Effects of different functional groups on metastatic behavior of SPC-A-1/human lung cancer cells in self-assembled monolayers†
Guan Zheng‡
a,
Lihua Li‡b,
Mei Lib,
Xinglong Fengb,
Xiaobing Pub,
Baoliang Zhangab,
Peng Yuc,
Guanping Hea,
Yu Zhangb and
Hong Xia*ab
aSouthern Medical University, Guangzhou, Guangdong, People's Republic of China. E-mail: gzxiahong2@126.com
bDepartment of Orthopedics, Guangzhou General Hospital of Guangzhou Military Command, 111 Liuhua Road, Guangzhou 510010, China
cNational Engineering Research Center for Tissue Restoration and Reconstruction, South China University of Technology, Guangzhou 510641, China
First published on 21st April 2015
Abstract
Cancer cell processes and tumor microenvironment play key roles in metastasis, which is the primary cause of death from lung cancer. We utilized self-assembled monolayers terminated with different functional groups (–CH3, –OH, –COOH, –NH2, –SH) to explore their effects on the metastatic behavior of human lung cancer cells (SPC-A-1) in vitro. Cell adhesion was investigated by scanning electron microscopy and 4,6-diamidino-2-phenylindole staining; rhodamine–phalloidin was further used to directly label microfilaments. Viability/cytotoxicity staining and lactate dehydrogenase assays were performed to determine cell toxicity, and apoptosis was investigated by flow cytometry. Transwell assays were used to analyze the relationship between cell migration and surface chemistry. Gene expression associated with metastatic behavior was measured by quantitative real-time PCR. Our results demonstrated that –NH2 and –COOH groups promote cell adhesion and spreading. Although the –OH group did not affect cell survival and mobility, –CH3 and –SH groups were toxic to SPC-A-1 cells after 24 h of incubation. Furthermore, the –SH group inhibited cell migration and increased gene expression of E-cadherin. The addition of the –SH group has a potential application in new biomaterials or drugs for lung cancer metastasis therapy.
1. Introduction
Lung cancer is the most common cause of death from cancer in both men and women, accounting for approximately 28% of all cancer deaths in America and nearly 2.4 million years of life lost in 2010.1 Although surgical resection and chemotherapy can be successful in some patients, the long-term prognosis for patients is poor because of tumor metastasis, especially in the brain and bones, which represent the primary cause of death from lung cancer and is responsible for 90% of all morbidity.2 Therefore, improved treatment largely depend on interrupting the metastatic process, which is also the direction of basic tumor research.3–6 However, understanding the complexity of the mechanisms by which metastasis occurs is daunting. The metastatic process is classically simplified into an orderly sequence of basic steps: local invasion, intravasation, survival in the circulation, extravasation and colonization. This sequence has helped to rationalize the complex set of biological properties that must be acquired for lung cancer to progress toward overt metastatic disease.7 All these steps are supported by the functions of cancer cells themselves and their microenvironment.3,7,8Self-assembled monolayers (SAMs) are widely used as a model surface to explore the effect of surface chemistry on cell functions.9–13 Functional group-terminated surfaces have been demonstrated to affect metastatic behaviors such as cell morphology, adhesion, proliferation and migration in hepatoma cells (HepG2s), MCF-7 breast cancer cells and human osteosarcoma cells.14–16 These reports demonstrated that different functional groups have different effects on cancer cells.
Nonetheless, the effects of different functional groups on the metastatic behaviors of SPC-A-1/human lung cancer cells in SAMs remain to be elucidated. Various chemically modified surfaces were prepared with typical functional groups including methyl (–CH3), hydroxyl (–OH), carboxyl (–COOH), amino (–NH2) and mercapto (–SH) groups. Cell morphology, adhesion, toxicity, apoptosis and migration were then examined to characterize the effects of these surfaces on SPC-A-1 cells in vitro, which is necessary for a better understanding of cell–biomaterial interactions in the tumor microenvironment. The ultimate goal of our study is to design appropriate biomaterials or drugs to reduce the incidence of metastasis in lung cancer.
2. Materials and methods
2.1. Preparation of self-assembled monolayers (SAMs) on Au surfaces
Au surfaces (thickness of ∼40 nm) were prepared on glass coverslips by ion beam sputtering (IBS) technique with a Ti layer (thickness of ∼10 nm) as a buffer. The wafers were rinsed with ethyl alcohol and triple-distilled water and cleaned with a highly acidic piranha solution (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (v/v) H2SO4
3 (v/v) H2SO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O2) for 10 min.17 The wafers were washed with ethyl alcohol in an ultrasonic bath for 10 min and then exposed to ultraviolet rays for 30 min. Finally, the Au substrates were immersed in an ethanol solution containing 1 mM 11-mercapto-1-undecane (–OH, Sigma, St. Louis, MO, USA), 12-mercaptododecanoic acid (–COOH, Sigma), 11-amino-1-undecanethiol hydrochloride (–NH2, Sigma), 1-dodecanethiol (–CH3, Sigma), or 11-undecanedithiol (–SH, Sigma) for 4 h at room temperature (25 °C).
H2O2) for 10 min.17 The wafers were washed with ethyl alcohol in an ultrasonic bath for 10 min and then exposed to ultraviolet rays for 30 min. Finally, the Au substrates were immersed in an ethanol solution containing 1 mM 11-mercapto-1-undecane (–OH, Sigma, St. Louis, MO, USA), 12-mercaptododecanoic acid (–COOH, Sigma), 11-amino-1-undecanethiol hydrochloride (–NH2, Sigma), 1-dodecanethiol (–CH3, Sigma), or 11-undecanedithiol (–SH, Sigma) for 4 h at room temperature (25 °C).
2.2. Substrate characterization
Model surfaces were characterized by contact angle measurements. Ambient air–water substrate contact angle measurements (1 μL ultra-pure H2O) were obtained using the contact angle system OCA15 (Dataphysics, Filderstadt, Germany) fitted with a digital camera and then analyzed using in-house image analysis software.2.3. Cell culture
SPC-A-1 cells (ATCC, Manassas, VA, USA) were cultured at 37 °C under a 5% CO2 atmosphere in RPMI 1640 Medium (Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS, HyClone, Smithfield, Australia). The medium was changed every 48 h.2.4. Cell adhesion
2.5. Cell migration assays
Migration assays were performed using Transwell (Falcon-Corning, Tewksbury, MA, USA; pore size, 8 μm) 24-well plates. Prior to performing the assay, the cells were pretreated on different SAM surfaces for 24 h, digested with trypsin and resuspended at a density of 5 × 104 cells in 150 μL of serum-free medium. The cells were then placed in the upper chamber, and 800 μL of the same medium containing 20% FBS was placed in the lower chamber. The plates were incubated for 24 h at 37 °C in 5% CO2. Next, the cells were fixed in 100% methanol for 30 min and then stained with 0.05% crystal violet in PBS for 30 min. The cells from the upper side of the filters were removed using cotton-tipped swabs, and the filters were washed with PBS. The cells from the underside of the filters were examined and counted using a microscope. Each group of pretreated cells was plated in triplicate in each experiment, and each experiment was repeated at least three times.18–202.6. Cell toxicity
2.7. Cell apoptosis
SPC-A-1 cells were cultured for 24 h, washed three times with PBS, and then resuspended in 100 μL of RPMI 1640 with 1% FBS. The positive control was pretreated by co-culturing with cisplatin (10 μmol L−1) for 24 h. The cells were stained in a 96-well microplate with Guava Nexin Reagent (Millipore, Billerica, MA, USA), a pre-made cocktail containing Annexin V-PE and 7-AAD in a final volume of 200 μL. After 20 min incubation at room temperature, the samples were analyzed using a Guava EasyCyte 5HT flow cytometer (Millipore, USA), and the data were analyzed using Guava Nexin Software v2.2.2.2.8. Quantitative real-time PCR (qRT-PCR)
PrimeScript RT Master Mix and SYBR Premix Ex Taq II (Takara) were used for cDNA synthesis and SYBR Green qRT-PCR according to the manufacturer's protocols, respectively. The qRT-PCR assays were performed by Rotor-Gene Q (Qiagen). All reactions were carried out in triplicate and the qRT-PCR results were analyzed using the Rotor-Gene Real-Time analysis software 6.0. Then relative gene expression of SPC-A-1 cells attached to the SAMs after incubation for 24 h was calculated using the 2−ΔΔct method. The cycling protocol was set as follows: 95 °C for 10 min, followed by 45 cycles with each cycle made of 95 °C for 15 s, 60 °C for 20 s and 72 °C for 20 s. The primers used in the experiments were as follows: E-cadherin: F,5′-TGAAGGTGACAGAGCCTCTGGAT-3′ and R,5′-TGGGTGAATTCGGGCTTGTT-3′; β-catenin: F,5′-CCAGCGTGGACAATGGCTAC-3′ and R,5′-TGAGCTCGAGTCATTGCATAC-3′; GAPDH: F,5′-GGAGCGAGATCCCTCCAAAAT-3′ and R,5′-GGCTGTTGTCATACTTCTCATGG-3′.222.9. Statistical analysis
All statistical computations were performed with GraphPad Prism 5. The data were analyzed by ANOVA (analysis of variance) followed by Dunnett's multiple comparison test. The values were considered significantly different when p < 0.05.3. Results
3.1. Water contact angles of different model surfaces
The contact angles using water as the solvent for chemical group-modified coverslips are shown in Fig. 1. The surfaces ranged from hydrophobic for CH3-terminated SAMs (103.4 ± 3.2°) to hydrophilic for OH-SAMs (39.7 ± 6.9°) and COOH-SAMs (50.1 ± 2.3°). The surfaces of NH2-SAMs and pristine gold (control group) had higher contact angle values of 68.9 ± 4.5° and 77.3 ± 0.5°, respectively. The contact angle for SH-SAMs was 87.8 ± 0.5°, which is relatively hydrophobic.233.2. Cell adhesion and spreading
After 24 h of incubation, SPC-A-1 cells exhibited various morphologies on different functional group-modified surfaces, as determined by SEM (Fig. 2). On the pristine gold surface (control), –NH2, and –COOH-modified surfaces, a flat morphology and lamellipodia formations were observed around the cell bodies. On the –OH and –SH modified surfaces, the cells displayed a polygonal or spindle-like shape with filopodia at the leading edge. Whereas a more rounded or spindle-like shape and a smaller contact were found for the cells on the –CH3 surface.Focal adhesion analysis further confirmed the initial cell spreading difference modulated by different chemical functional groups. Representative images of cytoskeleton organization are presented in Fig. 3.
DAPI staining was used to evaluate the initial adhesion of the SPC-A-1 cells. The number of adherent cells on different SAM-modified surfaces after 30, 60 and 90 min of incubation are shown in Fig. 4. Compared with the pristine gold surface, there were no differences in cell number among the –CH3, –SH, –OH and –COOH groups at 30 min, whereas the cell number on the surface with the –NH2 group was significantly increased (p < 0.001). The cell numbers on the –COOH (p < 0.01) and the –NH2 surfaces (p < 0.001) increased at 60 min. Although the –CH3 and –SH groups showed no significant increase during the 90 min incubation, the cell numbers on the other groups increased over time, especially for the –COOH and –NH2 groups. Interestingly, no obvious differences between the –OH and control groups were observed during the 90 min incubation. The adhesion numbers of SPC-A-1 cells followed the following trend: –NH2 ≥ –COOH > –OH ≥ Control ≫ –SH ≈ –CH3.
3.3. Cell migration
When SPC-A-1 cells were cultured with different chemical group-modified surfaces for 24 h, significant differences in cell migration from the original aggregate were observed. Many cells migrated out from their original aggregate to the other side on –COOH and control surfaces. However, relatively few cells migrated on the –CH3 and –SH substrates. Nevertheless, differences in behavior were apparent between the cells grown on the –NH2 and –OH surfaces. Consequently, cell migration on these SAM-modified surfaces followed the following trend: –COOH > –NH2 ≈ –OH ≫ –SH > –CH3 as shown in Fig. 5.3.4. Cell toxicity
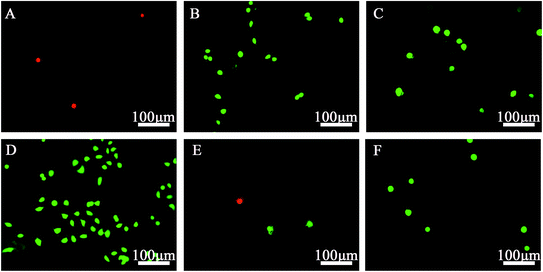
LDH is a cytoplasmic enzyme, and LDH activity is often associated with cell membrane damage and cell death.21 The results of LDH activities are shown in Fig. 6. The –CH3 and –SH groups showed significant toxicity toward SPC-A-1 cells compared with the pristine gold (Control) group (p < 0.05).Cell toxicity was further confirmed by viability/cytotoxicity staining (Fig. 7). Numerous viable (green) cells were observed on all SAM-modified surfaces except those with –CH3 and –SH groups, on which relatively few non-viable (red) cells were found. These results confirmed that these two groups are toxic to SPC-A-1 cells.
3.5. Cell apoptosis
To understand the possible mechanisms of the toxicity on different SAM-modified surfaces, SPC-A-1 cells were stained with PI and Annexin V-FITC and then analyzed by fluorescence-activated cell sorting (Fig. 8). CH3-SAMs and SH-SAMs promoted SPC-A-1 cell necrosis with 17.2% and 15.3% necrotic cells, respectively, compared with the controls (4.0%). SH-SAMs also promoted apoptosis with 6.7% apoptotic cells compared with the controls (1.0%). We found that the CH3-SAMs (20.5%) and SH-SAMs (22.0%) induced almost the same apoptosis/necrosis rates as a 10 μmol L−1 cisplatin-treated group (26.7%), and more information about the cisplatin's sensitive concentration to the SPC-A-1 cells can be get in ESI (Fig. S2 and S3†).3.6. Expression of genes involved in metastatic behaviors
We then proceeded to investigate the effects of different SAMs on the expression of target genes involved in metastatic behaviors of SPC-A-1 cells, and to study the molecular mechanism. To this end, we measured the mRNA expression levels of E-cadherin and β-catenin using qRT-PCR analysis. The results showed that the relative expression of E-cadherin was significantly down regulated with the –CH3, –OH, –NH2 groups (p < 0.01), but significantly up regulated with –COOH (p < 0.01) and –SH groups (p < 0.05). The ratio of β-catenin was significantly down regulated in –OH (p < 0.05), –NH2 groups, but those on the –COOH group showed a significant increase (p < 0.01). There was no significant change in β-catenin expression in the cells on the –CH3 and –SH monolayers.4. Discussion
Metastasis (from an initial primary tumor via angiogenesis, intravasation, survival in the bloodstream, extravasation and metastatic growth) is an inefficient process, and few released cancer cells complete the entire course. Changes in adhesive characteristics and motility of cells have long been suspected to play an important role in mediating the spread of malignant neoplasms.3,7,24,25 We used the SAM model to explore the effect of chemical groups on SPC-A-1 cells in vitro.First, cellular adhesion on biomaterial surfaces is a complex process that is affected by multiple factors. Nonspecific forces arise from the substrate's physicochemical characteristics, and specific forces can be presented as a result of receptor–ligand binding events between receptors on the cell surface and the physisorbed or chemisorbed proteins, peptides, and other bioactive factors on the material's surface.26 Surface wettability has been shown to be an important factor in protein adhesion to biomaterial surfaces, and protein adhesion forces were found to increase with contact time, consistent with the surface-induced conformational changes in proteins.27 Based on the morphology of single SPC-A-1 cell adhered onto different SAM-modified surfaces, the adhesion of SPC-A-1 cells changed in response to the surface chemistry (Fig. 2 and 3). Fewer cells adhered onto the –CH3 surface, and most exhibited a spindle shape; furthermore, the contact area was much smaller compared with the other groups. Conversely, SPC-A-1 cells on the –NH2 and –COOH surfaces exhibited more spreading, which is consistent with previous studies.14,16,26 Our results demonstrated that the hydrophobic CH3-SAMs and SH-SAMs inhibit the pro-adhesion of SPC-A-1 cells, whereas the hydrophilic NH2-SAMs and COOH-SAMs promote cell adhesion. However, the variations in cell adhesion and morphology observed for SPC-A-1 cells cannot be explained merely by the characteristics discussed above. For example, the cells on the –SH surfaces displayed a polygonal shape that was obviously different from the cells on the CH3-SAMs, even though they both produced high contact angles. The adhesion number on the OH-SAMs was smaller than that on the NH2-SAMs and COOH-SAMs, despite the higher contact angle. Thus, other characteristics including surface charge, surface energy and receptor–ligand binding should be considered.28,29
Second, cell viability plays important role in the process of metastasis, which is invariably affected by the tumor microenvironment. Our results (Fig. 6 and 7) demonstrated that the CH3-SAMs and SH-SAMs exert cell toxicity toward SPC-A-1 cells, indeed, these functional groups may promote cell apoptosis.30 Early apoptosis is also indicated by Annexin V-FITC-positivity and PI-negativity, and the results of the cell apoptosis assay supported the cell toxicity results described above.
Third, mobility is one of the primary events of the invasion-metastasis cascade.31 The basic migratory cycle includes the extension of a protrusion, the formation of stable attachments near the leading edge of the protrusion, translocation of the cell body forward, the release of adhesions and retraction at the cell rear. Lamellipodia and filopodia play a key role in cell migration. Previous studies have reported a similar relationship between cytoskeletal assembly and cell migration, and increased vinculin expression has been shown to reduce cell motility.24,32–34 In our study, although SPC-A-1 cell lamellipodia were easily observed on the NH2-SAMs and COOH-SAMs, lamellipodia were not found with the –CH3-SAMs (Fig. 2). Furthermore, numerous actin stress fiber bundles anchored to the plasma membrane were observed on the –COOH surfaces (Fig. 3), whereas stress fiber formation was largely inhibited on the –CH3 surfaces.
Finally, we found that SPC-A-1 cells exhibited high migration rates on the control and –COOH substrates yet lower migration rates on the –CH3 and –SH substrates (Fig. 5). To explore the mechanism by which these different functional groups affect metastatic behaviors, we focused on the expression of E-cadherin and β-catenin in these cells. E-cadherin, a transmembrane cell adhesion molecule, is associated with three types of cytoplasmic proteins (alpha-catenin, beta-catenin and plakoglobin), and the formation of the cadherin–catenins adhesion complex is indispensable for tight cell-to-cell adhesion in adherence junctions. Loss of cadherin-mediated adhesion may promote cell migration and subsequent dissemination of lung cancer cells.35–37 The qRT-PCR results (Fig. 9) showed a significant down-regulation of E-cadherin and β-catenin in cells on –NH2 groups, and such reduced levels are associated with tumour invasion, metastasis, and unfavorable prognosis. Although the migratory ability of SPC-A-1 cells seemed to be prohibited on the CH3-SAMs (Fig. 5), the ratio of E-cadherin was significantly down regulated on the –CH3 group. Accordingly, the use of CH3 group in lung cancer treatment should be reconsidered. However, E-cadherin was significantly up regulated on the SH-SAMs. Additional analyses are needed to explain the changes observed with the other chemical groups. To confirm these results, future studies will focus on alterations in larger set of genes and proteins.2,3,8,30
5. Conclusion
The data presented herein may be useful for a better understanding of cell–biomaterial interactions and for engineering new biomaterials or drugs. Our data indicate that SPC-A-1 cell behaviors, such as cell attachment efficiency, spreading behavior, actin stress fiber formation, and cell migration rates, are dependent on material surface chemistry. The –NH2 and –COOH groups promoted adhesion and spreading, and the –OH group did not affect cell survival or mobility, in contrast, –CH3 and –SH groups exhibited toxicity toward SPC-A-1 cells; furthermore, the –SH group inhibited cell migration and increased metastasis-related gene expression. These results demonstrate that the –SH chemical group is potentially useful for designing novel lung cancer treatment strategies.Acknowledgements
This study was supported by The National Natural Science Foundation of China (81271957, 81272507) and the Twelve five-year plan of military (bws11c065) and National Basic Research Program of China (Grant no. 2012CB619106) the Guangdong Key Laboratory of Orthopaedic Technology and Implant Materials in China ([2011]233-32). We also sincerely thank Jie Li, Xiaolan Wang and Qiaoting Hu for their assistance in the preparation of this manuscript.Notes and references
- R. Siegel, J. Ma, Z. Zou and A. Jemal, Ca-Cancer J. Clin., 2014, 64, 9–29 CrossRef PubMed.
- P. Mehlen and A. Puisieux, Nat. Rev. Cancer, 2006, 6, 449–458 CrossRef CAS PubMed.
- S. L. Wood, M. Pernemalm, P. A. Crosbie and A. D. Whetton, Cancer Treat. Rev., 2014, 40, 558–566 CrossRef CAS PubMed.
- J. Ferlay, H. R. Shin, F. Bray, D. Forman, C. Mathers and D. M. Parkin, Int. J. Cancer, 2010, 127, 2893–2917 CrossRef CAS PubMed.
- I. Sekine, H. Nokihara, N. Yamamoto, H. Kunitoh, Y. Ohe and T. Tamura, Lung Cancer, 2009, 65, 219–222 CrossRef PubMed.
- S. Valastyan and R. A. Weinberg, Cell, 2011, 147, 275–292 CrossRef CAS PubMed.
- A. F. Chambers, A. C. Groom and I. C. MacDonald, Nat. Rev. Cancer, 2002, 2, 563–572 CrossRef CAS PubMed.
- D. X. Nguyen, P. D. Bos and J. Massague, Nat. Rev. Cancer, 2009, 9, 274–284 CrossRef CAS PubMed.
- M. Mrksich, Acta Biomater., 2009, 5, 832–841 CrossRef CAS PubMed.
- M. Mrksich and G. M. Whitesides, Annu. Rev. Biophys. Biomol. Struct., 1996, 25, 55–78 CrossRef CAS PubMed.
- J. M. Curran, R. Chen and J. A. Hunt, Biomaterials, 2005, 26, 7057–7067 CrossRef CAS PubMed.
- Y. J. Ren, H. Zhang, H. Huang, X. M. Wang, Z. Y. Zhou, F. Z. Cui and Y. H. An, Biomaterials, 2009, 30, 1036–1044 CrossRef CAS PubMed.
- C. A. Scotchford, C. P. Gilmore, E. Cooper, G. J. Leggett and S. Downes, J. Biomed. Mater. Res, 2002, 59, 84–99 CrossRef CAS PubMed.
- X.-L. Yu, S.-J. Xu, J.-D. Shao, C. Du, S.-F. Chen, B. Zhang, Y.-X. Wang and X.-M. Wang, Surf. Coat. Technol., 2013, 228, S48–S54 CrossRef CAS PubMed.
- P. Filippini, G. Rainaldi, A. Ferrante, B. Mecheri, G. Gabrielli, M. Bombace, P. L. Indovina and M. T. Santini, J. Biomed. Mater. Res, 2001, 55, 338–349 CrossRef CAS.
- H. Yan, S. Zhang, J. He, Y. Yin, X. Wang, X. Chen, F. Cui, Y. Li, Y. Nie and W. Tian, Biomed. Mater., 2013, 8, 035008 CrossRef PubMed.
- P. Jal, S. Patel and B. Mishra, Talanta, 2004, 62, 1005–1028 CrossRef CAS PubMed.
- C. H. Tang and M. E. Lu, Prostate, 2009, 69, 1781–1789 CrossRef CAS PubMed.
- I. Saiki, J. Murata, K. Watanabe, H. Fujii, F. Abe and I. Azuma, Jpn. J. Cancer Res., 1989, 80, 873–878 CrossRef CAS PubMed.
- C. Y. Huang, Y. C. Fong, C. Y. Lee, M. Y. Chen, H. C. Tsai, H. C. Hsu and C. H. Tang, Biochem. Pharmacol., 2009, 77, 794–803 CrossRef CAS PubMed.
- G. Haslam, D. Wyatt and P. A. Kitos, Cytotechnology, 2000, 32, 63–75 CrossRef CAS.
- M. Li, Y. Zhu, H. Zhang, L. Li, P. He, H. Xia, Y. Zhang and C. Mao, Sci. Rep., 2014, 4, 7380 CrossRef CAS PubMed.
- S. Jo and K. Park, Biomaterials, 2000, 21, 605–616 CrossRef CAS.
- J. B. McCarthy, M. L. Basara, S. L. Palm, D. F. Sas and L. T. Furcht, Cancer Metastasis Rev., 1985, 4, 125–152 CrossRef CAS.
- P. A. Netland and B. R. Zetter, J. Cell Biol., 1985, 101, 720–724 CrossRef CAS.
- M. H. Lee, D. A. Brass, R. Morris, R. J. Composto and P. Ducheyne, Biomaterials, 2005, 26, 1721–1730 CrossRef CAS PubMed.
- L.-C. Xu and C. A. Siedlecki, Biomaterials, 2007, 28, 3273–3283 CrossRef CAS PubMed.
- M. P. Van Damme, J. Tiglias, N. Nemat and B. N. Preston, Anal. Biochem., 1994, 223, 62–70 CrossRef CAS.
- S. A. Makohliso, R. F. Valentini and P. Aebischer, J. Biomed. Mater. Res., 1993, 27, 1075–1085 CrossRef CAS PubMed.
- D. E. Ingber, Semin. Cancer Biol., 2008, 18, 356–364 CrossRef CAS PubMed.
- S. Y. Lee, S. Voronov, K. Letinic, A. C. Nairn, G. Di Paolo and P. De Camilli, J. Cell Biol., 2005, 168, 789–799 CrossRef CAS PubMed.
- D. J. Webb, J. T. Parsons and A. F. Horwitz, Nat. Cell Biol., 2002, 4, E97–E100 CrossRef CAS PubMed.
- A. J. Ridley, M. A. Schwartz, K. Burridge, R. A. Firtel, M. H. Ginsberg, G. Borisy, J. T. Parsons and A. R. Horwitz, Science, 2003, 302, 1704–1709 CrossRef CAS PubMed.
- J. L. R. Fernandez, B. Geiger, D. Salomon and A. Ben-Ze'ev, Cell Motil. Cytoskeleton, 1992, 22, 127–134 CrossRef PubMed.
- H. Shibanuma, T. Hirano, K. Tsuji, Q. Wu, B. Shrestha, C. Konaka, Y. Ebihara and H. Kato, Lung Cancer, 1998, 22, 85–95 CrossRef CAS.
- J. M. Retera, M. P. Leers, M. A. Sulzer and P. H. Theunissen, J. Clin. Pathol., 1998, 51, 891–894 CrossRef CAS.
- R. M. Bremnes, R. Veve, F. R. Hirsch and W. A. Franklin, Lung Cancer, 2002, 36, 115–124 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra16554c |
| ‡ These two authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2015 |