Construction of nanoparticles based on amphiphilic PEI–PA polymers for bortezomib and paclitaxel co-delivery
Ruirui Zhang†
a,
Yinghua Liu†a,
Zhiwei Yangbc,
Yunhao Lid,
Xianghui Rongbc,
Lin Wang*bc,
Chujun Guobc,
Sheng Libc,
Junxing Liu*bc,
Mingjun Li*bc and
Yan Wu*a
aNational Center for Nanoscience and Technology, Laboratory of Nanobiomedicine and Nanosafety, Division of Nanomedicine and Nanobiology, Beijing 100190, China. E-mail: wuy@nanoctr.cn; Fax: +86 10 62656765; Tel: +86 10 82545614
bSchool of Basic Medical Sciences, Jiamusi University, Jiamusi 154007, China
cThe First Affiliated Hospital of Jiamusi University, Jiamusi 154003, China. E-mail: wanglin4477@163.com; liujunxing0982@163.com; limingjun6116@163.com
dDepartment of Clinical Medicine, Zhongshan School of Medicine, Sun Yat-sen University, Guangzhou, 510080, China
First published on 16th January 2015
Abstract
Co-delivery is a feasible means to overcome the drawbacks of using a single drug. Herein, we report novel polymer nanoparticles based on branched polyethyleneimine and palmitic acid conjugates for bortezomib and paclitaxel co-delivery. These drug-loaded nanoparticles have a preferable particle size, high encapsulation efficiency and a pH-dependent release profile. The co-delivery system has a great advantage in suppressing tumor cell growth over single drug loaded nanoparticles, whereas the polymer nanocarrier itself shows excellent biocompatibility. The good cellular uptake properties and synergistic effects of co-delivery by polymer nanoparticles provide significant advances toward cancer therapy.
Introduction
Owing to the molecular complexity of cancer, the smart combinations of drugs has become increasingly attractive for better modulating the cell-signalling network to maximize the therapeutic effect and reduce drug resistance.1,2 The strategy of combining an imaging unit and two or more therapeutic agents to form a nanocarrier platform has great potential for revolutionizing the future of cancer therapy, which not only allows multiple functions to be brought together but also achieves synergistic effects.3,4 In addition, the co-delivery of two or more drugs is a reasonable means of overcoming the drawbacks of using a single drug such as drug resistance, low efficacy, high toxicity and a limited regime for clinical uses.1Research advances in nanoscience and biomedicine have driven the development of multifunctional delivery systems. Various nanocarriers have been reported with reasonable delivery performance such as liposomes,5 polymer nanoparticles,6 and mesoporous silica.7,8 Among them, the amphiphilic polymers have attracted great attention as drug carriers because they can provide a narrow size distribution, increase the solubility of hydrophobic payloads, enhance the drug bioavailability, and control the release of payloads over time period.6,9 Furthermore, polymers can be easily modified to display functionalities and exhibit the desired surface character.10 In particular, polymeric nanoparticles have been shown to target tumor tissues preferentially, as a result of passive accumulation through tumors' enhanced permeability and retention (EPR) effect.11
Bortezomib (BTZ), a selective 26S proteasome inhibitor, has the potential to affect multiple signaling pathways, including cell growth, survival, apoptosis, gene expression related to cellular adhesion, migration and angiogenesis.12 It has been tested for anticancer activity against various cell lines, but the efficacy differs.13–15 Notably, only 8% of non-small cell lung cancer (NSCLC) patients with single-agent BTZ show remarkably positive responses.16 To enhance its therapeutic efficacy, BTZ is commonly used in combination with other chemotherapeutic agents, including cyclophosphamide, cisplatin, doxorubicin, docetaxel and paclitaxel (PTX).17–19 It is postulated that BTZ overcomes resistance to conventional chemotherapy by blocking the chemotherapy-induced NF-kappaB activation as well as increasing the cell cycle regulatory proteins, p21, p27, transcription factor p53 and decreasing the expression of bcl-2.13 PTX promotes microtubule stabilization and cell death, which induces the phosphorylation of bcl-2 and suppresses its antiapoptic function.13,20 When BTZ was added to PTX treatment, Bcl-xL expression was decreased; moreover, the survival was reduced fivefold compared to the cells treated with either BTZ or PTX alone.21 Therefore, simultaneous administration has been shown to be important when combining BTZ with PTX in preclinical studies.22 However, the administration of BTZ with PTX is limited by their poor solubility and once dissolved in an aqueous solution, BTZ is only stable for 8 h at 25 °C.23 Low temperature and scarce contact with air can decrease the degradation process of BTZ.24 The intravenous administration of hydrophobic drugs could cause coagulation in the blood capillaries and elicit serious complications, which also lower the effective drug doses.25 In addition, BTZ and PTX often has serious side effects on patients, including allergic reactions, neurotoxicity, and nephrotoxicity.26,27 Consequently, a delivery system that improves the solubility of BTZ and PTX, as well as regulates the BTZ to PTX ratio needs to be developed for the treatment of cancer.
Hence, we constructed co-delivery nanoparticles based on branched polyethyleneimine (PEI) and palmitic acid (PA) conjugates for the delivery of two different drugs. Endogenous lipid PA and fluorescein isothiocyanate (FITC) were conjugated with branched PEI (FITC–PEI–PA) to form nanoparticles with a well-controlled size and higher encapsulation efficiency as well as cell imaging. The chemical structure and physicochemical properties of these FITC–PEI–PA polymers were investigated. BTZ and PTX were simultaneously loaded and incorporated into the polymeric FITC–PEI–PA nanoparticles by an emulsion–solvent evaporation technique. We also investigated the physicochemical properties of these BTZ and PTX loaded polymeric nanoparticles, including their size, encapsulation efficiency, and release profile. The in vitro cytotoxic activity and in vitro antitumor efficiency of the BTZ and PTX loaded FITC–PEI–PA nanoparticles were then evaluated to explore their potential as efficient drugs carriers.
Experimental
Materials
Branched polyethyleneimine (PEI) with an average molecular weight of 25 kDa was purchased from Sigma-Aldrich (St. Louis, MO, USA). Palmitic acid (PA), N,N-diisopropylethylamine (DIEA) and o-(7-azabenzotriazol-1-yl)-N,N,N′,N′-tetramethyluronium hexafluorophosphate (HATU) were obtained from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Paclitaxel (PTX) was purchased from Beijing HuaFeng United Co., Ltd. (Beijing, China). Bortezomib (BTZ) was obtained from Beijing QinWuTian Pharmaceutical Co., Ltd. (Beijing, China). Commercial CellTiter 96® AQueous One Solution (MTS) was purchased from Promega (San Luis Obispo, CA). Human cervical carcinoma (HeLa) cells and Human Gastric Carcinoma (SGC-7901) cells were purchased from the American Type Culture Collection (ATCC). All other chemical reagents were analytical grade and used as received.Synthesis of FITC–PEI–PA
PA conjugated with PEI (PEI–PA) was synthesized according to a previous report with some modifications.28 256 mg (1 mmol) PA was dissolved in 20 mL of N,N-dimethylformamide (DMF) by magnetic stirring and then 380 mg (1 mmol) HATU and 380 μL (1 mmol) DIEA were added to the solution. The mixture was allowed to react at room temperature for 2 h. Subsequently, 250 mg PEI (dissolved in 10 mL of DMF) was added in a stepwise manner and reacted overnight. The reaction solution was dialyzed against distilled water for 3 days and then lyophilized. FITC (0.3 mg) was then conjugated to the obtained PEI–PA polymers in 1 mL DMF at room temperature for 2 h in the dark. The resulting solution was dialyzed against distilled water for 3 days to remove unreacted FITC, and then lyophilized.Preparation of the BTZ and/or PTX loaded polymer nanoparticles
BTZ and/or PTX loaded polymer nanoparticles were prepared via an emulsion/solvent evaporation technique. Taking BTZ and PTX loaded FITC–PEI–PA polymers nanoparticles as an example, 30 mg of FITC–PEI–PA polymers were dissolved in 3 mL methylene chloride (DCM), then BTZ and PTX were added with polymer/PTX/BTZ mass ratios of 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5
0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5, 50
0.5, 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5
0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5, 80
0.5, 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5
0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5, and 100
0.5, and 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5
0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5. The mixture was slowly added to 6 mL aqueous solution with 1% (w/v) of polyvinyl alcohol (PVA) and stirred vigorously at room temperature for 10 min. The mixture was then emulsified by ultrasonication for 5 min at 235 W and evaporated under reduced pressure to remove the remaining DCM. The nanoparticles were recovered at room temperature by centrifugation at 15
0.5. The mixture was slowly added to 6 mL aqueous solution with 1% (w/v) of polyvinyl alcohol (PVA) and stirred vigorously at room temperature for 10 min. The mixture was then emulsified by ultrasonication for 5 min at 235 W and evaporated under reduced pressure to remove the remaining DCM. The nanoparticles were recovered at room temperature by centrifugation at 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 25 min and washed three times with deionized water. Other nanoparticles (FITC–PEI–PA polymer nanoparticles, BTZ loaded FITC–PEI–PA polymer nanoparticles, PTX loaded FITC–PEI–PA polymer nanoparticles) were made using the same procedure.
000 rpm for 25 min and washed three times with deionized water. Other nanoparticles (FITC–PEI–PA polymer nanoparticles, BTZ loaded FITC–PEI–PA polymer nanoparticles, PTX loaded FITC–PEI–PA polymer nanoparticles) were made using the same procedure.
Encapsulation efficiency of the BTZ loaded nanoparticles, PTX loaded nanoparticles, BTZ and PTX loaded nanoparticles
The BTZ encapsulation efficiency was determined by HPLC (Waters 2478, Milford, MA, USA) in triplicate with UV detection at 270 nm. A C18-column (Nova-Pak 3.9 × 250 mm, Waters, Milford, MA) was used with a mobile phase consisting of water and acetonitrile (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80 v/v) and the flow rate was 1 mL min−1.
80 v/v) and the flow rate was 1 mL min−1.
The PTX encapsulation efficiency was determined by HPLC (Waters 2478, Milford, MA, USA) in triplicate with UV detection at 227 nm. A C18-column (Nova-Pak 3.9 × 250 mm, Waters, Milford, MA) was used with a mobile phase consisting of water and acetonitrile (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80 v/v) and the flow rate was 1 mL min−1.
80 v/v) and the flow rate was 1 mL min−1.
The lyophilized BTZ loaded nanoparticles, PTX loaded nanoparticles, BTZ and PTX loaded nanoparticles were also dissolved in acetonitrile. The encapsulation efficiency was defined as follows:
| Encapsulation efficiency (%) = (W0 − Wt)/W0 × 100% |
In vitro drug release
The in vitro release of BTZ and PTX from nanoparticles was investigated using a dialysis method. The freeze dried BTZ loaded nanoparticles, PTX loaded nanoparticles, and BTZ and PTX loaded nanoparticles were dispersed in 3 mL deionized water and then placed into a dialysis bag (MWCO: 3500 Da). The end-sealed dialysis bag was incubated in 40 mL different media (buffer with pH 7.4 and 5.4) at 37 °C and shaken in a water bath at a speed of 150 rpm. 1 mL of the supernatant were extracted at predetermined time intervals and replaced with 1 mL fresh buffer solutions. The concentration of BTZ and PTX released from the nanoparticles was determined by HPLC, as described above.Cytotoxicity assay
HeLa cells and SGC-7901 cells were maintained in the 96-well plates at 37 °C for 24 h in 100 μL DMEM and RMPI 1640 medium, respectively. Both DMEM and RMPI 1640 were supplemented with 10% (v/v) fetal bovine serum (FBS). The culture medium was replaced with 100 μL of medium, containing blank polymer nanoparticles, BTZ loaded nanoparticles, PTX loaded nanoparticles, and BTZ and PTX loaded nanoparticles. The concentration ratio of BTZ to PTX in nanoparticles was calculated from the drug amount used in the preparation of BTZ and PTX loaded nanoparticles. To study the cell proliferation on different substrates, the number of viable cells were determined using the colorimetric MTS assay (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt). After 24 h and 48 h of cell seeding in a 24-well plate, the cells were washed with PBS and incubated with 20% of MTS reagent, containing serum free medium. After 3 h incubation at 37 °C in 5% CO2, aliquots were pipetted into a 96-well plate. The absorbance of the content of each well was measured at 492 nm using a spectrophotometric plate reader.Characterization
The chemical structures of the polymers were detected by Fourier transform infrared spectroscopy (FT-IR) and nuclear magnetic resonance (NMR) spectral analysis. The FT-IR spectra of the polymers were recorded on a spectrophotometer (Perkin Elmer, Fremont, CA, USA) using KBr as a reference. 1H NMR spectra of the polymers were obtained by a Bruker AVANCE 400 NMR spectrometer (Billerica, MA, USA). The fluorescence spectra of FITC–PEI–PA polymers and FITC–PEI–PA nanoparticles were detected by a LS 55 Fluorescence Spectrometer (Perkin Elmer, Fremont, CA, USA). The nanoparticle size (diameter, nm) and polydispersity index were determined using a ZetaSizer Nano series Nano-ZS (Malvern Instruments Ltd., Malvern, UK) equipped with a HeNe Laser beam at a wavelength of 633 nm and a fixed scattering angle of 90°. The determinations were performed at 25 °C for samples appropriately diluted in distilled water. The morphology of the nanoparticles was observed by transmission electron microscopy (TEM) (EM-200CX; JEOL Ltd., Tokyo, Japan) after being negative stained with uranyl acetate.Cellular uptake and subcellular localization of the nanoparticles
HeLa cells and SGC-7901 were induced in 96-well culture plates (a sterile coverslip was put in each well) for 24 h, with a density of 5 × 103 cells per well. After incubation for a given time, the culture medium, containing the blank FITC–PEI–PA polymer nanoparticles, BTZ loaded nanoparticles, PTX loaded nanoparticles, and BTZ and PTX loaded nanoparticles was added. To detect the intracellular localization, the cells were further incubated with 75 nM LysoTracker Red DND-99 (Invitrogen, Carlsbad, California, USA) for 30 min. The cells were imaged by laser confocal scanning microscopy (CLSM) using an Olympus FV1000 (Olympus, Japan). The excitation and emission wavelengths were λex 488 nm and λem 510 nm for the LysoTracker and FITC-labeled nanoparticles, respectively.Results and discussion
Synthesis and characterizations of the PEI–PA and FITC–PEI–PA polymer
PA was conjugated to branched PEI using HATU/DIEA as a coupling system (Scheme 1). The resulting PEI–PA conjugates were isolated by dialysis and characterized by 1H NMR and FT-IR spectroscopy. In Fig. 1A, the resonances a–d correspond to palmitoyl protons, –CH3 (δ ∼ 0.89 ppm), γ-CH2 (δ ∼ 1.27 ppm), β-CH2 (δ ∼ 1.64 ppm), and α-CH2 (δ ∼ 2.35 ppm), respectively. 1H NMR of PEI–PA conjugates in CDCl3 displayed a broad peak at δ ∼ 2.98 ppm assignable to the methylene protons of PEI (Fig. 1B), which proved the successful attachment of PA groups to the PEI polymer.28 The FT-IR spectra further confirmed the construction of PEI–PA and FITC–PEI–PA polymer (Fig. 2). The PEI–PA and FITC–PEI–PA polymer showed the characteristic absorptions of amino groups at around 3300 cm−1. Specifically, the characteristic symmetric (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) band of the fatty acids at 1695 cm−1 disappeared and the absorptions characteristic of amide groups were observed at 1550 and 1640 cm−1 for PEI–PA and FITC–PEI–PA polymer, respectively. Although the characteristic band of the benzene ring overlaps with the characteristic band of the amide group, the FITC–PEI–PA polymer showed a particular band at 1583 cm−1, corresponding to the benzene ring of FITC. All the results prove that the FITC–PEI–PA polymer had been successfully prepared. As shown in Fig. 3, the fluorescent spectra taken from FITC–PEI–PA were slightly shifted to the blue region compared with the emission spectrum of free FITC solution, due to FITC conjugation to PEI. This slight variation of the fluorescence of FITC further confirmed that the FITC–PEI–PA polymers had been successfully obtained. It should be noted that a broad fluorescent peak at 620 nm appeared, which was ascribed to the emission of PEI–PA polymer nanoparticles based on effective Förster resonance energy transfer (FRET) from FITC to the PEI–PA polymer nanoparticles. PEI-based polymer nanoparticles generally had a broad fluorescence emission and were sensitive to the excitation wavelength.29 Assembling therapeutic and imaging molecules to a single nanoparticle can favor cancer therapy by allowing the close tracking of the antitumor efficacy during the course of treatment.
O) band of the fatty acids at 1695 cm−1 disappeared and the absorptions characteristic of amide groups were observed at 1550 and 1640 cm−1 for PEI–PA and FITC–PEI–PA polymer, respectively. Although the characteristic band of the benzene ring overlaps with the characteristic band of the amide group, the FITC–PEI–PA polymer showed a particular band at 1583 cm−1, corresponding to the benzene ring of FITC. All the results prove that the FITC–PEI–PA polymer had been successfully prepared. As shown in Fig. 3, the fluorescent spectra taken from FITC–PEI–PA were slightly shifted to the blue region compared with the emission spectrum of free FITC solution, due to FITC conjugation to PEI. This slight variation of the fluorescence of FITC further confirmed that the FITC–PEI–PA polymers had been successfully obtained. It should be noted that a broad fluorescent peak at 620 nm appeared, which was ascribed to the emission of PEI–PA polymer nanoparticles based on effective Förster resonance energy transfer (FRET) from FITC to the PEI–PA polymer nanoparticles. PEI-based polymer nanoparticles generally had a broad fluorescence emission and were sensitive to the excitation wavelength.29 Assembling therapeutic and imaging molecules to a single nanoparticle can favor cancer therapy by allowing the close tracking of the antitumor efficacy during the course of treatment.
Preparation and characterization of the BTZ and PTX loaded FITC–PEI–PA nanoparticles
Multidrug delivery is a promising strategy to overcome the undesirable toxicity and other side effects that limit the utility of many potential drugs.30 For the different structure and property of BTZ and PTX, it is necessary to find a type of polymer material that could load those drugs simultaneously. In our design strategy of nanoparticles, PEI and PA were used to form size-controllable core–shell nanoparticles (Scheme 2), which have the potential for pharmaceutical applications. PA, biocompatible FDA approved polymer, was chosen for the hydrophobic segment. PEI was chosen for the hydrophilic segment because of their excellent properties, including the enhanced water solubility, large numbers of functional groups available for post-modification and relative simplicity of bulk production. Owing to the hydrophobicity of BTZ and PTX, a modified emulsion/solvent evaporation method was used to prepare BTZ and PTX loaded FITC–PEI–PA nanoparticles.29 These nanoparticles could be lyophilized for long time storage and subsequently re-suspended in solvents according to specific applications. The morphology and size distribution of BTZ and PTX loaded FITC–PEI–PA nanoparticles were analyzed by TEM and DLS, respectively. As shown in Fig. 4c, the resulting BTZ and PTX loaded FITC–PEI–PA nanoparticles are of spherical shapes and have a relative narrow size distribution (Fig. 4d). TEM images showed that both PEI–PA nanoparticles and drug loaded nanoparticles with a diameter between approximately 80 and 120 nm were smaller than that obtained by DLS (Fig. 4). This difference is because the diameter of the nanoparticles obtained by DLS reflected the hydrodynamic diameter of nanoparticles swelled in aqueous solution, whereas those observed by TEM were the diameters of dried nanoparticles.31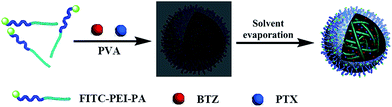 | ||
| Scheme 2 Illustration of the FITC–PEI–PA nanoparticles loaded with both BTZ and PTX using emulsion/solvent evaporation method. | ||
Influences of formulation parameters on the encapsulation efficiency and particle size
BTZ and PTX were successfully loaded in the PEI–PA nanoparticles, which was confirmed by HPLC. That is, the emulsion/solvent evaporation method was effective for the co-delivery of hydrophobic drugs. The highest encapsulation efficiencies of BTZ and PTX in the FITC–PEI–PA were 84% and 43% at a copolymer/BTZ/PTX ratio of 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5
0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5, respectively (Table 1). With the polymer/BTZ/PTX ratio change from 30
0.5, respectively (Table 1). With the polymer/BTZ/PTX ratio change from 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5
0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 to 100
0.5 to 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5
0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5, the encapsulation efficiency of BTZ and PTX was altered. In general, as the ratio of polymer/drug increased, the drug loading content decreased, while the encapsulation efficiency gradually rose.10 Hence, we changed the BTZ and PTX ratio range from 0
0.5, the encapsulation efficiency of BTZ and PTX was altered. In general, as the ratio of polymer/drug increased, the drug loading content decreased, while the encapsulation efficiency gradually rose.10 Hence, we changed the BTZ and PTX ratio range from 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 at a fixed polymer/drugs ratio of 30
0 at a fixed polymer/drugs ratio of 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to investigate the encapsulation efficiency of BTZ and PTX. It should be noted that the encapsulation efficiencies of BTZ and PTX have a different tendency with increasing feed ratio, which might be due to the greater hydrophobicity of PTX. As shown in Table 1, 30
1 to investigate the encapsulation efficiency of BTZ and PTX. It should be noted that the encapsulation efficiencies of BTZ and PTX have a different tendency with increasing feed ratio, which might be due to the greater hydrophobicity of PTX. As shown in Table 1, 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.3
0.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.7 is considered to be the optimal polymer/BTZ/PTX ratio for the maximum encapsulation efficiency of drugs.
0.7 is considered to be the optimal polymer/BTZ/PTX ratio for the maximum encapsulation efficiency of drugs.
| a Determined by dynamic light scattering (DLS). The nanoparticles were prepared by directly dissolving BTZ and PTX loaded nanoparticles in distilled water at a concentration of 0.5 mg mL−1 followed by 10 min sonication. The results are reported as the mean ± SD (n = 3). | |||||||||
|---|---|---|---|---|---|---|---|---|---|
Polymer![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BZT BZT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PTX ratio PTX ratio |
30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5 |
50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5 |
80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5 |
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5 |
30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.9 0.9 |
30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8 0.8 |
30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.3 0.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.7 0.7 |
30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| Particle size (nm) | 259.0 ± 0.2 | 252.3 ± 0.3 | 247.4 ± 0.3 | 200.1 ± 0.1 | 214.4 ± 0.2 | 216.6 ± 0.4 | 235.8 ± 0.1 | 255.6 ± 0.3 | 182.7 ± 0.1 |
| Polydispersity | 0.34 ± 0.02 | 0.26 ± 0.02 | 0.21 ± 0.03 | 0.24 ± 0.04 | 0.24 ± 0.01 | 0.18 ± 0.03 | 0.23 ± 0.02 | 0.34 ± 0.01 | 0.18 ± 0.03 |
| Encapsulation efficiency of BTZ (%) | 68.59 ± 0.7 | 69.29 ± 0.6 | 82.84 ± 0.9 | 84.00 ± 1.1 | — | 96.74 ± 0.3 | 72.17 ± 0.5 | 70.92 ± 0.9 | 64.69 ± 1.1 |
| Encapsulation efficiency of PTX (%) | 22.59 ± 1.2 | 23.44 ± 0.7 | 42.20 ± 0.6 | 43.01 ± 0.9 | 53.94 ± 0.7 | 7.56 ± 0.6 | 8.31 ± 0.9 | 21.62 ± 0.7 | — |
The size and size distribution of the drug loaded core–shell nanoparticles prepared in various polymer/drug weight ratios are summarized in Table 1. The polymer/drug weight ratios have a slight effect on the particle size and its polydispersity. These drug loaded nanoparticles showed an average size of 180–260 nm. Note that higher encapsulation efficiency could increase the size of the nanoparticles, indicating that the presence of BTZ and PTX in the hydrophobic core of the nanoparticles.
In vitro drug release studies
The releasing behaviors of the drug loaded nanoparticles in pH 7.4 and pH 5.4 buffered solutions were studied. As demonstrated in Fig. 5, the BTZ and PTX loaded nanoparticles exhibited slow release at both pH 7.4 and 5.4 buffer solutions. At pH 7.4, the release ratio of BTZ was 64% at day 11, and release ratio of PTX was 55% at day 11; however, at pH 5.4, the release of BTZ and PTX from nanoparticles was up to 70% and 60% at day 11, respectively. This was attributed to the ionization of the acidic amino residues on the side chains, and the strengthened repulsion between side chains, which caused a loose structure in favor of rapid drug release. This means that the BTZ and PTX loaded nanoparticles have a pH sensitive release profile, which may be beneficial for the drug release in the acidic endosome/lysosome compartments of cells, and thus reducing the systemic toxicity as a result of slow release in neutral body fluids.31 | ||
| Fig. 5 Release profiles of BTZ and PTX from FITC–PEI–PA polymer nanoparticles under neutral (pH 7.4) and acidic conditions (pH 5.4) at 37 °C. | ||
In vitro cytotoxicity of the BTZ and PTX loaded nanoparticles
The in vitro cytotoxicity of the BTZ and PTX loaded nanoparticles, BTZ loaded nanoparticles, PTX loaded nanoparticles, and polymer nanoparticles was evaluated by a MTS assay against HeLa and SGC-7901 cells. According to the encapsulation efficiency studies, nanoparticles with a BTZ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PTX feed mass ratio of 3
PTX feed mass ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 were chosen to demonstrate the anti-tumor efficacy. Fig. 6 shows the cell viability after (A and C) 24 h and (B and D) 48 h culture with different BTZ and PTX dosages from 0.001 to 10 μg mL−1, respectively. The empty polymer nanoparticles did not exhibit cytotoxicity even at high concentrations, indicating the good biocompatibility of PEI–PA polymer nanoparticles. The co-delivery of BTZ and PTX significantly reduced the cell viability, and the cell viability of both cell lines decreased with increasing incubation time as well as the BTZ and PTX concentration. Notably, the co-delivery of BTZ and PTX reduced the cell viability significantly, with a positive correlation with the incubation time and drug concentration. In addition, there are preferable synergistic effects in the combination of BTZ and PTX (ratio of 3
7 were chosen to demonstrate the anti-tumor efficacy. Fig. 6 shows the cell viability after (A and C) 24 h and (B and D) 48 h culture with different BTZ and PTX dosages from 0.001 to 10 μg mL−1, respectively. The empty polymer nanoparticles did not exhibit cytotoxicity even at high concentrations, indicating the good biocompatibility of PEI–PA polymer nanoparticles. The co-delivery of BTZ and PTX significantly reduced the cell viability, and the cell viability of both cell lines decreased with increasing incubation time as well as the BTZ and PTX concentration. Notably, the co-delivery of BTZ and PTX reduced the cell viability significantly, with a positive correlation with the incubation time and drug concentration. In addition, there are preferable synergistic effects in the combination of BTZ and PTX (ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7), exhibiting favorable anti-tumor efficacy (Fig. 6).
7), exhibiting favorable anti-tumor efficacy (Fig. 6).
Cellular uptake and subcellular localization of the BTZ and PTX loaded FITC–PEI–PA nanoparticles
The cellular uptake and distribution of BTZ and PTX loaded nanoparticles in HeLa and SGC-7901 cells were investigated using CLSM. BTZ and PTX loaded nanoparticles were incubated with HeLa and SGC-7901 cells at 37 °C for 30 min, 60 min, 120 min, and 240 min. Fig. 7 shows the dynamic cellular uptake process of BTZ and PTX loaded nanoparticles at different time points. A large amount of BTZ and PTX loaded nanoparticles accumulated on the plasma membranes during the first 30 min and then gradually entered inside the cells.The lysosomes were labeled with LysoTracker Red to study the subcellular localization of polymer nanoparticles. The majority of nanoparticles were localized in the lysosomes (Fig. 8). Regarding the pH sensitivity, a faster drug release might occur when the nanoparticles internalized into the lysosomes from the external neutral environment, leading to a desirable pH-dependent controlled release.
Conclusions
Co-delivery nanoparticles based on amphiphilic PEI–PA polymers were constructed. The PEI–PA polymer was synthesized by a facile strategy; moreover, FT-IR, NMR, and fluorescence confirmed the introduction of PA and FITC onto PEI. The FITC–PEI–PA polymer was employed as carriers to co-deliver BTZ and PTX by emulsion–solvent evaporation methods. The drug loaded nanoparticles have been successfully constructed with a satisfactory size distribution, high encapsulated efficiency and a pH-dependent release profile. The cytotoxicity of the drug loaded nanoparticles against HeLa and SGC-7901 cells were verified by a MTS assay. The BTZ and PTX loaded nanoparticles suppresses tumor cell growth more efficiently than single drug loaded nanoparticles, while the polymer nanocarrier itself shows excellent biocompatibility. The good cellular uptake properties and synergistic effects of co-delivery polymer nanoparticles will set up the basis for future in vivo biomedical applications.Acknowledgements
This study was supported by the National Natural Science Foundation of China (81272453, 81472850) and 973 Program (2010CB93404).References
- B. Wang, J. M. Rosano, R. e. Cheheltani, M. P. Achary and M. F. Kiani, Expert Opin. Drug Delivery, 2010, 7, 1159–1173 CrossRef CAS PubMed.
- F. Greco and M. J. Vicent, Adv. Drug Delivery Rev., 2009, 61, 1203–1213 CrossRef CAS PubMed.
- J. A. Barreto, W. O'Malley, M. Kubeil, B. Graham, H. Stephan and L. Spiccia, Adv. Mater., 2011, 23, H18–H40 CrossRef CAS PubMed.
- R. Bardhan, S. Lal, A. Joshi and N. J. Halas, Acc. Chem. Res., 2011, 44, 936–946 CrossRef CAS PubMed.
- Y. Malam, M. Loizidou and A. M. Seifalian, Trends Pharmacol. Sci., 2009, 30, 592–599 CrossRef CAS PubMed.
- Y. Wang, L. Hosta-Rigau, H. Lomas and F. Caruso, Phys. Chem. Chem. Phys., 2011, 13, 4782–4801 RSC.
- Y. Wang, Y. Yan, J. Cui, L. Hosta-Rigau, J. Heath, E. Nice and F. Caruso, Adv. Mater., 2010, 22, 4293–4297 CrossRef CAS PubMed.
- Y. Wang, A. K. Wise, J. Tan, J. W. Maina, R. K. Shepherd and F. Caruso, Small, 2014, 10, 4244–4248 CAS.
- M. Hruby, C. Konak and K. Ulbrich, J. Controlled Release, 2005, 103, 137–148 CrossRef CAS PubMed.
- Q. Xu, Y. X. Liu, S. S. Su, W. Li, C. Y. Chen and Y. Wu, Biomaterials, 2012, 33, 1627–1639 CrossRef CAS PubMed.
- M. E. Davis, Z. Chen and D. M. Shin, Nat. Rev. Drug Discovery, 2008, 7, 771–782 CrossRef CAS PubMed.
- P. G. Richardson, C. Mitsiades, T. Hideshima and K. C. Anderson, Annu. Rev. Med., 2006, 57, 33–47 CrossRef CAS PubMed.
- S. Cresta, C. Sessa, C. V. Catapano, E. Gallerani, D. Passalacqua, A. Rinaldi, F. Bertoni, L. Vigano, M. Maur, G. Capri, E. Maccioni, D. Tosi and L. Gianni, Eur. J. Cancer, 2008, 44, 1829–1834 CrossRef CAS PubMed.
- C. Ceresa, E. Giovannetti, J. Voortman, A. C. Laan, R. Honeywell, G. Giaccone and G. J. Peters, Mol. Cancer Ther., 2009, 8, 1026–1036 CrossRef CAS PubMed.
- G. V. Scagliotti, P. Germonpré, L. Bosquée, J. Vansteenkiste, R. Gervais, D. Planchard, M. Reck, F. De Marinis, J. S. Lee, K. Park, B. Biesma, S. Gans, R. Ramlau, A. Szczesna, A. Makhson, G. Manikhas, B. Morgan, Y. Zhu, K. C. Chan and J. Von Pawel, Lung Cancer, 2010, 68, 420–426 CrossRef PubMed.
- B. Besse, D. Planchard, A.-S. Veillard, L. Taillade, D. Khayat, M. Ducourtieux, J.-P. Pignon, J. Lumbroso, C. Lafontaine, C. Mathiot and J.-C. Soria, Lung Cancer, 2012, 76, 78–83 CrossRef PubMed.
- J. Wagenblast, M. Hambek, M. Baghi, W. Gstottner, K. Strebhardt, H. Ackermann and R. Knecht, J. Cancer Res. Clin. Oncol., 2008, 134, 323–330 CrossRef CAS PubMed.
- B. Ramaswamy, T. Bekaii-Saab, L. J. Schaaf, G. B. Lesinski, D. M. Lucas, D. C. Young, A. S. Ruppert, J. C. Byrd, K. Culler, D. Wilkins, J. J. Wright, M. R. Grever and C. L. Shapiro, Cancer Chemother. Pharmacol., 2010, 66, 151–158 CrossRef CAS PubMed.
- A. Romano, A. Chiarenza, C. Conticello, M. Cavalli, C. Vetro, C. Di Raimondo, R. Cunsolo, G. A. Palumbo and F. Di Raimondo, Eur. J. Haematol., 2014, 93, 207–213 CAS.
- S. Cory, D. C. S. Huang and J. M. Adams, Oncogene, 2003, 22, 8590–8607 CrossRef CAS PubMed.
- J. C. Cusack, Cancer Treat. Rev., 2003, 29, 21–31 CrossRef CAS.
- S. T. Nawrocki, B. Sweeney-Gotsch, R. Takamori and D. J. McConkey, Mol. Cancer Ther., 2004, 3, 59–70 CAS.
- J. P. Vanderloo, M. L. Pomplun, L. C. Vermeulen and J. M. Kolesar, J. Oncol. Pharm. Pract., 2011, 17, 400–402 CrossRef CAS PubMed.
- A. Bolognese, A. Esposito, M. Manfra, L. Catalano, F. Petruzziello, M. C. Martorelli, R. Pagliuca, V. Mazzarelli, M. Ottiero, M. Scalfaro and B. Rotoli, Adv. Hematol., 2009, 2009, 704928 Search PubMed.
- W. Wei, Z.-G. Yue, J.-B. Qu, H. Yue, Z.-G. Su and G.-H. Ma, Nanomedicine, 2010, 5, 589–596 CrossRef CAS PubMed.
- R. Schwartz and T. Davidson, Oncology, 2004, 18, 14–21 Search PubMed.
- S. S. Feng, L. Mu, K. Y. Win and G. F. Huang, Curr. Med. Chem., 2004, 11, 413–424 CrossRef CAS.
- M. Zheng, Y. Zhong, F. Meng, R. Peng and Z. Zhong, Mol. Pharm., 2011, 8, 2434–2443 CrossRef CAS PubMed.
- Y. Sun, W. Cao, S. Li, S. Jin, K. Hu, L. Hu, Y. Huang, X. Gao, Y. Wu and X.-J. Liang, Sci. Rep., 2013, 3, 3036 Search PubMed.
- H. Wang, Y. Zhao, Y. Wu, Y.-l. Hu, K. Nan, G. Nie and H. Chen, Biomaterials, 2011, 32, 8281–8290 CrossRef CAS PubMed.
- X. Liu, S. Su, F. Wei, X. Rong, Z. Yang, J. Liu, M. Li and Y. Wu, J. Colloid Interface Sci., 2014, 413, 54–64 CrossRef CAS PubMed.
Footnote |
| † These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2015 |








