Reversibly cross-linked poly(ethylene glycol)–poly(amino acid)s copolymer micelles: a promising approach to overcome the extracellular stability versus intracellular drug release challenge
Abstract
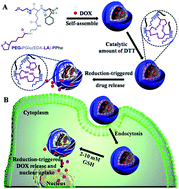
Reversibly shell cross-linked micelles based on a lipoic acid (LA) decorated triblock copolymer poly(ethylene glycol)-b-poly(γ-benzyl-L-glutamate)-b-poly(L-phenylalanine) (PEG–PGlu(EDA–LA)–PPhe) have been developed for active loading and efficient intracellular delivery of DOX. The triblock copolymer was synthesized through consecutive ring-opening polymerization of cyclic monomers γ-benzyl-L-glutamate N-carboxyanhydride (BLG-NCA) and L-phenylalanine N-carboxyanhydride (Phe-NCA) using amino-terminated poly(ethylene glycol) (PEG–NH2) as macroinitiator, followed by conjugation with LA for reversible cross-linking. The amphiphilic polymer was self-assembled to core shell corona micelles, which could be further crosslinked in the presence of a catalytic amount of dithiothreitol (DTT) in phosphate buffer (pH 7.4) to form shell-cross-linking micelles (SCLM). The SCLM showed excellent stability under physiological conditions but rapid dissociation and drug release in reductive environments mimicking those of the cytoplasm and the cell nucleus. Confocal laser scanning microscopy further demonstrated that DOX was delivered and released into the nuclei of HeLa cells following 12 h incubation with DOX-loaded SCLM. MTT assays revealed that DOX-loaded SCLM had similar anti-tumor activity as non-cross-linked micelles (NCLM) for HeLa cells following 48 h incubation. PEG–PGlu(EDA–LA)–PPhe micelles displayed low cytotoxicity up to a concentration of 1.0 mg mL−1. These biodegradable reversibly shell-cross-linked micelles provide a promising platform for intelligent intracellular drug delivery in clinical chemotherapy.

 Please wait while we load your content...
Please wait while we load your content...