Allylic oxidation of cyclohexene over ruthenium-doped titanium-pillared clay
Abstract
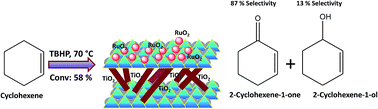
Ruthenium-doped H-Montmorillonite (H-Mont) and Ti-pillared clay (Ti-PILC) were prepared then studied for oxidation of cyclohexene, with tert-butylhydroperoxide (TBHP) as the oxygen source. The Ti-PILC support was prepared by hydrolysis of Ti(OC3H7)4 with HCl. The synthesized Ru/Ti-PILC and Ru/H-Mont catalysts were characterized by chemical analysis, surface area/pore volume measurements, Fourier transform infrared (FTIR) spectroscopy, X-ray powder diffraction (XRD), and UV-vis-diffuse reflectance spectroscopy (UV-vis-DRS). Both catalysts can selectively oxidize cyclohexene through allylic oxidation to give 2-cyclohexene-1-one as the major product, and 2-cyclohexene-1-ol as the minor product. The influence of reaction time, temperature, catalyst amount, and substrate/oxidant ratio was also investigated to find the optimal reaction for cyclohexene oxidation to get the highest conversion. Indeed, when 5% Ru/Ti-PILC was employed as catalyst, 59% cyclohexene conversion, 87% selectivity for 2-cyclohexene-1-one and 13% selectivity for 2-cyclohexene-1-ol were obtained under ambient pressure, at 70 °C, for a 6 h reaction time. The catalysts were reused in four consecutive runs.

 Please wait while we load your content...
Please wait while we load your content...