Sensitive mutant DNA biomarker detection based on magnetic nanoparticles and nicking endonuclease assisted fluorescence signal amplification†
Na Li,
Zhong Feng Gao,
Bei Hua Kang,
Nian Bing Li* and
Hong Qun Luo*
Key Laboratory of Eco-environments in Three Gorges Reservoir Region (Southwest University), Ministry of Education, School of Chemistry and Chemical Engineering, Chongqing 400715, China. E-mail: linb@swu.edu.cn; luohq@swu.edu.cn; Fax: +86-23-68253237; Tel: +86-23-68253237
First published on 10th February 2015
Abstract
Based on the nicking endonuclease (NEase)-assisted target recycling and magnetic nanoparticle (MNPs) separation process via the streptavidin–biotin system, we developed a new, signal amplified and ultrasensitive fluorescent biosensor for the detection of mutant human p53 gene. The target mutant DNA hybridizes with the loop portion of a hairpin probe (HP) modified with biotin and a fluorescein isothiocyanate dye (FITC) at its 5′ and 3′ ends separately and forms a nicking site for NEase, which cleaves the HP and releases the target DNA. The released target DNA again hybridizes with the intact HP and initiates the DNA recycling process with the assistance of NEase, leading to the cleavage of a large number of HPs and detachment of the biotin labeled part with the FITC tagged signal portion. Only these cleaved fragments corresponding to target DNAs could remain in solution and function as a signaling flare, while the biotin labeled sequences including intact hairpin probes could be trapped and removed by the streptavidin coated MNPs. The developed method exhibits a detection limit as low as 198 fM and high discrimination efficiency toward a single-base mismatched sequence. Therefore, the novel NEase-amplified magnetic nanoparticle assay has great potential for sensitive and accurate detection of trace amounts of DNA in clinical diagnosis and biomedical research.
Introduction
Methods for the sensitive detection of sequence-specific DNA at low concentration are significant in clinical diagnostics, food safety monitoring, and biodefense applications.1–3 Recently, signal amplification has become an important concept to detect trace specific DNA sequences, since it exhibited exceptional promise by using labels, such as enzymes, nanomaterials, and DNA biobarcodes.4–10 Although the traditional polymerase chain reaction (PCR) has ultra-high sensitivity because of enzymatic amplification, it suffers from relatively complex, costly equipment and complicated handling procedures.11–13 These drawbacks have, to some extent, limited the practical application of this procedure under resource-limited conditions. Therefore, there has been renewed interest in sensitively detecting specific DNA sequences.14–19It is well known that two strategies have been used widely to improve the sensitivity of different sensors, that is, lowered background as well as signal amplified strategy.20 Firstly, graphene oxide, gold nanoparticles, and carbon nanotubes are widespread materials since they can serve as nanoquenchers to lower the background signal efficiently and thus improve the sensitivity of sensors.21–24 However, designing a sensor with lowered background signal still remains challenging until now. Secondly, an outstanding example of signal amplified detection is by the virtue of enzymes including horseradish peroxidase as well as DNAzyme. Wide applications of enzymes make amplified detection available and, hence, large signal-to-noise ratio is reached.25–28 However, few fluorescence detections using signal amplified strategies have been developed based on enzymes compared with electrochemical and colorimetric detections. Here, we developed a simpler and easier assay suitable for the quantification of mutant human p53 gene, combining the advantageous properties of the magnetic nanoparticles (MNPs)-based easy separation/collection process after enzyme-assisted target DNA recycling amplification strategy.
In this approach, the mutant p53 genes can hybridize with the hairpin DNA probes and create nicking sites for NEase to initiate the mutant p53 DNA recycling amplification process with the cleavage of numerous hairpin probes. Compared to the traditional hybridization assay at a ratio of target-to-signal with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry, nuclease-catalyzed reaction with great substrate turnover power employs a “catalyst” to interact with multiple probes and reach linear signal enlargement. And streptavidin-coated magnetic particles are used to remove the biotin-labeled DNA sequences including the intact probes via the streptavidin–biotin system, resulting in a minimum matrix effect.29–31
1 stoichiometry, nuclease-catalyzed reaction with great substrate turnover power employs a “catalyst” to interact with multiple probes and reach linear signal enlargement. And streptavidin-coated magnetic particles are used to remove the biotin-labeled DNA sequences including the intact probes via the streptavidin–biotin system, resulting in a minimum matrix effect.29–31
Experimental section
Reagents and chemicals
Streptavidin-coated magnetic nanoparticles (MNPs, 1 μm in diameter, 10 mg mL−1) were purchased from Zhengzhou Innosep Biosciences Co., Ltd. (Zhengzhou, China). The nicking enzyme Nt.AlwI (10 U μL−1, an endonuclease that recognizes a specific DNA sequence of 5′-GGATC-3′ in a double-stranded DNA) and 10× CutSmart™ Buffer (20 mM Tris–HAc, 50 mM KAc, 10 mM MgAc2, 100 μg mL−1 bovine serum albumin (BSA), pH 7.9) were purchased from New England Biolabs, Inc. (Beijing, China). And another buffer solution involved in this study was 10 mM Tris–HCl buffer (50 mM NaCl, 10 mM MgCl2, pH 7.9).The HPLC-purified oligonucleotide sequences listed in Table S1 (ESI†) were synthesized and labeled by Sangon Biotech Co., Ltd., Shanghai, China. The hairpin capture probe (HP) was heated at 90 °C for 5 min, and then cooled down to room temperature slowly before use. All other reagents and chemicals were of analytical reagent grade and used as received. Aqueous solutions were prepared using ultrapure water (specific resistance of 18.2 MΩ cm).
Apparatus
An F-4500 spectrofluorophotometer (Hitachi, Tokyo, Japan) was used to record the fluorescence spectra and measure the fluorescence intensities with equipment of a 150 W xenon lamp. Besides, its slits (Ex/Em) were 10.0/10.0 nm and the photomultiplier voltage was set at 400 V in the assay. The emission spectra were obtained by scanning wavelength from 505 to 630 nm upon excitation at 485 nm. A rapid mixing device (Ronghua Instrument Plant, Jiangsu, China) was used for mixing solutions completely. A pHS-3C pH meter (Shanghai Analytical Instrument Factory, Shanghai, China) was used for adjusting pH values.Fluorescence detection of the mutant p53 genes
The procedure can be divided into two primary parts: homogenous, enzyme-assisted signal amplification and solid-phase-based separation process via the streptavidin–biotin system. The nicking endonuclease-assisted amplified target DNA detection was performed as follows: 4 μL of 10× CutSmart™ Buffer, 3.5 μL of HP, and 5 μL of target DNA with different concentrations were mixed together and the mixture was diluted to the volume of 40 μL with ultrapure water, followed by incubation at 45 °C for 30 min. Then, the Nt.AlwI enzyme (10 μL, 0.3 U μL−1) was added. Fluorescence signal amplification was carried out at 45 °C for 90 min. The above solution contained 0.7 μM HP, 0.06 U μL−1 Nt.AlwI enzyme, 1× CutSmart™ Buffer and different concentrations of target DNA in a volume of 50 μL. Thereafter, the biotin labeled sequences can be separated using MNPs by the streptavidin–biotin interaction. The MNPs needed a preparation process before experiment. Homogenously dispersed MNPs (10 mg mL−1) with the volume of 20 μL were washed three times with 200 μL of 10 mM Tris–HCl buffer (50 mM NaCl, 10 mM MgCl2, pH 7.9) to remove the preservative and were dispersed in 450 μL of Tris–HCl buffer before use. Then, the Nt.AlwI enzyme digestion samples were incubated with the above prepared MNPs in a dark environment for 30 min at room temperature with gently shaking, followed by a separation by ferromagnet. The resulting supernatants were used for fluorescence detection.Results and discussion
Design strategy of the mutant p53 genes detection
The schematic diagram of Scheme 1 illustrates the principle of our novel strategy for magnetic nanoparticle assay. By virtue of the ability to recognize the specific sequence 5′-GGATC-3′/3′-CCTAG-5′ of double-strand DNA, the nicking enzyme Nt.AlwI can selectively create a single strand break at the site four random nucleotides downstream of the recognition sequence (5′-GGATC-3′)19 and our newly developed system could be used as a signal amplifier. Then, we designed a detection method for target DNA, which includes a hairpin capture probe (HP). HP contains the recognition sequence (-GGATC-) of nicking endonuclease and is tagged with biotin and a fluorescein isothiocyanate dye (FITC) at its 5′ and 3′ ends separately. An important component of the HP was the target-binding part (the loop of the HP), which was complementary with the target DNA. Duplex strand DNA would generate in the presence of the target DNA, and the HP could be cleaved by the Nt.AlwI enzyme, making detachment of biotin tag and the signaling segment. In order to recognize and detect the reacted DNA probes from the unreacted ones, we proposed a novel solid-phase-based method for separation, making full use of the streptavidin–biotin interaction. Only these cleaved DNA fragments corresponding to target DNAs could remain in solution and serve as the signaling element. However, the integrated DNA probes would be trapped and removed by streptavidin coated magnetic nanoparticles. Because of the signal amplification, the detection of trace amounts of the mutant human p53 gene can thus be reached. | ||
| Scheme 1 Schematic illustration of sensitive detection of mutant DNA biomarkers based on magnetic nanoparticles and nicking endonuclease assisted signal amplification. | ||
Optimization of assay conditions
To achieve the optimal condition for the assay, we explored the effect of different factors employing fluorescence signal increase percentage (F/F0 − 1) as standard, where F and F0 are the fluorescence intensities at 525 nm under excitation of 485 nm gained in the presence and absence of target DNA, respectively. It is well known that a higher fluorescence signal increase percentage (F/F0 − 1) is more favorable to obtain higher sensitivity of the detection method. The effect of Nt.AlwI enzyme at different concentrations was investigated (Fig. S1, ESI†). After measurements, the fluorescence intensity at each concentration point was recorded. We noted that fluorescence signal increase percentage (F/F0 − 1) increased and got to a maximum when the concentration of Nt.AlwI enzyme was 0.06 U μL−1. Therefore, 0.06 U μL−1 Nt.AlwI enzyme was chosen as the optimum concentration in the following experiments.In order to obtain the highly sensitive detection of target DNA by using the Nt.AlwI enzyme, the time for nicking reaction was studied and optimized. We tested the fluorescence intensity of the system at various incubation times, ranging from 0.25 to 2 h in the case of a fixed amount of 0.06 U μL−1 Nt.AlwI enzyme (Fig. S2, ESI†). Similar to the phenomenon observed for optimization of Nt.AlwI enzyme concentration, the fluorescence signal increase percentage (F/F0 − 1) elevated and kept stable in 90 min and longer reaction time did not increase the fluorescence ratio any more. As a result, 90 min was selected as the nicking time in the nicking step.
It is noted that an appropriate incubation temperature for enzyme is crucial, as proper temperature could guarantee the activity of Nt.AlwI enzyme and help the cleaved DNA probes dissociate quickly. Therefore, the effect of incubation temperature in the range of 35 to 60 °C was measured. When the temperature increased, the fluorescence signal increase percentage (F/F0 − 1) increased first and then decreased, with the maximum at 45 °C (see Fig. S3, ESI†). Therefore, 45 °C was chosen as the optimum reaction temperature.
Quantitation of the target DNA
Considering the appreciable changes in fluorescence property of the system toward target DNA, the potential of developing a novel fluorescence method for the quantification of mutant human p53 gene was assessed. In the absence of target DNA, the solution emitted little fluorescence. The fluorescence spectrum of the sensor upon the addition of target is shown in Fig. 1. It was noted that the fluorescence intensity at 525 nm was highly sensitive to target DNA and increased with increasing of target DNA. And it can be seen that the fluorescence intensity exhibited a good linear relationship with the value of target DNA concentration in the dynamic range from 1 to 20 pM (Fig. 1, inset). The regression equation was expressed as F = 3.174C + 27.33, with a correlation coefficient R2 of 0.9965, where F is the fluorescence intensity at 525 nm and C is the concentration of target DNA. The detection limit, based on 3σ/slope (where σ is the standard deviation of the blank measures, n = 11), was calculated to be 198 fM, which was comparable with most DNA detection methods using the magnetic nanoparticles strategy (Table 1).32–40 The high sensitivity of the sensor arises from several factors. First, homogenous reaction greatly reduces the surface effects and increases the opportunity for molecular recognition. Second, the increased interstrand spacing due to the homogenous reaction in the liquid phase environment offers the room for using Nt.AlwI enzyme amplification, which greatly increases the fluorescence signal.41–43 Furthermore, a series of 6 repetitive samples with 8 pM target DNA were used to investigate the precision of the system response, and a relative standard deviation (RSD) of 3.8% was obtained, demonstrating a good repeatability of the assay.| Method | Limit of detection (pM) | Linear range (pM) | Ref. |
|---|---|---|---|
| Giant magnetoresistive biochip | 10 | — | 32 |
| Fluorescence | 100 | 780–2500 | 33 |
| Colorimetry | 10 | — | 34 |
| Colorimetry | 0.05 | 0.05–5 | 35 |
| Electrochemistry | 1.55 | 44–2000 | 36 |
| Bio-biocode | 1 | 10–100 | 29 |
| Flow cytometry | 3.2 | — | 37 |
| Flow cytometry | 0.5 | 1–3000 | 38 |
| Electrochemiluminescence | 0.0032 | 0.01–10 | 39 |
| Surface enhanced Raman spectroscopy | 10 | 10–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
40 |
| Fluorescence | 0.198 | 1–20 | This work |
Selectivity of target detection
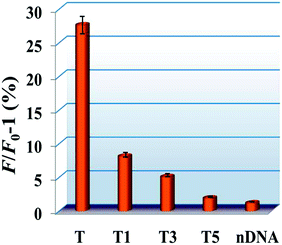
The specificity of the experiment was evaluated by using the same concentration of five kinds of DNA sequences, including the perfectly complementary sequence (T), single-base mismatched sequence (T1), three-base mismatched sequence (T3), five-base mismatched sequence (T5), and non-complementary DNA sequence (nDNA). As depicted in Fig. 2, we found that the fluorescence signal increase percentage (F/F0 − 1) was the highest after the addition of T. Interestingly, the fluorescence signal increase percentage increased slightly with addition of T1. And T3 and T5 exhibited small signal change compared to nDNA. Moreover, we observed that the DNA with one base mismatch led to a much lower fluorescence signal increase percentage compared with T. These results suggest that the developed sensor has an eminent specificity for recognizing and detecting target DNA from mismatched DNA. And it can be attributed to the use of stem-loop DNA probe as one of the sensing elements.Analytical application to complex matrix
To investigate whether the proposed method can be applied to detect target DNA in real clinical samples, we employed the human serum as model matrix. We performed recovery experiments in a complex sample matrix, 0.2% human serum (diluted with 1× CutSmart™ Buffer). And then the above human serum sample was spiked with the target at three concentrations (2, 8, and 15 pM). As shown in Fig. 3, the fluorescence signal increase percentage (F/F0 − 1) obtained from the serum sample changed slightly compared to that from the 1× CutSmart™ Buffer. And the corresponding recovery was calculated to be 107.84 ± 4.8%, 109.94 ± 1.2%, and 97.59 ± 3.4% for 2, 8, and 15 pM, respectively. The result indicated that our assay had great potential for DNA detection in real biological samples. | ||
| Fig. 3 Fluorescence signal increase percentage (F/F0 − 1) of the sensor for different concentrations of target DNA (2, 8, 15 pM) in 1× CutSmart™ Buffer and serum sample, respectively. | ||
Conclusions
In conclusion, a homogeneous enzyme-assisted signal amplification combined with solid-phase-based magnetic separation process was proposed for DNA detection. The method combined the easiness of magnetic separation and the nicking endonuclease assisted target recycling for amplification, endowing the method with simplicity and high sensitivity with a detection limit of 198 fM. And the proposed method can also discriminate single-base mismatched DNA from the target. Although we only challenged the detection of the mutant human p53 gene, the developed method can be extended for the detection of trace amounts of other DNA sequences with the selection of different nucleases and carefully designed DNA sequences.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (no. 21273174) and the Municipal Science Foundation of Chongqing City (no. CSTC–2013jjB00002).Notes and references
- J. Huang, Y. R. Wu, Y. Chen, Z. Zhu, X. H. Yang, C. Y. J. Yang, K. M. Wang and W. H. Tan, Angew. Chem., Int. Ed., 2011, 50, 401–404 CrossRef CAS PubMed.
- H. F. Dong, W. C. Gao, F. Yan, H. X. Ji and H. X. Ju, Anal. Chem., 2010, 82, 5511–5517 CrossRef CAS PubMed.
- F. Zhou, B. Li and J. Ma, RSC Adv., 2015, 5, 4019–4025 RSC.
- F. Patolsky, A. Lichtenstein and I. Willner, Nat. Biotechnol., 2001, 19, 253–257 CrossRef CAS PubMed.
- H. F. Cui, T.-B. Xu, Y.-L. Sun, A.-W. Zhou, Y.-H. Cui, W. Liu and J. H. T. Luong, Anal. Chem., 2015, 87, 1358–1365 CrossRef CAS PubMed.
- W. Gao, H. Dong, J. Lei, H. Ji and H. Ju, Chem. Commun., 2011, 47, 5220–5222 RSC.
- J. Wang, G. Liu and A. Merkoci, J. Am. Chem. Soc., 2003, 125, 3214–3215 CrossRef CAS PubMed.
- X. Zhu, H. Zhang, C. Feng, Z. Ye and G. Li, RSC Adv., 2014, 4, 2421–2426 RSC.
- H. Li, Z. Sun, W. Zhong, N. Hao, D. Xu and H. Y. Chen, Anal. Chem., 2010, 82, 5477–5483 CrossRef CAS PubMed.
- J. Xu, B. Jiang, J. Su, Y. Xiang, R. Yuan and Y. Chai, Chem. Commun., 2012, 48, 3309–3311 RSC.
- Z. Cheglakov, Y. Weizmann, M. K. Beissenhirtz and I. Willner, Chem. Commun., 2006, 42, 3205–3207 RSC.
- P. S. C. Wu, K. Ozawa, S. P. Lim, S. G. Vasudevan, N. E. Dixon and G. Otting, Angew. Chem., Int. Ed., 2007, 46, 3356–3358 CrossRef CAS PubMed.
- M. Wassenegger, Mol. Biotechnol., 2001, 17, 73–82 CrossRef CAS PubMed.
- Y. Weizmann, Z. Cheglakov, V. Pavlov and I. Willner, Angew. Chem., Int. Ed., 2006, 45, 2238–2242 CrossRef CAS PubMed.
- Y. Weizmann, Z. Cheglakov and I. Willner, J. Am. Chem. Soc., 2008, 130, 17224–17225 CrossRef CAS PubMed.
- Y. Weizmann, M. K. Beissenhirtz, Z. C. R. Nowarski, M. Kotler and I. Willner, Angew. Chem., Int. Ed., 2006, 45, 7384–7388 CrossRef CAS PubMed.
- J. Elbaz, M. Moshe, B. Shlyahovsky and I. Willner, Chem.–Eur. J., 2009, 15, 3411–3418 CrossRef CAS PubMed.
- W. Xu, X. Xue, T. Li, H. Zeng and X. Liu, Angew. Chem., Int. Ed., 2009, 48, 6849–6852 CrossRef CAS PubMed.
- S. Liu, T. Liu and L. Wang, Chem. Commun., 2015, 51, 176–179 RSC.
- L.-M. Lu, X.-B. Zhang, R.-M. Kong, B. Yang and W. H. Tan, J. Am. Chem. Soc., 2011, 133, 11686–11691 CrossRef CAS PubMed.
- J. Ge, Z.-M. Huang, Q. Xi, R.-U. Yu, J. H. Qiang and X. Chu, Chem. Commun., 2014, 50, 11879–11882 RSC.
- F. Gao, P. Cui, X. Chen, Q. Ye, M. Li and L. Wang, Analyst, 2011, 136, 3973–3980 RSC.
- L. Q. Guo, N. Yin, D. D. Nie, J. R. Gan, M. J. Li, F. F. Fua and G. N. Chen, Analyst, 2011, 136, 1632–1636 RSC.
- C. Hu, Q. Xi, J. Ge, F.-Y. Luo, L.-J. Tang, J.-H. Jiang and R.-Q. Yu, RSC Adv., 2014, 4, 64252–64257 RSC.
- H. Zhang, Y. P. Shi, F. Lan, Y. Pan, Y. K. Lin, J. Z. Lv, Z. H. Zhu, Q. Jiang and C. Q. Yi, Chem. Commun., 2014, 50, 1848–1850 RSC.
- H. Zhou, Y.-Y. Zhang, J. Liu, J.-J. Xu and H.-Y. Chen, Chem. Commun., 2013, 49, 2246–2248 RSC.
- M. D. Xu, J. Y. Zhuang, X. Chen, G. N. Chen and D. P. Tang, Chem. Commun., 2013, 49, 7304–7306 RSC.
- L. Shi, Y. Yu, Z. Chen, L. Zhang, S. He, Q. Shi and H. Yang, RSC Adv., 2015, 5, 11541–11548 RSC.
- Z. P. Zhou, T. Li, H. D. Huang, Y. Chen, F. Liu, C. Z. Huang and N. Li, Chem. Commun., 2014, 50, 13373–13376 RSC.
- C. E. Probst, P. Zrazhevskiy and X. H. Gao, J. Am. Chem. Soc., 2011, 133, 17126–17129 CrossRef CAS PubMed.
- W. J. Zhou, J. Su, Y. Q. Chai, R. Yuan and Y. Xiang, Biosens. Bioelectron., 2014, 53, 494–498 CrossRef CAS PubMed.
- L. Xu, H. Yu, M. S. Akhras, S.-J. Han, S. Osterfeld, R. L. White, N. Pourmand and S. X. Wang, Biosens. Bioelectron., 2008, 24, 99–103 CrossRef CAS PubMed.
- W. B. Qiang, W. Li, X. Q. Li, X. Chen and D. K. Xu, Chem. Sci., 2014, 5, 3018–3024 RSC.
- S. Persano, P. Valentini, J. H. Kim and P. P. Pompa, Chem. Commun., 2013, 49, 10605–10607 RSC.
- Y. Z. Liu, Z. T. Wu, G. H. Zhou, Z. K. He, X. D. Zhou, A. G. Shen and J. M. Hu, Chem. Commun., 2012, 48, 3164–3166 RSC.
- X. R. Zhang, H. R. Su, S. Bi, S. G. Li and S. S. Zhang, Biosens. Bioelectron., 2009, 24, 2730–2734 CrossRef CAS PubMed.
- J. Lu, I. T. Paulsen and D. Y. Jin, Anal. Chem., 2013, 85, 8240–8245 CrossRef CAS PubMed.
- W. Ren, H. M. Liu, W. X. Yang, Y. L. Fan, L. Yang, Y. C. Wang, C. H. Liu and Z. P. Li, Biosens. Bioelectron., 2013, 49, 380–386 CrossRef CAS PubMed.
- G. F. Jie, J. Zhang, G. X. Jie and L. Wang, Biosens. Bioelectron., 2014, 52, 69–75 CrossRef CAS PubMed.
- J.-M. Li, W.-F. Ma, L.-J. You, J. Guo, J. Hu and C.-C. Wang, Langmuir, 2013, 29, 6147–6155 CrossRef CAS PubMed.
- H. Pei, X. L. Zuo, D. Zhu, Q. Huang and C. H. Fan, Acc. Chem. Res., 2014, 47, 550–559 CrossRef CAS PubMed.
- Y. L. Wen, H. Pei, Y. Shen, J. J. Xi, M. H. Lin, N. Lu, X. Z. Shen, J. Li and C. H. Fan, Sci. Rep., 2012, 2, 867 Search PubMed.
- T. M. Squires, R. J. Messinger and S. R. Manalis, Nat. Biotechnol., 2008, 26, 417–426 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: The sequence of oligomers, Fig. S1–S3. See DOI: 10.1039/c4ra17059h |
| This journal is © The Royal Society of Chemistry 2015 |