Highly-ordered maghemite/reduced graphene oxide nanocomposites for high-performance photoelectrochemical water splitting†
Sundaram Chandrasekarana,
Seung Hyun Hur*a,
Eui Jung Kima,
Balasubramaniyan Rajagopalana,
Kadumudi Firoz Babua,
Velusamy Senthilkumarb,
Jin Suk Chunga,
Won Mook Choia and
Yong Soo Kimb
aSchool of Chemical Engineering, University of Ulsan, Daehak-ro, 102, Nam-gu, Ulsan 680-749, South Korea. E-mail: shhur@ulsan.ac.kr; Fax: +82 52 259 1689; Tel: +82 52 259 1028
bDepartment of Physics and Energy Harvest-Storage Research Center, University of Ulsan, Ulsan 680-749, South Korea
First published on 19th March 2015
Abstract
Highly ordered γ-Fe2O3/reduced graphene oxide (RGO) was synthesized via a facile solution technique combined with calcination at various temperatures. The maghemite iron oxide structure was obtained on the GO surface and improved crystallinity of γ-Fe2O3 was observed as the calcination temperature increased. The prepared highly ordered maghemite structure on RGO exhibited an excellent water splitting performance under UV light (∼360 nm) illumination. The photocurrent density of RGO/γ-Fe2O3 calcined at 500 °C was 6.74 mA cm−2 vs. RHE and a high incident photon to current conversion efficiency (IPCE) of 4.7%, was achieved. This photocurrent density and the IPCE values are 3.7 times and 4 times higher than that of pristine iron oxide, respectively.
1. Introduction
Production of hydrogen and oxygen from photocatalytic water splitting has attracted substantial attention as a potential means of renewable energy production with no reliance on fossil fuels and no carbon dioxide emission.1,2 Hydrogen production from water by solar energy is essential to constructing clean energy systems in order to solve the energy and environmental issues simultaneously. Even though various photocatalysts have been explored, efficient materials for splitting water into H2 and O2 have not been found yet.3,4 In this regard, a significant part of the scientific community is currently focusing on the synthesis of novel nanostructured materials that are capable of absorbing the photonic energy emitted by the sun or other external sources with a high efficiency of its conversion into chemical or electrical energy. Photocatalytic water splitting is still a challenging reaction even if the research history is long. Usually, photolysis or photo splitting of water into gaseous O2 and H2 is described by eqn (1),5
 | (1) |
The rate of water splitting by photoelectrochemical cell (PEC) can be increased significantly when artificial UV light sources are used.5
Recently, various metal oxides (such as TiO2, ZnO, WO3, and CoO) and graphene based metal oxide composites have been investigated for PEC water splitting.6–12 Among them, iron oxides (Fe3O4, α-Fe2O3 and γ-Fe2O3 etc.) have received much attention due to their favourable optical band gap, excellent chemical stability, natural abundance, and low material cost.13–16 The maximum theoretical efficiency of solar-to-hydrogen (STH) conversion is 16.8% so far, however, the reported value of STH for iron oxide is much lower than the expected value due to its very short lifetime of photo generated charge carriers (<10 ps) and short hole diffusion length (∼2–4 nm).17
Lately, graphene and graphene based hybrid materials have drawn great attention due to their unique microstructures and properties, which promote their extensive potential application in many fields.18,19 Moreover, the properties of graphene oxide (GO) and mildly reduced graphene oxide (RGO) prepared by the chemical method can be easily tailored compared with the graphene grown by the chemical vapour deposition (CVD) due to the formation of various functional groups such as the hydroxyl, carbonyl, and carboxyl groups on the graphene surface, which enables GO and RGO more easily modified by other materials.20–24 Therefore, semiconductor nanoparticles assembled on the surface of GO are believed to provide a new way to design high-performance energy harvesting electrode materials. Even though iron oxide/graphene composites have been extensively studied for use as an anode material for Li-ion batteries,25 super capacitors,26,27 sensors28 and catalysts,29,30 to the best of our knowledge, no PEC study of RGO/γ-Fe2O3 has been reported in the literature so far. There are few reports available on the electrochemical behaviour of RGO-maghemite (γ-Fe2O3) nanocomposites,25,31,32 while many literatures are available for magnetite and hematite based composites.13–16 Moreover, due to a lack of studies on maghemite/graphene composites, the actual nature of iron oxide is still unrevealed and most of the previous reports on iron oxide/graphene composites have assumed that iron oxide has magnetite phase.
Although nano-sized iron oxide and iron oxide composites made by atmospheric pressure chemical vapor deposition (APCVD)15,33 and atomic layer deposition (ALD)34 have shown excellent PEC performance, these techniques require special instruments such as atomizers or CVD or ALD. Moreover, ALD systems need toxic and flammable metal–organic precursors. In this paper, we report highly efficient photocatalytic materials that exhibit high energy conversion efficiency and excellent operational stability. We fabricated crumpled RGO/iron oxide (maghemite) nanocomposites by a facile solution technique. In addition, the structure of iron oxide anchored on GO was accurately identified in this work. After calcination at 300, 400 and 500 °C for 4 h (for sample identification the as-prepared, 300, 400 and 500 °C calcined samples are denoted as RGO-M1, RGO-M2, RGO-M3 and RGO-M4, respectively), the PEC performances were highly improved. The overall water splitting photocurrent density of 6.74 mA cm−2 with high incident photon to current conversion efficiency (IPCE) of 4.7% was achieved for the RGO-M4 (under UV light ∼360 nm), which is the highest IPCE for RGO based iron oxide photocatalysts under UV light.
2. Materials and methods
2.1. Synthesis of GO and RGO/γ-Fe2O3 nanocomposites
Graphene oxide (GO) was synthesized from graphite powder using a modified Hummers' method. Briefly, 1 g of graphite and 0.5 g of sodium nitrate were mixed, which was followed by the addition of 23 mL of concentrated sulfuric acid under constant stirring with a magnetic stirrer. After 1 h, 3 g of KMnO4 was gradually added to the above solution while keeping the temperature below 20 °C to prevent overheating and explosion. The mixture was stirred at 35 °C for 8 h and the resulting solution was diluted with 500 mL of deionized water under vigorous stirring. To ensure the completion of the reaction with KMnO4, the diluted mixture was further treated with 30% H2O2 solution (5 mL). The resulting suspension was washed with HCl and H2O, respectively, which was followed by filtration and drying to produce GO.To synthesize RGO/γ-Fe2O3 nanocomposites, 20 mg of GO was first dispersed in deionized water (40 mL) with vigorous stirring at 70 °C to obtain a homogeneous dispersion. Then, the GO solution was added to 0.5 mol L−1 of iron(III) chloride aqueous solution (80 mL) and the mixture was stirred for 1 h. An ammonia–ethanol solution with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) ratio (4 mL) was added to the above solution. Finally, RGO/γ-Fe2O3 precipitates were collected, washed with distilled water, and dried at 70 °C for 15 h. Then, the dried sample was further calcined at 300 °C, 400 °C and 500 °C for 4 h. Pure iron oxide was prepared by the same procedure without RGO.
1 (v/v) ratio (4 mL) was added to the above solution. Finally, RGO/γ-Fe2O3 precipitates were collected, washed with distilled water, and dried at 70 °C for 15 h. Then, the dried sample was further calcined at 300 °C, 400 °C and 500 °C for 4 h. Pure iron oxide was prepared by the same procedure without RGO.
2.2. Instrumental analysis
X-Ray diffraction (XRD; Rigaku) spectroscopy with Cu-Kα radiation was used to identify the crystalline phases. Field emission scanning electron microscopy (FE-SEM; JSM 6500F) was used to investigate the surface morphology of RGO/γ-Fe2O3 nanocomposites. Transmission electron microscopy (TEM; JEOL JEM 2100F) was used to analyse the micrograph images of the prepared samples. The functional groups were identified by using a Thermo Scientific Nicolet 380 FTIR spectrometer. Optical absorption measurements were taken on an Analytik Jena SPECORD210 Plus UV-vis spectrometer. The vibrational, rotational, and other low-frequency mode in the sample was analyzed using a Thermo Scientific DXR Raman spectrophotometer. Materials composition and oxidation states of carbon and iron atoms in the RGO/γ-Fe2O3 nanocomposites were analyzed using a Thermo Scientific K-alpha X-ray photoelectron spectrometer (XPS).2.3. Photoelectrochemical analysis
The photoelectrochemical tests were carried out using cyclic voltammetry (CV) with a three cell system by an electrochemical working station (Gamry instrument). The working electrodes were prepared with the RGO/γ-Fe2O3, carbon black and polyvinylidene fluoride (PVDF) binder in a weight ratio of 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The slurry was then coated on one side of thin Cu foil with a size of 1 × 1 cm2 and dried for 2 h at 60 °C in an oven. The mass of the electrode material was about 4 mg. The reference electrode and counter electrode were Ag/AgCl and Pt, respectively. The other side of the Cu foil was masked with a non-conducting tap. The measured potential vs. Ag/AgCl was converted to the reversible hydrogen electrode (RHE) scale using the Nernst equation,
1. The slurry was then coated on one side of thin Cu foil with a size of 1 × 1 cm2 and dried for 2 h at 60 °C in an oven. The mass of the electrode material was about 4 mg. The reference electrode and counter electrode were Ag/AgCl and Pt, respectively. The other side of the Cu foil was masked with a non-conducting tap. The measured potential vs. Ag/AgCl was converted to the reversible hydrogen electrode (RHE) scale using the Nernst equation,| ERHE = EAg/AgCl + 0.241 V + 0.0591 × pH at 25 °C |
The PEC water splitting performance of the photoanodes was evaluated using 1.0 M NaOH buffer solution (pH = 13.6) in a three electrode chemical cell under 360 nm UV light illumination.
3. Results and discussion
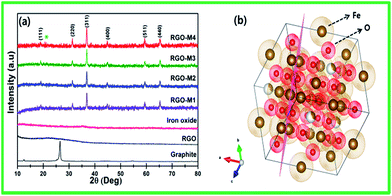
The XRD patterns of graphite, RGO, pristine iron oxide, as-prepared and calcined RGO/γ-Fe2O3 are shown in Fig. 1. The XRD pattern of the pristine iron oxide agreed well with the JCPDS file (no. 89-0951), indicating that the pristine iron oxide nanopowder has magnetite structure. On the contrary, RGO-M1 (as-prepared RGO/γ-Fe2O3) and RGO-M2 to RGO-M4 (calcined RGO/γ-Fe2O3) exhibited major (111), (220), (311) and (400) peaks, meaning that RGO induced the structural change of iron oxide. No structural changes but the intensity increase of iron oxide in the RGO/γ-Fe2O3 was observed during calcination, which indicates the calcination process was responsible for the preferred orientations.31,32The presence of iron nanoparticles reduces the aggregation of graphene sheets, which results in less stacking of GO sheets. As a result, a weak GO related peak was observed in the XRD patterns. Maghemite (γ-Fe2O3) and magnetite (Fe3O4), two main forms of iron oxide, have cubic inverse spinel structure and they are very similar in structure to each other. The distinct feature of maghemite is the presence of iron vacancies in the sub lattice. The cation or vacancy distribution in octahedral positions can give rise to several crystal symmetries in maghemite with the corresponding differences in the XRD patterns (Fig. 1b). Usually, disordered maghemite particles belong to the space group Fd3m.25 The spinel structure of γ-Fe2O3 contains cation vacancies that can be ordered (tetragonal superstructure), partially ordered (cubic superstructure), or completely disordered with a high probability of the aggregated vacancies.35 The precise nature of the ordering in γ-Fe2O3 particles with GO is still unclear.35,36 The observed and standard d-values for the iron oxide in the RGO/γ-Fe2O3 nanocomposites fabricated in this study agree well with JCPDS card no. 39-1346, confirming the cubic symmetry of maghemite (γ-Fe2O3), i.e. Fe3+ in octahedral sites and O in close-packed cubic arrangement (the maghemite structure was further confirmed by SAED patterns, which will be discussed in TEM analysis). It is well known that during the heat treatment of GO/γ-Fe2O3 iron atoms can interact with the oxygen related functional groups of GO and form Fe–O–C bonds, which results in the formation of maghemite structure of iron oxide on the GO sheets. Further, the functional groups on the surface of GO disappear when GO is thermally reduced and more π electrons on C atoms are regenerated by the restoration of sp2 networks. Thus, the unpaired π electrons are attracted to free Fe atoms, which results in the preferred growth of γ-Fe2O3 on the surface of RGO. From the Debye–Scherer formulae,31 the broad diffraction peaks are indicative of small-sized iron oxide nanoparticles (20–24 nm) in the RGO/γ-Fe2O3 nanocomposites. The functional groups of the crumpled RGO/γ-Fe2O3 were identified from FTIR spectra in Fig. 2a. Absorption peaks at 586.94, 632.06, and 793.60 cm−1 can be ascribed to the maghemite structure of iron oxide.37 The peak around ∼586 cm−1 is attributed to Fe–O bonds, and the enhanced intensity of Fe–O peak is due to iron oxide loaded to RGO.38 Serna et al.39 reported that a less-ordered maghemite (γ-Fe2O3) structure is evidenced by multiple lattice absorption bands within 800 cm−1. The broad peaks at around 800 and 580 cm−1 is the characteristic of well-ordered γ-Fe2O3.35 No peaks appeared at 3438 and 3150 cm−1, which strongly implies that α-FeOOH is absent in the prepared iron oxides. The peaks at 1638.39 cm−1 and 2357 cm−1 were due to the OH bending of water and the presence of atmospheric CO2, respectively. The stretching vibrations of epoxy C–O (1226.92 cm−1), weak aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C (1578 cm−1), and carboxyl O–C
C (1578 cm−1), and carboxyl O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (1386.11 cm−1) bonds were observed in RGO/iron oxide samples, which can be ascribed to the functional groups of GO and RGO.40 As the calcination temperature increased, some functional groups present in the RGO-M1 disappeared due to the thermal reduction of functional groups in GO.
O (1386.11 cm−1) bonds were observed in RGO/iron oxide samples, which can be ascribed to the functional groups of GO and RGO.40 As the calcination temperature increased, some functional groups present in the RGO-M1 disappeared due to the thermal reduction of functional groups in GO.
Fig. 2b represents the Raman spectra of RGO/γ-Fe2O3 nanocomposites. The spectral values are in good agreement with the standard Raman spectrum of maghemite.41,42
For maghemite structure,43 the net structure is spinel symmetry with randomly distributed vacancies in its structure. The projected vibrational modes of the first Brillouin zone center of a spinel structure is given as,
| Γvib = A1g + E1g + T1g + 3T2g + 2A2u + 2Eu + 4T1u + 2T2u |
In the above equation, excluding T1g, A2u, Eu, T1u and T2u modes (Raman inactive modes), all modes of vibrations are easily observable for maghemite spinel structure in the Raman spectra. The enduring symmetrical modes like A1g, Eg, and T2g are Raman active modes. As shown in Fig. 2b, three active modes were observed for maghemite phase, i.e. ∼263 cm−1 (Eg: Fe–O symmetric bending), ∼345 cm−1 (Eg: Fe–O symmetric stretching), ∼395 cm−1 (T2g: Fe–O asymmetric bending) and ∼665 cm−1 (A1g: symmetric stretching). The active modes of the Raman signal varies with materials preparation conditions and the vacancies in the maghemite crystal unit cell.37 The weak Raman signal is the result of poor scattering properties of maghemite.42 Compared with other samples, the RGO-M4 samples exhibited a red-shifted peak frequency for the main A1g peak, indicating a greater degree of maghemite crystallinity. The RGO in the RGO/γ-Fe2O3 nanocomposites are more amorphous than GO and the broadened and shifted band is due to the destruction of conjugated system in graphite. Compared with other samples, a smaller ID/IG ratio for the RGO-M4 (1.02) implies a larger sp2 domain size at higher calcination temperature.31 The optical band gap of pristine iron oxide (∼2.13 eV) and RGO/γ-Fe2O3 nanocomposites was evaluated from the UV-vis absorption spectra using Tauc relation ((αhν)1/2 vs. the photon energy where α is the absorption coefficient) as shown in Fig. 2c and S1.† Similar to previous results acquired for RGO–metal oxide nanocomposites, the RGO/γ-Fe2O3 composites exhibited a narrower band gap after the modification of γ-Fe2O3 with RGO and subsequent calcination. This phenomenon can be attributed to the formation of metal–O–C chemical bonds in the RGO/γ-Fe2O3 composites.44,45
Fig. 3(a–c) represents the FE-SEM and TEM images of the pure iron oxide sample. It clearly shows that the sample consists of agglomerated spherical particles of diameter ranging from 15 to 25 nm. Fig. 3d displays the electron diffraction (SAED) pattern indexed with the cubic symmetry of the magnetite phase, which is in good agreement with JCPDS file (no. 89-0951) and the above XRD results. Fig. 4 illustrates the TEM images and SAED patterns of RGO/γ-Fe2O3 nanocomposites. As shown in Fig. 4, large amounts of iron oxide nanoparticles are uniformly decorated on RGO46 and the intensity of SAED pattern evidently displays the graphene (002 plane reflection). It should be noted that the increased crystallinity of γ-Fe2O3 compared with pure iron oxide denotes that GO affects the formation of maghemite crystalline structure.
 | ||
| Fig. 3 (a) FE-SEM, (b and c) TEM images at various scales and (d) SAED pattern of pristine iron oxide. | ||
 | ||
| Fig. 4 TEM images and SAED patterns of (a and b) RGO-M1, (c and d) RGO-M2, (e and f) RGO-M3 and (g and h) RGO-M4 samples. | ||
The electronic state and the chemical composition of the samples were investigated by XPS. The XPS survey spectra in Fig. S2† reveal the presence of Fe, C and O in the RGO/γ-Fe2O3. As shown in Fig. 5, several XPS peaks appeared for iron oxide. In the observed XPS spectra, the major peaks at ∼711.7 eV and ∼725.1 eV associated with Fe 2p3/2 and Fe 2p1/2 core-level binding energies of γ-Fe2O3 on RGO.25 The nature of the γ-Fe2O3 was clearly revealed by simulating the observed spectra. It shows that, ∼710.7 eV, 713.2 eV and 718.7 eV (satellite peak) is ascribed to Fe 2p3/2 state. The presence of satellite peak at ∼718.7 eV is characteristic for maghemite.47 The peaks at 725.1 eV and 732.7 eV well documented to Fe 2p1/2 state of iron oxide.26 The Fe0 (709 eV) peak was not observed and only Fe2+ and Fe3+ states were detected in the XPS spectra as the γ-Fe2O3 nanoparticles were oxidized. The O 1s core level XPS spectra of as prepared sample showed two peaks at 529.96 and 531.84 eV, corresponding to Fe–O and Fe–O–C bond formations, respectively. Further calcination, the binding energy values were found to be shifted slightly in positive are due to the formation of coordinated oxygen in the composites (Fig. S3 and Table S1†).48,49 The XPS data strongly support the XRD, FTIR and Raman results and confirm the high order of γ-Fe2O3 on the surface of GO in all prepared nanocomposites.
The linear sweep voltagrams of pure iron oxide, as-prepared and calcined samples are shown in Fig. 6a. In the absence of UV light illumination, no oxidation photocurrent was obtained, indicating that all the electrodes are inactive toward OER in the dark condition. When the UV light was illuminated, all the electrodes exhibited a considerable oxidation photocurrent. The maximum photocurrent density was 1.83, 3.04, 3.39, 4.65 and 6.74 mA cm−2 for pristine iron oxide, RGO-M1, RGO-M2, RGO-M3 and RGO-M4, respectively. This result indicates that photocatalytic performance of iron oxide can be significantly improved by its hybridization with GO and calcination process. As described in Fig. 6b, the RGO serves as an electron sink to facilitate the exciton separation and store the separated electron.47 The saturated photocurrent density of the RGO-M4 was ca. 6.74 mA cm−2, which is 3.7 times higher than that of the pristine iron oxide and 2.3 times higher than that of the RGO-M1. An increase in photocurrent with calcination temperature is believed to be the result of reduced defect density (i.e. highly ordered γ-Fe2O3) in RGO/γ-Fe2O3 samples.
 | ||
| Fig. 6 (a) Linear sweep voltamograms of pristine iron oxide and RGO/γ-Fe2O3 samples at a scan rate of 20 mV s−1 in 1.0 M NaOH electrolyte. (b) Schematic diagram of water splitting by RGO/γ-Fe2O. | ||
The photoconversion efficiency of the sample was calculated using the following equation:
| η = I(1.23 − V)/Jlight | (2) |
To obtain a relationship between photo activity and light absorption of RGO/γ-Fe2O3, we have quantitatively investigated the photo activity of RGO/γ-Fe2O3 as a function of wavelength of incident light. In comparison to the photocurrent density, the photocurrent density, the photocurrent as a function of the wavelength and incident photon to current conversion efficiency (IPCE) is a more appropriate parameter to characterize the photoconversion efficiency of different photo anodes as it is independent of the light sources. IPCE can be calculated by the following equation:
| IPCE(λ) = 1240J(λ)/λEλ(λ) | (3) |
| Photocatalyst | Method | IPCE (%) value at 360 nm | Ref. |
|---|---|---|---|
| α-Fe2O3 | AACVD | 0.2 | 15 |
| α-Fe2O3 | Deposition annealing | 3.2 | 50 |
| Pt–α-Fe2O3 | Electro deposition | 4.0 | 52 |
| n-Fe2O3 | Spray pyrolysis | 1.8 | 53 |
| α-Fe2O3/graphene | Spray pyrolysis | 3.5 | 57 |
| α-Fe2O3/CNT | Spray pyrolysis | 4.2 | 57 |
| Fe3O4 | Solution method | 1.2 | Present work |
| RGO-M4 (RGO/γ-Fe2O3 (500 °C calcined)) | Solution method | 4.7 | Present work |
The photo-induced electrons are spontaneously injected from the conduction band of iron oxide into that of graphene. Further, the applied positive potential that provides a driving force for the electrons to transfer to the RGO could increase the charge separation and suppress the charge recombination, which leaves long-lived holes in the γ-Fe2O3 to oxidize water increasing the photocatalytic water splitting rate.
4. Conclusions
In this work, highly ordered maghemite/RGO nanocomposites were synthesized via a facile and cost effective solution technique. The maghemite iron oxide structure was formed on the GO surface due to the formation of Fe–O–C bonds between Fe and GO, which was confirmed by the TEM, Raman, XPS, XRD and FTIR analyses. The photocatalytic activity of RGO/γ-Fe2O3 nanocomposites was higher than that of pure iron oxide due to the enhanced light absorption and accelerated charge separation when iron oxide was anchored on GO. As the calcination temperature increased, the photocatalytic activity of RGO/γ-Fe2O3 nanocomposites was increased due to the improved iron oxide crystallinity and decreased defect density. The out-standing water splitting performance of the RGO/γ-Fe2O3 composites opens promising prospects for a new generation of photocatalyst exploiting the synergistic effects of the PEC cell.Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2013R1A1A2A10004468).Notes and references
- M. Gratzel, Nature, 2001, 414, 338–344 CrossRef CAS PubMed.
- J. Li and N. Wu, Catal. Sci. Technol., 2015, 4440–4441 Search PubMed.
- M. G. Walter, E. L. Warren, J. R. McKone, S. W. Boettcher, Q. Mi, E. A. Santori and N. S. Lewis, Chem. Rev., 2010, 110, 6446–6473 CrossRef CAS PubMed.
- W. Rüttinger and G. C. Dismukes, Chem. Rev., 1997, 97, 1–24 CrossRef PubMed.
- L. Wang, C.-Y. Lee and P. Schmuki, Electrochem. Commun., 2013, 30, 21–25 CrossRef PubMed.
- T. Hisatomi, J. Kubota and K. Domen, Chem. Soc. Rev., 2014, 43, 7520–7535 RSC.
- Y. Lin, G. Yuan, R. Liu, S. Zhou, S. W. Sheehan and D. Wang, Chem. Phys. Lett., 2011, 507, 209–215 CrossRef CAS PubMed.
- T.-F. Yeh, J. Cihlář, C.-Y. Chang, C. Cheng and H. Teng, Mater. Today, 2013, 16, 78–84 CrossRef CAS PubMed.
- P. V. Kamat and J. Bisquert, J. Phys. Chem. C, 2013, 117, 14873–14875 CAS.
- S. Chandrasekaran, Sol. Energy Mater. Sol. Cells, 2013, 109, 220–226 CrossRef CAS PubMed.
- S. Chandrasekaran, W. M. Choi, J. S. Chung, S. H. Hur and E. J. Kim, Mater. Lett., 2014, 136, 118–121 CrossRef CAS PubMed.
- S. Chandrasekaran, J. Nanoeng. Nanomanuf., 2011, 1, 242–247 CrossRef CAS PubMed.
- T. Jin, P. Diao, Q. Wu, D. Xu, D. Hu, Y. Xie and M. Zhang, Appl. Catal., B, 2014, 148–149, 304–310 CrossRef CAS PubMed.
- H. Gao, C. Liu, H. E. Jeong and P. Yang, ACS Nano, 2011, 6, 234–240 CrossRef PubMed.
- A. A. Tahir, K. G. U. Wijayantha, S. Saremi-Yarahmadi, M. Mazhar and V. McKee, Chem. Mater., 2009, 21, 3763–3772 CrossRef CAS.
- I. Cesar, A. Kay, J. A. Gonzalez Martinez and M. Grätzel, J. Am. Chem. Soc., 2006, 128, 4582–4583 CrossRef CAS PubMed.
- H. Dotan, O. Kfir, E. Sharlin, O. Blank, M. Gross, I. Dumchin, G. Ankonina and A. Rothschild, Nat. Mater., 2013, 12, 158–164 CrossRef CAS PubMed.
- N. A. Zubir, C. Yacou, J. Motuzas, X. Zhang and J. C. Diniz da Costa, Sci. Rep., 2014, 4, 1–8 Search PubMed.
- Y. V. Pleskov, Russ. J. Electrochem., 2003, 39, 328–330 CrossRef CAS.
- C. K. Chua and M. Pumera, Chem. Soc. Rev., 2014, 43, 291–312 RSC.
- H. P. Mungse and O. P. Khatri, J. Phys. Chem. C, 2014, 118, 14394–14402 CAS.
- K. P. Loh, Q. Bao, G. Eda and M. Chhowalla, Nat. Chem., 2010, 2, 1015–1024 CrossRef CAS PubMed.
- Y. Zhang, Z. Schnepp, J. Cao, S. Ouyang, Y. Li, J. Ye and S. Liu, Sci. Rep., 2013, 3, 1–8 Search PubMed.
- J. Wang, C. Zhang, Y. Shen, Z. Zhou, J. Yu, Y. Li, W. Wei, S. Liu and Y. Zhang, J. Mater. Chem. A, 2015, 3, 5126–5131 CAS.
- S. H. Yu, D. E. Conte, S. Baek, D.-C. Lee, S.-K. Park, K. J. Lee, Y. Piao, Y.-E. Sung and N. Pinna, Adv. Funct. Mater., 2013, 23, 4293–4305 CrossRef CAS.
- Q. Qu, S. Yang and X. Feng, Adv. Mater., 2011, 23, 5574–5580 CrossRef CAS PubMed.
- P. C. Gao, P. A. Russo, D. E. Conte, S. Baek, F. Moser, N. Pinna, T. Brousse and F. Favier, ChemElectroChem, 2014, 1, 747–754 CrossRef CAS.
- T. Peik-See, A. Pandikumar, H. Nay-Ming, L. Hong-Ngee and Y. Sulaiman, Sensors, 2014, 14, 15227–15243 CrossRef CAS PubMed.
- A. Dey, P. Varma, J. Athar, H. Prasant, A. Sikder and S. Chattopadhyay, RSC Adv., 2014, 5, 1950–1960 RSC.
- J. Tuček, K. C. Kemp, K. S. Kim and R. Zbořil, ACS Nano, 2014, 8, 7571–7612 CrossRef PubMed.
- I. T. Kim, A. Magasinski, K. Jacob, G. Yushin and R. Tannenbaum, Carbon, 2013, 52, 56–64 CrossRef CAS PubMed.
- O. Vargas, Á. Caballero and J. Morales, Electrochim. Acta, 2014, 130, 551–558 CrossRef CAS PubMed.
- S. C. Warren, K. Voïtchovsky, H. Dotan, C. M. Leroy, M. Cornuz, F. Stellacci, C. Hébert, A. Rothschild and M. Grätzel, Nat. Mater., 2013, 12, 842–849 CrossRef CAS PubMed.
- D. Ramimoghadam, S. Bagheri and S. B. A. Hamid, J. Magn. Magn. Mater., 2014, 368, 207–229 CrossRef CAS PubMed.
- A. Recnik, I. Nyiro-Kosa, I. Dodony and M. Posfai, CrystEngComm, 2013, 15, 7539–7547 RSC.
- K. K. A. W. Mader, Z. Naturforsch., B: J. Chem. Sci., 2006, 61, 665–671 Search PubMed.
- N. S. Chaudhari, S. S. Warule, S. Muduli, B. B. Kale, S. Jouen, B. Lefez, B. Hannoyer and S. B. Ogale, Dalton Trans., 2011, 40, 8003–8011 RSC.
- X. Yang, C. Chen, J. Li, G. Zhao, X. Ren and X. Wang, RSC Adv., 2012, 2, 8821–8826 RSC.
- C. J. Serna and M. P. Morales, Surf. Colloid Sci., ed. E. Matijević and M. Borkovec, Springer, USA, 2004, vol. 17, ch. 2, pp. 27–81 Search PubMed.
- G. Wang, Z. Gao, G. Wan, S. Lin, P. Yang and Y. Qin, Nano Res., 2014, 7, 704–716 CrossRef CAS PubMed.
- S. Oh, D. C. Cook and H. E. Townsend, Hyperfine Interact., 1998, 112, 59–66 CrossRef CAS.
- I. T. Lucas, E. Dubois, J. Chevalet and S. Durand-Vidal, Phys. Chem. Chem. Phys., 2008, 10, 3263–3273 RSC.
- A. M. Jubb and H. C. Allen, ACS Appl. Mater. Interfaces, 2010, 2, 2804–2812 CAS.
- H. Zhang, X. Lv, Y. Li, Y. Wang and J. Li, ACS Nano, 2009, 4, 380–386 CrossRef PubMed.
- H. N. Tien, V. H. Luan, L. T. Hoa, N. T. Khoa, S. H. Hahn, J. S. Chung, E. W. Shin and S. H. Hur, Chem. Eng. J., 2013, 229, 126–133 CrossRef CAS PubMed.
- S. Mao, Z. Wen, T. Huang, Y. Hou and J. Chen, Energy Environ. Sci., 2014, 7, 609–616 CAS.
- F. Meng, J. Li, S. K. Cushing, J. Bright, M. Zhi, J. D. Rowley, Z. Hong, A. Manivannan, A. D. Bristow and N. Wu, ACS Catal., 2013, 3, 746–751 CrossRef CAS.
- J. Zhou, H. Song, L. Ma and X. Chen, RSC Adv., 2011, 1, 782–791 RSC.
- S. L. Tait, Y. Wang, G. Costantini, N. Lin, A. Baraldi, F. Esch, L. Petaccia, S. Lizzit and K. Kern, J. Am. Chem. Soc., 2008, 130, 2108–2113 CrossRef CAS PubMed.
- G. Wang, Y. Ling, D. A. Wheeler, K. E. N. George, K. Horsley, C. Heske, J. Z. Zhang and Y. Li, Nano Lett., 2011, 11, 3503–3509 CrossRef CAS PubMed.
- A. Mettenbörger, T. Singh, A. P. Singh, T. T. Järvi, M. Moseler, M. Valldor and S. Mathur, Int. J. Hydrogen Energy, 2014, 39, 4828–4835 CrossRef PubMed.
- Y. S. Hu, A. Kleiman-Shwarsctein, A. J. Forman, D. Hazen, J.-N. Park and E. W. McFarland, Chem. Mater., 2008, 20, 3803–3805 CrossRef CAS.
- S. U. M. Khan and J. Akikusa, J. Phys. Chem. B, 1999, 103, 7184–7189 CrossRef CAS.
- S. Kumari, A. P. Singh, C. Tripathi, D. Chauhan, S. Dass, R. Shrivastav, V. Gupta, K. Sreenivas and V. R. Satsangi, Int. J. Photoenergy, 2007, 2007, 1–6 CrossRef PubMed.
- C.-Y. Chang, C.-H. Wang, C.-J. Tseng, K.-W. Cheng, L.-W. Hourng and B. T. Tsai, Int. J. Hydrogen Energy, 2012, 37, 13616–13622 CrossRef CAS PubMed.
- K. Zhang, X. Shi, J. K. Kim, J. S. Lee and J. H. Park, Nanoscale, 2013, 5, 1939–1944 RSC.
- J. Young Kim, J.-W. Jang, D. Hyun Youn, J. Yul Kim, E. Sun Kim and J. Sung Lee, RSC Adv., 2012, 2, 9415–9422 RSC.
- J. Y. Kim, G. Magesh, D. H. Youn, J.-W. Jang, J. Kubota, K. Domen and J. S. Lee, Sci. Rep., 2013, 3, 1–8 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra02934a |
| This journal is © The Royal Society of Chemistry 2015 |