sigma-Aldrich Co. Ltd.) and dicumyl peroxide (DCP, 98%, Sigma-Aldrich Co. Ltd.) were purified by recrystallization from methanol. Solvents such as N,N-dimethylformamide (DMF, >98%), N,N-dimethylacetamide, (DMA, >98%), N-methyl-2-pyrrolidone (NMP, >98%), tetrahydrofuran (THF) (>98%), toluene (>98%), and methanol (>98%) were purchased from Shanghai Chemical Reagents Co. and purify as standard methods. 2-Iodo-2-methylpropionitrile (CPI) was synthesize as the literature.15 All other chemicals were also obtained from Shanghai Chemical Reagents Co. Ltd and used as received unless mentioned.
General procedure for RTCP of MMA
A typical solvent polymerization procedure with the molar ratio of [MMA]0/[CPI]0/[AIBN]0 = 200/1/1 in the presence/absence of a limited amount of air is as follows. A mixture was obtained by adding AIBN (16.5 mg, 0.094 mmol), and CPI (7.4 mg, 0.094 mmol) MMA (2.0 mL, 18.8 mmol), and solvent of DMF (1.0 mL) to a dried ampoule with a stir bar. For the polymerization in the absence of air, the mixture was thoroughly bubbled with argon for 20 min to eliminate the dissolved oxygen, and then flame-sealed. For the polymerization in the presence of limited amounts of air, the ampoule was flame-sealed directly (no bubbling with inert gas). [O2]0 = 1.7 × 10−2 mol L−1 was calculated from the residual volume (air volume, 5.3 mL) of ampoule after adding the reaction mixture (3.0 mL) and ignoring the amount of oxygen dissolved in the liquids. The sealed ampoule was transferred into an oil bath held by a thermostat at the desired temperature (60 °C) to polymerize under stirring. After the desired polymerization time, the ampoule was cooled by immersing into iced water. Afterwards, the ampule was opened, and the contents were dissolved in THF (∼2.0 mL). The resulting solution was precipitated into a large amount of methanol (∼200 mL) with stirring. The polymer obtained by filtration was dried under vacuum until constant weight at 30 °C. The monomer conversion was determined gravimetrically.
Typical procedures for chain extension using PMMA as macroinitiator
The PMMA sample (Mn,SEC = 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 g mol−1, Mw/Mn = 1.21) obtained with the molar ratio of [MMA]0/[CPI]0/[AIBN]0 = 200/1/1 in the presence of limited amount air with DMF as solvent was used as the macroinitiator for the chain extension reaction at the molar ratio of [MMA]0/[PMMA]0(/[AIBN]0) = 400/1(/1). The polymerization procedure is as follows: PMMA (277 mg, 0.0235 mmol) was dissolved in 1.0 mL (0.94 g, 9.4 mmol) of fresh MMA, and then the predetermined quantity of AIBN (4.2 mg, 0.0235 mmol) if added, and DMF (1.0 mL) were added. The rest of the procedure was the same as that described above. The chain-extension reaction was carried out under stirring at 60 °C.
800 g mol−1, Mw/Mn = 1.21) obtained with the molar ratio of [MMA]0/[CPI]0/[AIBN]0 = 200/1/1 in the presence of limited amount air with DMF as solvent was used as the macroinitiator for the chain extension reaction at the molar ratio of [MMA]0/[PMMA]0(/[AIBN]0) = 400/1(/1). The polymerization procedure is as follows: PMMA (277 mg, 0.0235 mmol) was dissolved in 1.0 mL (0.94 g, 9.4 mmol) of fresh MMA, and then the predetermined quantity of AIBN (4.2 mg, 0.0235 mmol) if added, and DMF (1.0 mL) were added. The rest of the procedure was the same as that described above. The chain-extension reaction was carried out under stirring at 60 °C.
Characterization
The number-average molecular weight (Mn,SEC) and molecular weight distribution (Mw/Mn) values of the resulting polymers were determined using a Waters 1515 gel permeation chromatograph (SEC) equipped with a refractive-index detector (Waters 2414), using HR 1 (pore size: 100 Å, 100–5000 Da), HR 2 (pore size: 500 Å, 500–20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da) and HR 4 (pore size 10
000 Da) and HR 4 (pore size 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Å, 50–100
000 Å, 50–100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da) columns (7.8 × 300 mm, 5 μm beads size) with measurable molecular weights ranging from 102 to 5 × 105 g mol−1. THF was used as the eluent at a flow rate of 1.0 mL min−1 and 30 °C. SEC samples were injected using a Waters 717 plus autosampler and calibrated with poly(methyl methacrylate) standards purchased from Waters. The UV-Vis spectra were recorded on a Shimadzu UV3150 spectrophotometer with a quartz UV-Vis cell (1 mm path length). 1H NMR spectrum of the obtained polymer was recorded on an INOVA 400 MHz nuclear magnetic resonance instrument using CDCl3 as the solvent and tetramethylsilane (TMS) as an internal standard.
000 Da) columns (7.8 × 300 mm, 5 μm beads size) with measurable molecular weights ranging from 102 to 5 × 105 g mol−1. THF was used as the eluent at a flow rate of 1.0 mL min−1 and 30 °C. SEC samples were injected using a Waters 717 plus autosampler and calibrated with poly(methyl methacrylate) standards purchased from Waters. The UV-Vis spectra were recorded on a Shimadzu UV3150 spectrophotometer with a quartz UV-Vis cell (1 mm path length). 1H NMR spectrum of the obtained polymer was recorded on an INOVA 400 MHz nuclear magnetic resonance instrument using CDCl3 as the solvent and tetramethylsilane (TMS) as an internal standard.
Results and discussion
Effect of solvents and CPI on RTCP of MMA
Table 1 shows the effect of different nitrogen-based solvents on RTCP using CPI as the alkyl halide initiator and AIBN as the conventional radical initiator, respectively. From entry 1 in Table 1, it can be seen that about 94.5% of conversion was obtained after 3.0 hours without additional any solvent. The Mn,SEC value was close to its corresponding theoretical one but Mw/Mn value of the obtained polymers was as broad as 1.43, indicating that the controllability is limited due to a low exchange frequency of iodine. From entry 2 to 5 in Table 1, the conversion (84.6%, 85.5%, 90.2%, 82.9%) was obtained when DMF, DMA, NMP and toluene as solvent (V[monomer]0/V[solvent]0 = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was added into the reaction system, respectively. It can also be seen that the Mn,SEC values were close to their corresponding theoretical ones and Mw/Mn values of the obtained polymers were lower (≤1.15) in nitrogen-based solvents (DMF, DMA and NMP) than that with bulk (1.43) or toluene (1.49) as solvent, indicating a well-controlled polymerization process with nitrogen-based solvents. Nitrogen-based solvents can act as catalyst for RTCP of MMA. As shown in entries 6–8 in Table 1, the conversion of MMA increase from 40.1%, 44.5% to 49.7% after 3.0 h when DMF decrease from 0.5 mL, 0.2 mL to 0.1 mL, respectively; while Mw/Mn values are slightly higher. As shown in entries 9 to 10 in Table 1, the conversion of MMA decreased with the ratio of [MMA]0/[AIBN]0 from 200/0.5 to 200/0.2, indicating that AIBN can influence the polymerization rate markedly.
1) was added into the reaction system, respectively. It can also be seen that the Mn,SEC values were close to their corresponding theoretical ones and Mw/Mn values of the obtained polymers were lower (≤1.15) in nitrogen-based solvents (DMF, DMA and NMP) than that with bulk (1.43) or toluene (1.49) as solvent, indicating a well-controlled polymerization process with nitrogen-based solvents. Nitrogen-based solvents can act as catalyst for RTCP of MMA. As shown in entries 6–8 in Table 1, the conversion of MMA increase from 40.1%, 44.5% to 49.7% after 3.0 h when DMF decrease from 0.5 mL, 0.2 mL to 0.1 mL, respectively; while Mw/Mn values are slightly higher. As shown in entries 9 to 10 in Table 1, the conversion of MMA decreased with the ratio of [MMA]0/[AIBN]0 from 200/0.5 to 200/0.2, indicating that AIBN can influence the polymerization rate markedly.
Table 1 Effect of solvents on RTCP of MMA
| Entry |
Time [h] |
Solvent |
Conv. [%] |
Mn,th [g mol−1] |
Mn,SEC [g mol−1] |
Mw/Mn |
Polymerization conditions: [MMA]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [CPI]0 [CPI]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [AIBN]0: = 200 [AIBN]0: = 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Polymerization conditions: [MMA]0 1. Polymerization conditions: [MMA]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [CPI]0 [CPI]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [AIBN]0: = 200 [AIBN]0: = 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5. Polymerization conditions: [MMA]0 0.5. Polymerization conditions: [MMA]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [CPI]0 [CPI]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [AIBN]0: = 200 [AIBN]0: = 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2; MMA = 2.0 mL, temperature = 60 °C. Mn,th = ([MMA]0/[CPI]0) × MMMA × conversion + MCPI, where MMMA and MCPI represent the molecular weights of MMA and CPI, respectively.7 0.2; MMA = 2.0 mL, temperature = 60 °C. Mn,th = ([MMA]0/[CPI]0) × MMMA × conversion + MCPI, where MMMA and MCPI represent the molecular weights of MMA and CPI, respectively.7 |
| 1a |
3.0 |
Bulk |
94.5 |
19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
1.43 |
| 2a |
3.0 |
DMF (1.0 mL) |
84.6 |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
1.13 |
| 3a |
3.0 |
DMA (1.0 mL) |
85.5 |
17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
1.10 |
| 4a |
3.0 |
NMP (1.0 mL) |
90.2 |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
1.08 |
| 5a |
5.0 |
Toluene (1.0 mL) |
82.9 |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
1.49 |
| 6a |
0.5 |
DMF (0.5 mL) |
40.1 |
8000 |
9100 |
1.12 |
| 7a |
0.5 |
DMF (0.2 mL) |
44.5 |
8900 |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
1.23 |
| 8a |
0.5 |
DMF (0.1 mL) |
49.7 |
9900 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
1.26 |
| 9b |
3.0 |
DMF (1.0 mL) |
49.5 |
9900 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.17 |
| 10c |
17.0 |
DMF (1.0 mL) |
0 |
— |
— |
— |
Plausible mechanism of RTCP using nitrogen-based solvents
To explore the plausible mechanism, the UV-vis spectra of CPI–DMF solution was shown in Fig. 1. There is a new absorption peak appears at 362 nm over time. It is believed that the appearance of this new peak was attributed to the formation of a complex between CPI and DMF. To determine the formation of the complex, we heated a series of CDCl3 solution with CPI, DMF or CPI–DMF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 equivalent) in NMR tubes under argon atmosphere at 60 °C for 10 min. Fig. 2 shows the 1H NMR spectrum of (1) CPI, (2) DMF and (3) CPI + DMF in CDCl3. As shown in (3), the signals of protons deriving from –CH3 (a′) and –CHO (b′) of DMF shifted to low field, affording a solid evidence that the amine was combined with an electron-withdrawing group (I). In the classic literatures,16 n–σ types of charge-transfer complexes, involving iodine and amine as acceptor and donor, respectively, have been most extensively studied. The crystal structure of these complexes has been determined by X-ray methods. The intermolecular bond is localized, and the nitrogen–iodine arrangement is linear. Recently, we reported the RTCP system with triphenylphosphine as catalyst, in which triphenylphosphine-I2 produced in situ acts as deactivator.15 Therefore, the mechanism of MMA/CPI/AIBN in nitrogen-based solvents is suggested as RTCP, where amine-I2 produced in situ acts as deactivator. The Polymer˙, which is originally initiated by AIBN, is supposed to react with amine-I2, in situ producing the activator radicals (amine-I˙) and Polymer-I. The amine-I˙ could capture I from CP-I (or Polymer-I) effectively to produce CP˙ (or Polymer˙), and the circulation between activation and deactivation will be started (the mechanism was shown in Scheme 2).
1 equivalent) in NMR tubes under argon atmosphere at 60 °C for 10 min. Fig. 2 shows the 1H NMR spectrum of (1) CPI, (2) DMF and (3) CPI + DMF in CDCl3. As shown in (3), the signals of protons deriving from –CH3 (a′) and –CHO (b′) of DMF shifted to low field, affording a solid evidence that the amine was combined with an electron-withdrawing group (I). In the classic literatures,16 n–σ types of charge-transfer complexes, involving iodine and amine as acceptor and donor, respectively, have been most extensively studied. The crystal structure of these complexes has been determined by X-ray methods. The intermolecular bond is localized, and the nitrogen–iodine arrangement is linear. Recently, we reported the RTCP system with triphenylphosphine as catalyst, in which triphenylphosphine-I2 produced in situ acts as deactivator.15 Therefore, the mechanism of MMA/CPI/AIBN in nitrogen-based solvents is suggested as RTCP, where amine-I2 produced in situ acts as deactivator. The Polymer˙, which is originally initiated by AIBN, is supposed to react with amine-I2, in situ producing the activator radicals (amine-I˙) and Polymer-I. The amine-I˙ could capture I from CP-I (or Polymer-I) effectively to produce CP˙ (or Polymer˙), and the circulation between activation and deactivation will be started (the mechanism was shown in Scheme 2).
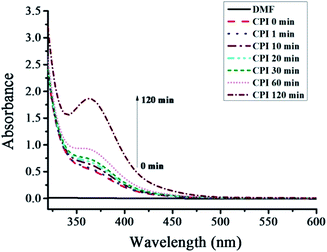 |
| | Fig. 1 UV-vis spectra of CPI (1 × 10−4 mol L−1) in DMF. | |
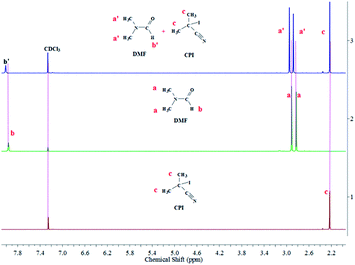 |
| | Fig. 2 1H NMR spectra of CPI (1), DMF (2) and DMF + CPI (3) in CDCl3, temperature = 60 °C. | |
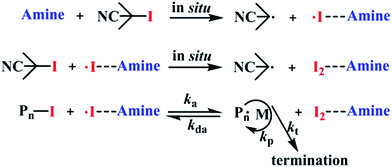 |
| | Scheme 2 The mechanism of RTCP in amine-based solvents. | |
Polymerization kinetics for RTCP of MMA in the different solvents
In order to further investigate the controllability of polymerization in nitrogen-based solvents, the kinetics was studied in the bulk, DMF and NMP using AIBN as the initiator, CPI as the alkyl halide initiator. Fig. 3 shows the kinetics of bulk and solution polymerization of MMA with a molar ratio of [MMA]0/[CPI]0/[AIBN]0 = 200/1/1, and a volume ratio of [MMA]0/[solvent]0 = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. All the kinetics showed linear plots, indicating that the polymerizations were approximately first order with respect to the monomer concentration, and that the number of active species remained constant during the polymerization process. However, the polymerization rates for the solution and bulk RTCPs were faster than that in solution of DMF and NMP. By calculating the apparent rate constant of polymerization, kappp (Rp = −d[M]/dt = kp[P˙n][M] = kappp[M]), as determined from the kinetic slops, a kappp of 2.74 × 10−4 s−1 for the bulk polymerization and 2.22 × 10−4 s−1 for the solution polymerization in DMF and kappp of 1.75 × 10−4 s−1 in NMP were obtained. The kappp of the polymerization in bulk was 1.23 and 1.57 times as that in DMF and NMP, respectively. Fig. 4 shows the evolution of the number-average molecular weight (Mn,SEC) values of PMMA and molecular weight distribution (Mw/Mn) values on the conversion for the bulk and solution RTCPs of MMA at 60 °C. As shown in Fig. 4, the Mn,SEC values of the polymers in DMF or NMP increased linearly with monomer conversion while keeping low Mw/Mn values (Mw/Mn = 1.11–1.25). Meanwhile, the experimental molecular weights agreed well with the corresponding theoretical ones. However, the Mn,SEC values of the polymers obtained in bulk (iodide transfer radical polymerization, ITP17) were obviously higher than the corresponding theoretical ones with broader Mw/Mn values (Mw/Mn = 1.30–1.40), which indicated a relatively lower initiation efficiency than that in nitrogen-based solvents (DMF or NMP). These results indicated that the solution RTCPs of MMA in the presence of DMF or NMP was a better RDRPs process.
1. All the kinetics showed linear plots, indicating that the polymerizations were approximately first order with respect to the monomer concentration, and that the number of active species remained constant during the polymerization process. However, the polymerization rates for the solution and bulk RTCPs were faster than that in solution of DMF and NMP. By calculating the apparent rate constant of polymerization, kappp (Rp = −d[M]/dt = kp[P˙n][M] = kappp[M]), as determined from the kinetic slops, a kappp of 2.74 × 10−4 s−1 for the bulk polymerization and 2.22 × 10−4 s−1 for the solution polymerization in DMF and kappp of 1.75 × 10−4 s−1 in NMP were obtained. The kappp of the polymerization in bulk was 1.23 and 1.57 times as that in DMF and NMP, respectively. Fig. 4 shows the evolution of the number-average molecular weight (Mn,SEC) values of PMMA and molecular weight distribution (Mw/Mn) values on the conversion for the bulk and solution RTCPs of MMA at 60 °C. As shown in Fig. 4, the Mn,SEC values of the polymers in DMF or NMP increased linearly with monomer conversion while keeping low Mw/Mn values (Mw/Mn = 1.11–1.25). Meanwhile, the experimental molecular weights agreed well with the corresponding theoretical ones. However, the Mn,SEC values of the polymers obtained in bulk (iodide transfer radical polymerization, ITP17) were obviously higher than the corresponding theoretical ones with broader Mw/Mn values (Mw/Mn = 1.30–1.40), which indicated a relatively lower initiation efficiency than that in nitrogen-based solvents (DMF or NMP). These results indicated that the solution RTCPs of MMA in the presence of DMF or NMP was a better RDRPs process.
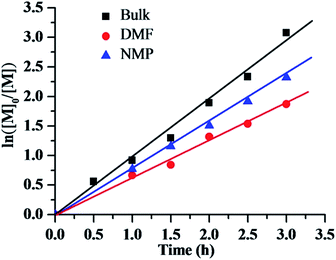 |
| | Fig. 3 ln([M]0/[M]) as a function of time for RTCP of MMA with DMF or NMP as solvent or in bulk in the absence of oxygen. Polymerization conditions: [MMA]0/[CPI]0/[AIBN]0 = 200/1/1, MMA = 2.0 mL, MMA/solvent (v/v) = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, temperature = 60 °C. 1, temperature = 60 °C. | |
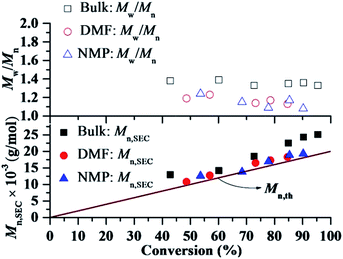 |
| | Fig. 4 Number–average molecular weight (Mn,SEC) and molecular weight distribution (Mw/Mn) versus conversion for RTCP of MMA with DMF or NMP as solvent or in bulk in the absence of oxygen. Polymerization conditions were the same as that in Fig. 3. Mn,th = ([MMA]0/[CPI]0) × MMMA × conversion + MCPI, where MMMA and MCPI represent the molecular weights of MMA and CPI, respectively. | |
Polymerization kinetics for RTCP of MMA in the presence of air
For a reversible-deactivation radical polymerization process, it is always appreciated if the polymerization can be carried out in the presence of air due to its simple and economical manipulation. There have been some examples for AGET ATRP18 and even RAFT polymerization19 systems available manipulation in the presence of a limited amount of air (oxygen). Therefore, the polymerization kinetic of RTCPs was intensively investigated to make it possible in the presence of a limited amount of air (oxygen). Fig. 5 shows the kinetic plots of RTCP of MMA with the molar ratio of [MMA]0/[CPI]0/[AIBN]0 = 200/1/1 at 60 °C without removal of oxygen. The first order kinetics with respect to [MMA]0 is still observed, indicating that the propagating radical concentrations remain constant in the presence of a limited amount of air. However, an induction period (about 2.0 h) is observed in this case. This may result that the oxygen in air will inhibit the polymerization by the side reaction with radical. Fig. 6 shows that Mn,SEC values increase linearly with the monomer conversion and are close to their corresponding theoretical ones while keeping narrow Mw/Mn values (Mw/Mn < 1.20), which indicates that the presence of a limited amount of oxygen did not sacrifice the controlled nature of RTCP. From the results mentioned above, it can be concluded that the RTCP of MMA with the absence of catalyst not only can be conducted in the presence of a limited amount of air but also does not damage the controlled characteristics of RDRP.
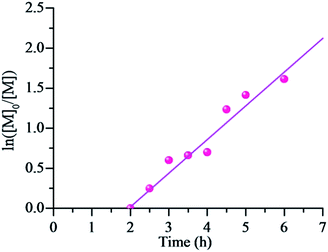 |
| | Fig. 5 ln([M]0/[M]) as a function of time for RTCP of MMA in the presence of oxygen with DMF as solvent. Polymerization conditions: [MMA]0/[CPI]0/[AIBN]0 = 200/1/1, MMA = 2.0 mL, MMA/DMF (v/v) = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, temperature = 60 °C, [O2] = 1.7 × 10−2 mol L−1. 1, temperature = 60 °C, [O2] = 1.7 × 10−2 mol L−1. | |
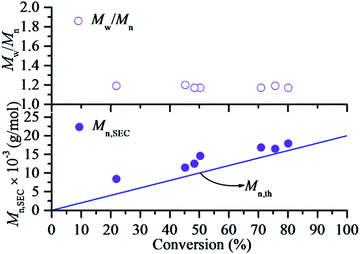 |
| | Fig. 6 Number–average molecular weight (Mn,SEC) and molecular weight distribution (Mw/Mn) versus conversion for RTCP of MMA in the presence of oxygen with DMF as solvent. Polymerization conditions were the same as that in Fig. 5. Mn,th = ([MMA]0/[CPI]0) × MMMA × conversion + MCPI, where MMMA and MCPI represent the molecular weights of MMA and CPI, respectively. | |
Analysis of chain end, chain extension and monomer addition experiments
The chain end of the PMMA-I (Mn,SEC = 1.27 × 104 g mol−1, Mw/Mn = 1.23) prepared by RTCP in the absence catalyst was analyzed by 1H NMR spectroscopy to prove the existence of I functionality in PMMA-I, as shown in Fig. 7. The chemical shifts at about 0.82, 1.02, and 1.18 ppm (Fig. 7) can be ascribed to syndiotactic (rr), atactic (mr), and isotactic (mm) methyl groups, respectively. The tacticities of PMMA were calculated as 4.08% mm, 33.65% mr, and 62.27% rr triads, respectively, which in good agreement with the tacticity distribution for traditional radical polymerizations of MMA, indicating that the RTCP of MMA follows the nature of radical polymerization mechanism. The chemical shifts at 1.02–2.06 ppm (c in Fig. 7) are assigned to the methylene and methyne protons in the PMMA chains and the methyl protons in CPI moieties. The chemical shifts at 4.2–4.5 ppm (b in Fig. 7) are assigned to the methyne protons in the chain ends of PMMAs because of the electron-attracting function of ω-I atom. Meanwhile, the percentage of chain-end functionality (f = 93.7%) can be estimated by Mn,NMR/Mn,SEC. The molecular weights of PMMA sample calculated from the 1H NMR spectrum (Mn,NMR) was 1.23 × 104 g mol−1, which was very close to the SEC value (1.27 × 104 g mol−1), according to eqn (1) where 68.1, 100.1 and 221.9 are the molecular weights of (CH3)2CCN moieties, MMA and MMA-I, respectively.| | |
Mn,NMR (g mol−1) = 68.1 + 100.1 × (Ib/Ic + 1) + 226.8
| (1) |
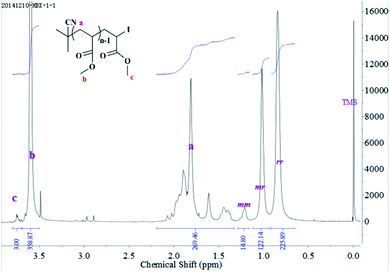 |
| | Fig. 7 1H NMR spectrum of PMMA (Mn,SEC = 1.27 × 104 g mol−1, Mw/Mn = 1.23) obtained in the presence of air with CDCl3 as solvent and tetramethylsilane (TMS) as internal standard. Polymerization conditions: [MMA]0/[CPI]0/[AIBN]0 = 200/1/1, [MMA]0 = 2.0 mL, DMF = 1.0 mL, temperature = 60 °C. | |
From this result, most of resultant polymer obtained from RTCP of MMA in the absence of catalyst were also active with the “living” polymer chain ends. Therefore, the obtained polymer can be used as macroinitiators to conduct chain-extension reaction. The “living” nature of the polymer was studied with the purified PMMA as macroinitiator in the presence or absence of additional AIBN. As shown in Fig. 8, the PMMA (Mn,SEC = 1.18 × 104 g mol−1, Mw/Mn = 1.21) obtained in the presence of air was used as the predecessor, there was a remarkably peak shift from the macroinitiator to the chain-extended PMMA with Mn,SEC = 1.99 × 104 g mol−1 and Mw/Mn = 1.16 without additional AIBN, while the polymerization rate was slow with 18.8% conversion after 12 h. When equimolar amounts of AIBN with PMMA was added, the chain extension experiment proceeded with a considerable rate (t = 4.0 h, conversion = 40.9%), combining remarkably increased molecular weight (Mn,SEC = 3.01 × 104 g mol−1). However, the Mw/Mn was slightly broader (1.30). The reason maybe be that the Polymer˙, which is initiated by AIBN in the chain extension procedure, is supposed to have relatively lower reversible chain transfer rate than general RTCPs. Similar results were shown in literature.11a To further investigate the “living” nature, we added a fresh MMA (1.0 mL) to the polymerization system20 when the monomer conversion of chain extension reached 84%. MMA was smoothly consumed to give additional 86% conversion (totally 170%) (Fig. 9). The SEC analysis of the obtained polymers showed the high controllability even after the addition of MMA, where the Mn,SEC increased in direct proportion with the conversion and the peak top was shifted to higher molecular weight, while keeping narrow Mw/Mn (<1.25). The above results fully confirmed the “living” nature of the polymers.
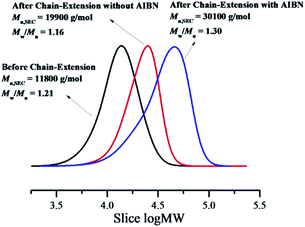 |
| | Fig. 8 SEC traces of before and after chain extension using PMMA prepared by RTCP as the macroinitiator. Original PMMA: [MMA]0/[CPI]0/[AIBN]0 = 200/1/1, MMA = 4.0 mL, DMF = 2.0 mL, temperature = 60 °C, time = 3.0 h, conversion = 52.9%. Chain extended PMMA without AIBN: [MMA]0/[PMMA]0 = 400/1, MMA = 1.0 mL, PMMA = 278 mg, DMF = 1.0 mL, temperature = 60 °C, time = 12.0 h, conversion = 18.8%. Chain extended PMMA with AIBN: [MMA]0/[PMMA]0/[AIBN]0 = 400/1/1, MMA = 1.0 mL, PMMA = 278 mg, DMF = 1.0 mL, temperature = 60 °C, time = 4.0 h, conversion = 40.9%. | |
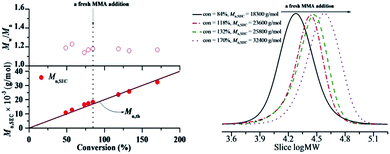 |
| | Fig. 9 Monomer-addition experiments in the polymerization of MMA with DMF as solvent. The original polymerization conditions were the same as that in Fig. 3 in DMF. MMAadd = 1.0 mL. | |
RTCPs of other kind monomers in DMF & research of other kind amines as solvent
To extend the application of this RTCP technique, RTCPs of other kind monomers (such as styrene (St), acrylonitrile (AN), glycidyl methacrylate (GMA) and vinyl acetate (VAc)) were studied in DMF under the same conditions as Fig. 5. As shown in Table 2, the RTCP of St, AN and GMA in DMF was well controlled with the predictable molecular weight and narrow Mw/Mn (entry 1–3). However, the RTCP of VAc under the same conditions was difficult mainly due to its highly reactive growing radical species. And there was no polymer produced after 72 h (entry 4). These results proved that the RTCP technique in nitrogen-based solvents could be also applicable for other conjugated vinyl monomers, such as St, AN and GMA.
Table 2 RTCPs of various kinds monomer in DMFa
| Entry |
Monomer |
Time [h] |
Conv. [%] |
Mn,th [g mol−1] |
Mn,SEC [g mol−1] |
Mw/Mn |
Polymerization conditions: [monomer]0/[CPI]0/[initiator]0 = 200/1/1, monomer = 2.0 mL, monomer/DMF (v/v) = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, [O2] = 1.7 × 10−2 mol L−1. Initiator = DCP, temperature = 100 °C. Initiator = AIBN, temperature = 60 °C. Mn,th = ([monomer]0/[initiator]0) × Mmonomer × conversion + Minitiator, where Mmonomer and Minitiator represent the molecular weights of monomer and initiator, respectively. 1, [O2] = 1.7 × 10−2 mol L−1. Initiator = DCP, temperature = 100 °C. Initiator = AIBN, temperature = 60 °C. Mn,th = ([monomer]0/[initiator]0) × Mmonomer × conversion + Minitiator, where Mmonomer and Minitiator represent the molecular weights of monomer and initiator, respectively. |
| 1 |
Stb |
13.0 |
37.1 |
7700 |
7500 |
1.25 |
| 2 |
ANc |
2.0 |
42.1 |
4500 |
9100 |
1.29 |
| 3 |
GMAc |
2.0 |
68.9 |
19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
1.18 |
| 4 |
Vacc |
72.0 |
0 |
— |
— |
— |
Besides DMF and NMP, other general amines (pyridine, triethylamine, ethylenediamine, 1-aminobutane, and ammonium hydroxide), were also used as the additives for the RTCP of MMA. The results are listed in Table 3. It can be seen that the polymerization rate enhanced from pyridine, triethylamine, ethylenediamine, 1-aminobutane to ammonium hydroxide while reducing the controllability of molecular weight and molecular weight distributions (Mw/Mn from 1.19 to 1.24). The improved controllability of the polymerization resulted from the increased series of amine probably due to the increased number of alkyl groups attached to the multi-amine, which enhanced the combination ability of amine and iodine. Therefore, well-controlled polymerization processes by simply adding amines have been demonstrated.
Table 3 Effect of amines on RTCP of MMAa
| Entry |
Amine |
Time [h] |
Conv. [%] |
Mn,th [g mol−1] |
Mn,SEC [g mol−1] |
Mw/Mn |
| Polymerization conditions: [MMA]0/[CPI]0/[AIBN]0 = 200/1/1/0.5, [MMA]0 = 2.0 mL, [amine]0 = 1.0 mL, temperature = 60 °C, [O2] = 1.7 × 10−2 mol L−1. Mn,th = ([MMA]0/[CPI]0) × MMMA × conversion + MCPI, where MMMA and MCPI represent the molecular weights of MMA and CPI, respectively. |
| 1 |
Pyridine |
4.0 |
60.2 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
1.19 |
| 2 |
Triethylamine |
4.0 |
68.8 |
13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 760 760 |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
1.20 |
| 3 |
Ethylenediamine |
4.0 |
72.9 |
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 580 580 |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
1.23 |
| 4 |
1-Aminobutane |
4.0 |
86.7 |
17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 340 340 |
23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.22 |
| 5 |
Ammonium hydroxide |
4.0 |
89.4 |
17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 880 880 |
28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
1.24 |
Conclusions
Nitrogen-based solvents (such as DMF & NMP), can be used for reversible chain transfer catalyzed polymerizations (RTCPs) without additional catalyst. It could be applicable for conjugated vinyl monomers, such as MMA, St, AN and GMA. The polymerizations showed typical features of reversible-deactivation radical polymerization. Well-defined PMMA with narrow polydispersity could be conveniently obtained. The use of commercially available solvent in the presence of air can potentially allow the more facile preparation, storage, and shipment of RTCP systems.
Acknowledgements
The financial was supported by the National Natural Science Foundation of China (no. 21404051 and 21404052), the Natural Science Foundation of Shandong Province (no. ZR2014BQ016 and BS2014CL040), the Talent Introduction Special Funds of Ludong University (no. 2014012 and 2014017), the Program for Scientific research Innovation Team in Colleges and universities of Shandong Province and Jiangsu Key Laboratory of Advanced Functional Polymer Design and Application (Soochow University).
References and Notes
-
(a) A. D. Jenkins, R. G. Jones and G. Moad, Pure Appl. Chem., 2010, 82, 483 CAS;
(b) J. Hui, Z. J. Dong, Y. Shi, Z. F. Fu and W. T. Yang, RSC Adv., 2014, 4, 55529 RSC;
(c) Y. S. Gao, T. Y. Zhao and W. X. Wang, RSC Adv., 2014, 4, 61687 RSC.
- K. Matyjaszewski and T. P. Davis, Handbook of Radical Polymerization, Wiley Interscience, Hoboken, 2002 Search PubMed.
-
(a) J. S. Wang and K. Matyjaszewski, J. Am. Chem. Soc., 1995, 117, 5614 CrossRef CAS;
(b) M. Kato, M. Kamigaito, M. Sawamoto and T. Higashimura, Macromolecules, 1995, 28, 1721 CrossRef CAS;
(c) J. S. Wang and K. Matyjaszewski, Macromolecules, 1995, 28, 7901 CrossRef CAS;
(d) K. Matyjaszewski and J. Xia, Chem. Rev., 2001, 101, 2921 CrossRef CAS PubMed;
(e) M. Kamigaito, T. Ando and M. Sawamoto, Chem. Rev., 2001, 101, 3689 CrossRef CAS PubMed;
(f) T. Pintauer and K. Matyjaszewski, Chem. Soc. Rev., 2008, 37, 1087 RSC;
(g) K. Matyjaszewski and N. V. Tsarevsky, Nat. Chem., 2009, 1, 276 CrossRef CAS PubMed;
(h) N. V. Tsarevsky and K. Matyjaszewski, Chem. Rev., 2007, 107, 2270 CrossRef CAS PubMed;
(i) L. J. Bai, L. F. Zhang, Z. P. Cheng and X. L. Zhu, Polym. Chem., 2012, 3, 2685 RSC.
-
(a) M. K. Georges, R. P. N. Veregin, P. M. Kazmaier and G. K. Hamer, Macromolecules, 1993, 26, 2987 CrossRef CAS;
(b) V. Sciannamea, R. Jerome and C. Detrembleur, Chem. Rev., 2008, 108, 1104 CrossRef CAS PubMed;
(c) C. J. Hawker, A. W. Bosman and E. Harth, Chem. Rev., 2001, 101, 3661 CrossRef CAS PubMed;
(d) D. Benoit, S. Grimaldi, S. Robin, J.-P. Finet, P. Tordo and Y. Gnanou, J. Am. Chem. Soc., 2000, 122, 5929 CrossRef CAS.
-
(a) J. Chiefari, Y. K. Chong, F. Ercole, J. Krstina, J. Jeffery, T. P. T. Le, R. T. A. Mayadunne, G. F. Meijs, C. L. Moad, G. Moad, E. Rizzardo and S. H. Thang, Macromolecules, 1998, 31, 5559 CrossRef CAS;
(b) G. Moad, E. Rizzardo and S. H. Thang, Aust. J. Chem., 2005, 58, 379 CrossRef CAS;
(c) H. Willcock and R. K. O'Reilly, Polym. Chem., 2010, 1, 149 RSC.
-
(a) B. Kowollik, Handbook of RAFT Polymerization, Wiley-VCH, Weinheim, 2008 Search PubMed;
(b) G. Moad, E. Rizzardo and S. H. Thang, Aust. J. Chem., 2006, 59, 669 CrossRef CAS;
(c) G. Moad, E. Rizzardo and S. H. Thang, Polymer, 2008, 49, 1079 CrossRef CAS PubMed.
- A. Goto, H. Zushi, N. Hirai, T. Wakada, Y. Tsujii and T. Fukuda, J. Am. Chem. Soc., 2007, 29, 13347 CrossRef PubMed.
-
(a) S. Yamago, K. Iida and J. Yoshida, J. Am. Chem. Soc., 2002, 124, 2874 CrossRef CAS PubMed;
(b) S. Yamago, Proc. Jpn. Acad., Ser. B, 2005, 81, 117 CrossRef CAS;
(c) S. Yamago, J. Polym. Sci., Part A: Polym. Chem., 2006, 44, 1 CrossRef CAS PubMed;
(d) S. Yamago, B. Ray, K. Iida, J. Yoshida, T. Tada, K. Yoshizawa, Y. Kwak, A. Goto and T. Fukuda, J. Am. Chem. Soc., 2004, 126, 13908 CrossRef CAS PubMed;
(e) S. Yamago, E. Kayahara, M. Kotani, B. Ray, Y. Kwak, A. Goto and T. Fukuda, Angew. Chem., Int. Ed., 2007, 46, 1304 CrossRef CAS PubMed;
(f) E. Kayahara and S. Yamago, J. Am. Chem. Soc., 2009, 131, 2508 CrossRef CAS PubMed.
-
(a) A. Goto, H. Zushi, Y. Kwak and T. Fukuda, ACS Symp. Ser., 2006, 944, 595–603 CrossRef CAS PubMed;
(b) A. Goto, H. Zushi, N. Hirai, T. Wakada, Y. Kwak and T. Fukuda, Macromol. Symp., 2007, 248, 126 CrossRef CAS PubMed.
- A. Goto, N. Hirai, Y. Tsujii and T. Fukuda, Macromol. Symp., 2008, 261, 18 CrossRef CAS PubMed.
-
(a) A. Goto, N. Hirai, T. Wakada, K. Nagasawa, Y. Tsujii and T. Fukuda, Macromolecules, 2008, 41, 6261 CrossRef CAS;
(b) M. Yorizane, T. Nagasuga, Y. Kitayama, A. Tanaka, H. Minami, A. Goto, T. Fukuda and M. Okubo, Macromolecules, 2010, 43, 8703 CrossRef CAS;
(c) Y. Kitayama, M. Yorizane, H. Minami and M. Okubo, Macromolecules, 2012, 45, 2286 CrossRef CAS;
(d) Y. Kitayama, M. Yorizane, H. Minamia and M. Okubo, Polym. Chem., 2012, 3, 1394 RSC;
(e) X. F. Zheng, M. Yue, P. Yang, Q. Li and W. T. Yang, Polym. Chem., 2012, 3, 1982 RSC.
- A. Goto, N. Hirai, K. Nagasawa, Y. Tsujii, T. Fukuda and H. Kaji, Macromolecules, 2010, 43, 7971 CrossRef CAS.
- A. Goto, Y. Tsujii and T. Fukuda, Polymer, 2008, 49, 5177 CrossRef CAS PubMed.
- A. Goto, T. Suzuki, H. Ohfuji, M. Tanishima, T. Fukuda, Y. Tsujii and H. Kaji, Macromolecules, 2011, 44, 8709–8715 CrossRef CAS.
- L. J. Bai, L. F. Zhang, Y. Liu, X. Q. Pan, Z. P. Cheng and X. L. Zhu, Polym. Chem., 2013, 4, 3069 RSC.
-
(a) O. Stramme, Acta Chem. Scand., 1959, 13, 268 CrossRef PubMed;
(b) A. M. Halpern and K. Weiss, J. Am. Chem. Soc., 1968, 90, 6297 CrossRef CAS;
(c) C. D. Schmulbach and D. M. Hart, J. Am. Chem. Soc., 1964, 86, 2347 CrossRef CAS.
- G. David, C. Boyer, J. Tonnar, B. Ameduri, P. Lacroix-Desmazes and B. Boutevin, Chem. Rev., 2006, 106, 3936 CrossRef CAS PubMed.
-
(a) W. Jakubowski and K. Matyjaszewski, Macromol. Rapid Commun., 2006, 27, 594 CrossRef PubMed;
(b) L. J. Bai, L. F. Zhang, Z. B. Zhang, Y. F. Tu, N. C. Zhou, Z. P. Cheng and X. L. Zhu, Macromolecules, 2010, 43, 9283 CrossRef CAS;
(c) L. J. Bai, L. F. Zhang, Z. B. Zhang, J. Zhu, N. C. Zhou, Z. P. Cheng and X. L. Zhu, J. Polym. Sci., Part A: Polym. Chem., 2011, 49, 3970 CrossRef CAS PubMed;
(d) L. J. Bai, L. F. Zhang, Z. B. Zhang, J. Zhu, N. C. Zhou, Z. P. Cheng and X. L. Zhu, J. Polym. Sci., Part A: Polym. Chem., 2011, 49, 3980 CrossRef CAS PubMed;
(e) L. J. Bai, L. F. Zhang, J. Zhu, Z. P. Cheng and X. L. Zhu, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 2002 CrossRef CAS PubMed.
- Z. B. Zhang, X. L. Zhu, J. Zhu, Z. P. Cheng and S. P. Zhu, J. Polym. Sci., Part A: Polym. Chem., 2006, 44, 3343 CrossRef CAS PubMed.
- M. Ishio, M. Katsube, M. Ouchi, M. Sawamoto and Y. Inoue, Macromolecules, 2009, 42, 188 CrossRef CAS.
|
| This journal is © The Royal Society of Chemistry 2015 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 g mol−1, Mw/Mn = 1.21) obtained with the molar ratio of [MMA]0/[CPI]0/[AIBN]0 = 200/1/1 in the presence of limited amount air with DMF as solvent was used as the macroinitiator for the chain extension reaction at the molar ratio of [MMA]0/[PMMA]0(/[AIBN]0) = 400/1(/1). The polymerization procedure is as follows: PMMA (277 mg, 0.0235 mmol) was dissolved in 1.0 mL (0.94 g, 9.4 mmol) of fresh MMA, and then the predetermined quantity of AIBN (4.2 mg, 0.0235 mmol) if added, and DMF (1.0 mL) were added. The rest of the procedure was the same as that described above. The chain-extension reaction was carried out under stirring at 60 °C.
800 g mol−1, Mw/Mn = 1.21) obtained with the molar ratio of [MMA]0/[CPI]0/[AIBN]0 = 200/1/1 in the presence of limited amount air with DMF as solvent was used as the macroinitiator for the chain extension reaction at the molar ratio of [MMA]0/[PMMA]0(/[AIBN]0) = 400/1(/1). The polymerization procedure is as follows: PMMA (277 mg, 0.0235 mmol) was dissolved in 1.0 mL (0.94 g, 9.4 mmol) of fresh MMA, and then the predetermined quantity of AIBN (4.2 mg, 0.0235 mmol) if added, and DMF (1.0 mL) were added. The rest of the procedure was the same as that described above. The chain-extension reaction was carried out under stirring at 60 °C.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da) and HR 4 (pore size 10
000 Da) and HR 4 (pore size 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Å, 50–100
000 Å, 50–100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da) columns (7.8 × 300 mm, 5 μm beads size) with measurable molecular weights ranging from 102 to 5 × 105 g mol−1. THF was used as the eluent at a flow rate of 1.0 mL min−1 and 30 °C. SEC samples were injected using a Waters 717 plus autosampler and calibrated with poly(methyl methacrylate) standards purchased from Waters. The UV-Vis spectra were recorded on a Shimadzu UV3150 spectrophotometer with a quartz UV-Vis cell (1 mm path length). 1H NMR spectrum of the obtained polymer was recorded on an INOVA 400 MHz nuclear magnetic resonance instrument using CDCl3 as the solvent and tetramethylsilane (TMS) as an internal standard.
000 Da) columns (7.8 × 300 mm, 5 μm beads size) with measurable molecular weights ranging from 102 to 5 × 105 g mol−1. THF was used as the eluent at a flow rate of 1.0 mL min−1 and 30 °C. SEC samples were injected using a Waters 717 plus autosampler and calibrated with poly(methyl methacrylate) standards purchased from Waters. The UV-Vis spectra were recorded on a Shimadzu UV3150 spectrophotometer with a quartz UV-Vis cell (1 mm path length). 1H NMR spectrum of the obtained polymer was recorded on an INOVA 400 MHz nuclear magnetic resonance instrument using CDCl3 as the solvent and tetramethylsilane (TMS) as an internal standard.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was added into the reaction system, respectively. It can also be seen that the Mn,SEC values were close to their corresponding theoretical ones and Mw/Mn values of the obtained polymers were lower (≤1.15) in nitrogen-based solvents (DMF, DMA and NMP) than that with bulk (1.43) or toluene (1.49) as solvent, indicating a well-controlled polymerization process with nitrogen-based solvents. Nitrogen-based solvents can act as catalyst for RTCP of MMA. As shown in entries 6–8 in Table 1, the conversion of MMA increase from 40.1%, 44.5% to 49.7% after 3.0 h when DMF decrease from 0.5 mL, 0.2 mL to 0.1 mL, respectively; while Mw/Mn values are slightly higher. As shown in entries 9 to 10 in Table 1, the conversion of MMA decreased with the ratio of [MMA]0/[AIBN]0 from 200/0.5 to 200/0.2, indicating that AIBN can influence the polymerization rate markedly.
1) was added into the reaction system, respectively. It can also be seen that the Mn,SEC values were close to their corresponding theoretical ones and Mw/Mn values of the obtained polymers were lower (≤1.15) in nitrogen-based solvents (DMF, DMA and NMP) than that with bulk (1.43) or toluene (1.49) as solvent, indicating a well-controlled polymerization process with nitrogen-based solvents. Nitrogen-based solvents can act as catalyst for RTCP of MMA. As shown in entries 6–8 in Table 1, the conversion of MMA increase from 40.1%, 44.5% to 49.7% after 3.0 h when DMF decrease from 0.5 mL, 0.2 mL to 0.1 mL, respectively; while Mw/Mn values are slightly higher. As shown in entries 9 to 10 in Table 1, the conversion of MMA decreased with the ratio of [MMA]0/[AIBN]0 from 200/0.5 to 200/0.2, indicating that AIBN can influence the polymerization rate markedly.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [CPI]0
[CPI]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [AIBN]0: = 200
[AIBN]0: = 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.b Polymerization conditions: [MMA]0
1.b Polymerization conditions: [MMA]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [CPI]0
[CPI]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [AIBN]0: = 200
[AIBN]0: = 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5.c Polymerization conditions: [MMA]0
0.5.c Polymerization conditions: [MMA]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [CPI]0
[CPI]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [AIBN]0: = 200
[AIBN]0: = 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2; MMA = 2.0 mL, temperature = 60 °C. Mn,th = ([MMA]0/[CPI]0) × MMMA × conversion + MCPI, where MMMA and MCPI represent the molecular weights of MMA and CPI, respectively.7
0.2; MMA = 2.0 mL, temperature = 60 °C. Mn,th = ([MMA]0/[CPI]0) × MMMA × conversion + MCPI, where MMMA and MCPI represent the molecular weights of MMA and CPI, respectively.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900
900![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300
300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600
600![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300
300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600
600![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700
700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700
700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300
300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 equivalent) in NMR tubes under argon atmosphere at 60 °C for 10 min. Fig. 2 shows the 1H NMR spectrum of (1) CPI, (2) DMF and (3) CPI + DMF in CDCl3. As shown in (3), the signals of protons deriving from –CH3 (a′) and –CHO (b′) of DMF shifted to low field, affording a solid evidence that the amine was combined with an electron-withdrawing group (I). In the classic literatures,16 n–σ types of charge-transfer complexes, involving iodine and amine as acceptor and donor, respectively, have been most extensively studied. The crystal structure of these complexes has been determined by X-ray methods. The intermolecular bond is localized, and the nitrogen–iodine arrangement is linear. Recently, we reported the RTCP system with triphenylphosphine as catalyst, in which triphenylphosphine-I2 produced in situ acts as deactivator.15 Therefore, the mechanism of MMA/CPI/AIBN in nitrogen-based solvents is suggested as RTCP, where amine-I2 produced in situ acts as deactivator. The Polymer˙, which is originally initiated by AIBN, is supposed to react with amine-I2, in situ producing the activator radicals (amine-I˙) and Polymer-I. The amine-I˙ could capture I from CP-I (or Polymer-I) effectively to produce CP˙ (or Polymer˙), and the circulation between activation and deactivation will be started (the mechanism was shown in Scheme 2).
1 equivalent) in NMR tubes under argon atmosphere at 60 °C for 10 min. Fig. 2 shows the 1H NMR spectrum of (1) CPI, (2) DMF and (3) CPI + DMF in CDCl3. As shown in (3), the signals of protons deriving from –CH3 (a′) and –CHO (b′) of DMF shifted to low field, affording a solid evidence that the amine was combined with an electron-withdrawing group (I). In the classic literatures,16 n–σ types of charge-transfer complexes, involving iodine and amine as acceptor and donor, respectively, have been most extensively studied. The crystal structure of these complexes has been determined by X-ray methods. The intermolecular bond is localized, and the nitrogen–iodine arrangement is linear. Recently, we reported the RTCP system with triphenylphosphine as catalyst, in which triphenylphosphine-I2 produced in situ acts as deactivator.15 Therefore, the mechanism of MMA/CPI/AIBN in nitrogen-based solvents is suggested as RTCP, where amine-I2 produced in situ acts as deactivator. The Polymer˙, which is originally initiated by AIBN, is supposed to react with amine-I2, in situ producing the activator radicals (amine-I˙) and Polymer-I. The amine-I˙ could capture I from CP-I (or Polymer-I) effectively to produce CP˙ (or Polymer˙), and the circulation between activation and deactivation will be started (the mechanism was shown in Scheme 2).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. All the kinetics showed linear plots, indicating that the polymerizations were approximately first order with respect to the monomer concentration, and that the number of active species remained constant during the polymerization process. However, the polymerization rates for the solution and bulk RTCPs were faster than that in solution of DMF and NMP. By calculating the apparent rate constant of polymerization, kappp (Rp = −d[M]/dt = kp[P˙n][M] = kappp[M]), as determined from the kinetic slops, a kappp of 2.74 × 10−4 s−1 for the bulk polymerization and 2.22 × 10−4 s−1 for the solution polymerization in DMF and kappp of 1.75 × 10−4 s−1 in NMP were obtained. The kappp of the polymerization in bulk was 1.23 and 1.57 times as that in DMF and NMP, respectively. Fig. 4 shows the evolution of the number-average molecular weight (Mn,SEC) values of PMMA and molecular weight distribution (Mw/Mn) values on the conversion for the bulk and solution RTCPs of MMA at 60 °C. As shown in Fig. 4, the Mn,SEC values of the polymers in DMF or NMP increased linearly with monomer conversion while keeping low Mw/Mn values (Mw/Mn = 1.11–1.25). Meanwhile, the experimental molecular weights agreed well with the corresponding theoretical ones. However, the Mn,SEC values of the polymers obtained in bulk (iodide transfer radical polymerization, ITP17) were obviously higher than the corresponding theoretical ones with broader Mw/Mn values (Mw/Mn = 1.30–1.40), which indicated a relatively lower initiation efficiency than that in nitrogen-based solvents (DMF or NMP). These results indicated that the solution RTCPs of MMA in the presence of DMF or NMP was a better RDRPs process.
1. All the kinetics showed linear plots, indicating that the polymerizations were approximately first order with respect to the monomer concentration, and that the number of active species remained constant during the polymerization process. However, the polymerization rates for the solution and bulk RTCPs were faster than that in solution of DMF and NMP. By calculating the apparent rate constant of polymerization, kappp (Rp = −d[M]/dt = kp[P˙n][M] = kappp[M]), as determined from the kinetic slops, a kappp of 2.74 × 10−4 s−1 for the bulk polymerization and 2.22 × 10−4 s−1 for the solution polymerization in DMF and kappp of 1.75 × 10−4 s−1 in NMP were obtained. The kappp of the polymerization in bulk was 1.23 and 1.57 times as that in DMF and NMP, respectively. Fig. 4 shows the evolution of the number-average molecular weight (Mn,SEC) values of PMMA and molecular weight distribution (Mw/Mn) values on the conversion for the bulk and solution RTCPs of MMA at 60 °C. As shown in Fig. 4, the Mn,SEC values of the polymers in DMF or NMP increased linearly with monomer conversion while keeping low Mw/Mn values (Mw/Mn = 1.11–1.25). Meanwhile, the experimental molecular weights agreed well with the corresponding theoretical ones. However, the Mn,SEC values of the polymers obtained in bulk (iodide transfer radical polymerization, ITP17) were obviously higher than the corresponding theoretical ones with broader Mw/Mn values (Mw/Mn = 1.30–1.40), which indicated a relatively lower initiation efficiency than that in nitrogen-based solvents (DMF or NMP). These results indicated that the solution RTCPs of MMA in the presence of DMF or NMP was a better RDRPs process.



![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, [O2] = 1.7 × 10−2 mol L−1.b Initiator = DCP, temperature = 100 °C.c Initiator = AIBN, temperature = 60 °C. Mn,th = ([monomer]0/[initiator]0) × Mmonomer × conversion + Minitiator, where Mmonomer and Minitiator represent the molecular weights of monomer and initiator, respectively.
1, [O2] = 1.7 × 10−2 mol L−1.b Initiator = DCP, temperature = 100 °C.c Initiator = AIBN, temperature = 60 °C. Mn,th = ([monomer]0/[initiator]0) × Mmonomer × conversion + Minitiator, where Mmonomer and Minitiator represent the molecular weights of monomer and initiator, respectively.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500
500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300
300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700
700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 760
760![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700
700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 580
580![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900
900![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 340
340![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 880
880![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200
200







