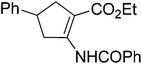
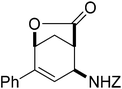
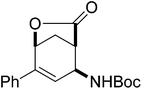
An insight into the synthesis of novel aryl-substituted alicyclic β-amino acid derivatives through substrate-directed palladium-catalysed regio- and stereoselective cross-coupling†
Melinda Nonnab,
Loránd Kissa,
Mikko M. Hänninenc and
Ferenc Fülöp*ab
aInstitute of Pharmaceutical Chemistry, University of Szeged, Eötvös u. 6, H-6720 Szeged, Hungary. E-mail: fulop@pharm.u-szeged.hu
bStereochemistry Research Group of the Hungarian Academy of Sciences, University of Szeged, Eötvös u. 6, H-6720 Szeged, Hungary
cDepartment of Chemistry, University of Jyväskylä, FIN-40014, Jyväskylä, Finland
First published on 12th January 2015
Abstract
Novel aryl-substituted alicyclic β-amino acid derivatives were synthesized through substrate-determined palladium-catalysed cross-coupling of aryl iodides with five- or six-membered cycloalkene β-amino esters. The arylations were investigated with different catalysts, solvents, bases and aryl halides, and with some cyclohexene 2-aminocarboxylate isomers. The stereochemistry and the position of the ring olefinic bond of the starting 2-aminocycloalkanecarboxylate influenced the coupling reaction, and predetermined the structure of the arylated product.
Introduction
Palladium-catalysed cross-couplings are among the most common and practical methods for the creation of a carbon–carbon bond, and are widely used for the synthesis of a large variety of organic substances, bioactive compounds or natural products. These coupling reactions have become almost indispensable techniques for the synthesis of a large family of pharmaceuticals. Besides carbon–carbon bond-forming transformations, they are important for the functionalization of a large number of biomolecules, including the important arylation of different substrates.1The natural and unnatural β-amino acids are an expanding area of research in synthetic organic and medicinal chemistry. They are elements of a series of complex pharmacons (e.g. enzyme inhibitors, antibiotics and antitumoural agents) or natural products.2 Due to π–π or CH–π interactions, a carbon–carbon π-electronic system in the framework of conformationally rigid amino acids or oligopeptides may have a significant role in their structural stabilization, influencing their secondary structures; this may be manifested in improved stability towards enzymes or metabolic degradation. The incorporation of such monomers into oligopeptides may lead to improved pharmacokinetic characteristics and tuned biological properties, and may therefore be of significance in drug discovery research.3 Some small molecular entities in this class of products, such as cispentacin or icofungipen, themselves exhibit interesting biological properties.2 Moreover, thanks to the pharmacological potential of their functionalized derivatives, such as the antifungal methylenated icofungipen (1), the antibacterial hydroxylated oryzoxymicin (2), or the analgetic phenyl-substituted tilidin (3) (Fig. 1), this type of molecule has received considerable attention in recent years.2a,b,4
The aryl- or heteroaryl-substituted amino acids may be regarded as interesting pharmacophores and building elements for oligopeptides,3a–i while cyclic β-amino acid scaffolds linked via amides to aryl- or heteroaryl systems are known to exhibit interesting pharmacological properties.2a Moreover, the combination of an aryl or heteroaryl framework with a saturated ring system with multiple chiral centres has aroused increasing interest in drug development in recent years.3j–l Despite to the potential tremendous benefits in medicinal and drug discovery research, only a very few approaches to aryl-substituted cycloalkane β-amino acid derivatives have been reported so far. One synthetic access route to aryl-substituted cyclic β-amino acids is based on the intramolecular dipolar cycloaddition of a nitrone (derived from a phenyl-substituted unsaturated aldehyde) to an olefinic function, followed by isoxazolidine ring opening and hydroxymethyl group oxidation.5 The rhodium-catalysed conjugate addition of organoborons to unsaturated cyanoesters followed by ring closure is another method for the construction of arylated cycloalkane β-amino acids,6 while cerium(IV)-catalysed sequential three-component reactions of alkylamines, β-oxoesters and aryl ketones (chalcones) lead to polysubstituted β-amino acids with a phenyl substituent attached to the cyclohexane ring.7 Lithium amide conjugate addition to five- or six-membered phenyl-substituted α,β-unsaturated esters furnishes the corresponding phenyl-substituted β-amino acids.8
Among the various palladium-coupling arylation methods, the Heck, Heck–Mizoroki, Heck–Matsuda, and reductive Heck reactions are widely available routes for the creation of a carbon–carbon bond for the preparation of arylated substances, pharmaceuticals or biomolecules through the palladium-catalysed reaction of aryl halides and olefin-containing molecules in the presence of a ligand and a base.1f–l,9
Since extremely few arylated alicyclic β-amino acids are available in this class of derivatives, and as a consequence of the high potential of aryl-substituted cyclic amino acids, our present aim was to synthetize carbocyclic β-amino acid derivatives bearing different aryl functions on the cycloalkane skeleton. For this purpose, palladium-catalysed arylations involving different aryl halides and cyclopentene or cyclohexene 2-aminocarboxylates as non-activated alkenes were investigated.
Results and discussion
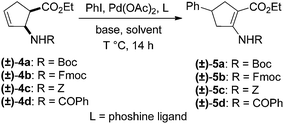
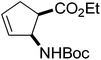
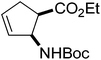
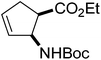
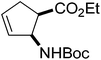
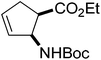
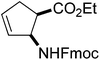
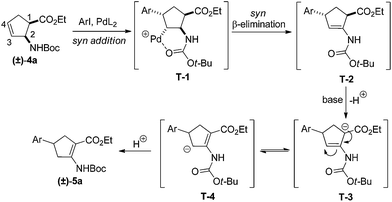
Our investigations of the preparation of arylated cycloalkane β-amino acid derivatives began with the reaction of the five-membered unsaturated derivative ethyl cis-2-tert-butoxycarbonylaminocyclopentane carboxylate ((±)-4a)10 with iodobenzene (PhI) as aryl source compound, different palladium compounds, phosphine ligands and bases (Heck coupling reaction conditions). When stirred with PhI, palladium acetate (Pd(OAc)2), triphenylphosphine (PPh3) and Et3N as non-nucleophilic amine as common base at 40 °C in acetone (±)-4a afforded under standard Heck reaction conditions, a phenylated product in 34% isolated yield after purification by column chromatography. This was identified by 2D NMR and X-ray analyses as (±)-5a, with the phenyl ring attached at C-4 of the ring, and a C–C double bond between the ester and the carbamate functions (Scheme 1, Table 1, entry 1, Fig. 2).In the continuation, instead of Et3N, some inorganic bases (K2CO3, NaOAc or NaHCO3) were systematically used, but after 14 h only starting material was recovered, and no arylated product. Silver salts such as AgOAc, Ag3PO4 or Ag2CO3 were next used as bases in the transformation (Table 1, entries 2–4); the reaction led to (±)-5a, the highest yield (42% after purification by column chromatography) being attained with Ag2CO3. The most suitable palladium catalyst in this reaction affording (±)-5a seemed to be Pd(OAc)2. Only traces of product were detected with several commercially available Pd(0) or Pd(II) catalysts such as bis(triphenylphosphine)palladium(II) dichloride (Pd(PPh3)2Cl2), bis(benzonitrile)palladium(II) chloride, bis(dibenzylideneacetone)palladium(0) (Pd(dba)2) or PdCl2. Experiments were further conducted by changing the solvent from acetone to MeCN, PhMe, THF, 1,4-dioxane or DMF at 40 °C. However, the corresponding (±)-5a could be isolated only in MeCN and in a yield of only 24% (Table 1, entry 5). No transformation was detected in the other solvents. When the transformation was attempted at temperatures higher than 40 °C, palladium black precipitated from the reaction mixture. Interestingly, when (±)-4a was stirred for 14 h under similar conditions (Pd(OAc)2, PPh3, Ag2CO3 and acetone) at 25 °C, the yield was slightly higher (65%, Table 1, entry 6), but the effect of modification of the temperature on the yield in other situations was insignificant. Replacement of PPh3 with bis(diphenylphosphino)ethane (dppe) did not lead to any drastic modification in the yield; the target compound (±)-5a was isolated in 51% by column chromatography (Table 1, entry 7). During the following experiments, several other cyclopentene β-amino esters with different N-protecting groups were investigated in the phenylation reaction. On modification of the Boc group to Fmoc, Cbz or PhCO, the arylation of (±)-4b–d resulted in the corresponding phenyl-substituted five-membered β-amino esters ((±)-5b–d) in moderate yields (Table 1, entries 8–10).
Although a common Heck arylation proceeds by syn addition to the olefinic bond, followed by β-elimination, to afford an α,β-unsaturated aryl-substituted product, the phenylation of ethyl cis-2-aminocyclopent-3-enecarboxylate took place by substrate control with the assistance of the carbamate group to result in a somewhat unexpected compound ((±)-5). In the first step, after Pd insertion into the aromatic C–I bond, syn addition gives intermediate T-1 both stereo- and regioselectively. The stereo- and regioselectivity of this step are probably a result of the stabilizing effect of the carbamate O-atom on the Pd. Next, the β-elimination process gives T-2. In turn under basic conditions, the active hydrogen at C-1 leads to deprotonation–reprotonation and hence double bond migration (T-3 and T-4), to furnish compound (±)-5a, in which the phenyl ring is attached to C-4, while the olefinic bond is situated between the ester and the carbamate functions (C-1–C-2) (Scheme 2). Besides the above presented route, an alternative mechanism which relies in an addition/β-elimination/reinsertion/β-elimination it is also plausible for the formation of arylated compound (±)-5a.
Further experiments were carried out with aryl iodide containing an electron-withdrawing or an electron-donating group. Thus, the Pd-catalysed cross-coupling reactions of (±)-4a with 4-nitroiodobenzene (electron-withdrawing) and 4-methoxyiodobenzene (electron-donating) in the presence of Pd(OAc)2, Ag2CO3 and PPh3 in acetone resulted in the C-4 aryl group-substituted five-membered β-amino ester (±)-6a or (±)-6b (31% and 39%, respectively) (Scheme 3). The yields in this case could be increased somewhat (42% and 56%) by changing the phosphine ligand from PPh3 to dppe (Scheme 3).
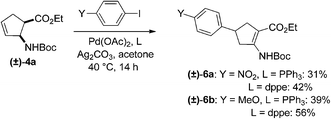 | ||
| Scheme 3 Arylation of N-Boc-protected ethyl cis-2-aminocyclopent-3-enecarboxylates with p-substituted iodobenzenes. | ||
Interestingly, in contrast with the arylation of ethyl cis-2-aminocyclopent-3-enecarboxylate ((±)-4a), its six-membered analogue (±)-7 (ref. 11) underwent phenylation under similar conditions (Pd(OAc)2, PPh3, Ag2CO3, acetone, 40 °C) to provide a different type of arylated product from that obtained in the earlier case (±)-5a. Structure elucidation by means of 2D NMR analysis showed that the product ((±)-8) contained an α,β-unsaturated phenyl moiety in its structure, with the phenyl group attached to C-4 of the six-membered cycloalkane ring (Scheme 4). With a similar reaction protocol, the trans counterpart of (±)-7, amino ester (±)-9,11 surprisingly gave phenylated compound (±)-10, containing the phenyl group at C-5, with the olefinic bond located between C-3 and C-4. At 25 °C, both reactions gave slightly better yields (65% and 81%, respectively) (Scheme 4).
 | ||
| Scheme 4 Arylation of N-Boc-protected ethyl cis- and trans-2-aminocyclohex-4-enecarboxylates with PhI. | ||
The substrate ethyl cis-2-aminocyclohex-3-enecarboxylate (±)-11,11 a regioisomer of (±)-7, was next subjected to phenylation under Heck reaction conditions. Analogously to its five-membered homologue (±)-4a, this allylamine afforded the corresponding six-membered arylated β-amino ester (±)-12 (Scheme 5), in which the phenyl ring is on C-4, and the C–C double bond is between the ester and carbamate functions (see also the transformation of (±)-4, Scheme 1).
For investigation of the influence of the position of the ring olefinic bond on the reaction, amino lactone (±)-13,12 was reacted analogously to (±)-11 with PhI in the presence of the Pd(OAc)2/PPh3/Ag2CO3 system in acetone. In contrast with (±)-11, no migration of the olefinic bond was detected during the transformation. Instead, after the syn addition and β-elimination steps, the obtained was identified by COSY and HSQC analyses as (±)-14, an α,β-unsaturated phenyl-substituted aminolactone (Scheme 6).
In conclusion, the reactions illustrate a substrate-directed Pd-catalyzed arylation protocol providing access to novel aryl-substituted alicyclic β-amino acid derivatives. Depending on the structure of the starting β-aminocycloalkenecarboxylate and the position of the ring C–C double bond, the palladium coupling under Heck reaction conditions somewhat surprisingly afforded a different type of aryl-substituted cyclic β-amino acid derivatives. For the elucidation of further aspects of such unexpected arylations, additional experiments are currently ongoing in our laboratories. These novel π-electron-rich conformationally restricted β-amino acid derivatives may well be of interest in the synthesis of oligopeptides.
Experimental
The chemicals were purchased from Sigma-Aldrich. The NMR spectra were recorded at 400 MHz with CDCl3 or DMSO as solvent and tetramethylsilane as internal standard. The solvents were used as received from the suppliers. Melting points were determined with a Kofler apparatus. Elemental analyses were recorded on a Perkin-Elmer CHNS-2400 Ser II Elemental Analyzer. Silica gel 60 F254 has been purchased from Merck. Mass spectra were recorded on a Finnigan MAT 95S spectrometer.General procedure for the arylation of ethyl 2-aminocycloalkanecarboxylates
To a solution of N-protected 2-aminocycloalkenecarboxylate (1 mmol), base (3 equiv.) and aryl iodide (1 equiv.) in solvent (10 mL), phosphine (20 mol%) and Pd(OAc)2 (10 mol%) were added. The solution was stirred at 25 °C or 40 °C for 14 h. The solid material was then filtered off and the filtrate was concentrated under reduced pressure. The residue was purified by column chromatography on silica gel (eluent: n-hexane–EtOAc 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) or crystallized from a mixture of n-hexane–EtOAc.
1) or crystallized from a mixture of n-hexane–EtOAc.
A white solid (n-hexane–EtOAc), mp 95–96 °C, yield: 65%. 1H NMR (CDCl3, 400 MHz): δ = 1.31 (t, 3H, CH3, J = 7.15 Hz), 1.51 (s, 9H, t-Bu), 2.61–2.69 (m, 1H, CH2), 2.97–3.04 (m, 1H, CH2), 3.15–3.24 (m, 1H, CH2), 3.48–3.56 (m, 1H, CH2), 3.62–3.67 (m, 1H, H-4), 4.18–4.28 (m, 2H, OCH2), 7.22–7.36 (m, 5H, Ar–H). 13C NMR (DMSO, 400 MHz): δ = 15.1, 28.6, 37.3, 40.6, 41.7, 60.5, 82.0, 104.5, 127.1, 127.5, 129.3, 145.9, 151.8, 154.3, 167.6. MS: (ES, pos) m/z = 332 (M + 1).
A colourless oil, yield: 53%. 1H NMR (DMSO, 400 MHz): δ = 1.29 (t, 3H, CH3, J = 7.15 Hz), 2.40–2.48 (m, 1H, CH2), 2.77–2.94 (m, 2H, CH2), 3.25–3.30 (m, 1H, CH2), 3.39–3.50 (m, 1H, H-4), 4.12–4.23 (m, 2H, OCH2), 4.25–4.30 (m, 1H, ArCH), 5.52–4.59 (m, 2H, OCH2), 7.23–7.28 (m, 3H, Ar–H), 7.30–7.37 (m, 6H, Ar–H), 7.59–7.64 (m, 2H, Ar–H), 7.77–7.86 (m, 2H, Ar–H), 9.54 (brs, 1H, N–H). 13C NMR (DMSO, 400 MHz): δ = 15.0, 39.8, 40.4, 41.7, 47.3, 60.6, 67.6, 105.5, 121.0, 125.6, 127.1, 127.6, 128.0, 128.6, 129.3, 141.7, 144.7, 145.9, 152.7, 153.3, 167.4. MS: (ES, pos) m/z = 454 (M + 1).
A white solid (n-hexane–EtOAc), mp 56–57 °C, yield: 50%. 1H NMR (CDCl3, 400 MHz): δ = 1.33 (t, 3H, CH3, J = 7.15 Hz), 2.64–2.72 (m, 1H, CH2), 3.01–3.10 (m, 1H, CH2), 3.19–3.28 (m, 1H, CH2), 3.50–3.58 (m, 1H, CH2), 3.66–3.75 (m, 1H, H-4), 4.20–4.28 (m, 2H, OCH2), 5.19 (s, 2H, OCH2), 7.27–7.43 (m, 5H, Ar–H). 13C NMR (CDCl3, 400 MHz): δ = 14.8, 37.4, 41.1, 41.7, 60.3, 67.7, 105.8, 126.7, 127.3, 128.7, 127.8, 128.9, 129.0, 136.2, 145.7, 153.2, 153.5, 167.8. MS: (ES, pos) m/z = 454 (M + 1).
A white solid (n-hexane–EtOAc), mp 90–91 °C, yield: 36%. 1H NMR (DMSO, 400 MHz): δ = 1.26 (t, 3H, CH3, J = 7.20 Hz), 2.49–2.59 (m, 1H, CH2), 2.92–3.00 (m, 1H, CH2), 3.20–3.35 (m, 1H, CH2), 3.52–3.60 (m, 1H, CH2), 3.70–3.75 (m, 1H, H-4), 4.17–4.25 (m, 2H, OCH2), 7.19–7.25 (m, 1H, Ar–H), 7.28–7.35 (m, 4H, Ar–H), 7.57–7.89 (m, 3H, Ar–H), 7.87–7.91 (m, 2H, Ar–H), 11.2 (brs, 1H, N–H). 13C NMR (DMSO, 400 MHz): δ = 15.0, 37.2, 41.0, 42.3, 60.9, 107.8, 127.1, 127.6, 128.0, 129.4, 130.0, 133.7, 133.9, 146.0, 153.9, 164.6, 167.9.
A white solid (n-hexane–EtOAc), mp 142–145 °C, yield: 74%. 1H NMR (CDCl3, 400 MHz): δ = 1.33 (t, 3H, CH3), 1.50 (s, 9H, t-Bu), 2.56–2.61 (m, 1H, CH2), 3.02–3.12 (m, 1H, CH2), 3.12–3.18 (m, 1H, CH2), 3.62–3.67 (m, 1H, CH2), 3.70–3.76 (m, 1H, H-4), 4.26–4.31 (m, 2H, OCH2), 7.42–7.49 (d, 2H, Ar–H, J = 9 Hz), 8.20–8.28 (d, 2H, Ar–H, J = 9 Hz), 9.58 (brs, 1H, N–H). 13C NMR (DMSO, 400 MHz): δ = 15.1, 28.6, 39.8, 40.9, 41.3, 60.6, 82.1, 104.3, 124.5, 129.0, 146.9, 151.8, 153.9, 154.0, 167.4.
A white solid (n-hexane–EtOAc), mp 130–132 °C, yield: 46%. 1H NMR (CDCl3, 400 MHz): δ = 1.32 (t, 3H, CH3), 1.49 (s, 9H, t-Bu), 2.53.2.58 (m, 1H, CH2), 2.93–3.02 (m, 1H, CH2), 3.11–3.20 (m, 1H, CH2), 3.48–3.3.52 (m, 1H, CH2), 3.60–3.67 (m, 1H, H-4), 3.79 (s, 3H, OMe), 4.18–4.24 (m, 2H, OCH2), 6.82–6.90 (d, 2H, Ar–H, J = 9 Hz), 7.18–7.22 (d, 2H, Ar–H, J = 9 Hz), 9.60 (brs, 1H, N–H). 13C NMR (DMSO, 400 MHz): δ = 15.1, 28.6, 37.5, 40.2, 41.9, 55.9, 60.4, 81.9, 104.5, 114.7, 128.5, 137.8, 151.8, 154.3, 158.6, 167.6.
A white solid (n-hexane–EtOAc), mp 115–118 °C, yield: 34%. 1H NMR (CDCl3, 400 MHz): δ = 1.34 (t, 3H, CH3, J = 7.20 Hz), 1.49 (s, 9H, t-Bu), 1.83–1.95 (m, 1H, CH2), 2.25–2.36 (m, 2H, CH2), 2.55–2.69 (m, 1H, CH2), 2.90–3.00 (m, 1H, H-1), 4.22–4.37 (m, 2H, OCH2), 4.66 (brs, 1H, N–H), 4.79–4.82 (m, 1H, H-2), 7.22–7.38 (m, 6H, Ar–H and H-5). 13C NMR (CDCl3, 400 MHz): δ = 14.9, 29.1, 34.2, 34.6, 36.8, 43.6, 60.7, 80.5, 127.1, 127.7, 129.3, 140.8, 143.3, 148.9, 150.5, 157.5. MS: (ES, pos) m/z = 367 (M + Na).
A white solid (n-hexane–EtOAc), mp 139–140 °C, yield 81%. 1H NMR (CDCl3, 400 MHz): δ = 1.34 (t, 3H, CH3, J = 7.20 Hz), 1.50 (s, 9H, t-Bu), 1.85–1.93 (m, 1H, CH2), 2.35–2.41 (m, 1H, CH2), 2.64–2.70 (m, 1H, H-1), 3.52–3.58 (m, 1H, H-2), 4.15–4.23 (m, 2H, OCH2), 4.56–4.60 (m, 1H, H-5), 4.65 (brs, 1H, N–H), 5.91–5.93 (m, 2H, H-2 and H-3), 7.21–7.39 (m, 5H, Ar–H). 13C NMR (CDCl3, 400 MHz): δ = 14.6, 28.8, 31.8, 39.4, 43.3, 48.0, 61.1, 81.5, 120.0, 127.0, 128.3, 129.0, 129.3, 132.5, 158.5, 166.8. MS: (ESI, pos) m/z = 367 (M + Na).
A white solid (n-hexane–EtOAc), mp 70–75 °C, yield 62%. 1H NMR (CDCl3, 400 MHz): δ = 1.34 (t, 3H, CH3, J = 7.20 Hz), 1.48 (s, 9H, t-Bu), 1.70–1.76 (m, 1H, CH2), 1.97–2.03 (m, 1H, CH2), 2.38–2.46 (m, 1H, CH2), 2.58–2.67 (m, 1H, CH2), 2.79–2.95 (m, 1H, CH2), 3.37–3.44 (m, 1H, H-4), 4.20–4.28 (m, 2H, OCH2), 7.19–7.37 (m, 5H, Ar–H), 10.9 (brs, 1H, N–H). 13C NMR (CDCl3, 400 MHz): δ = 14.7, 25.1, 28.6, 29.4, 35.8, 40.0, 60.5, 80.8, 102.5, 126.8, 127.3, 128.9, 145.8, 151.9, 152.7, 170.2.
A white solid (n-hexane–EtOAc), mp 160–162 °C, yield 43%. 1H NMR (CDCl3, 400 MHz): δ = 2.19–2.21 (m, 2H, CH2), 3.27–3.29 (m, 1H, H-1), 3.89–3.91 (m, 1H, H-2), 4.72–4.74 (m, 1H, H-5), 5.15–5.24 (m, 2H, OCH2), 5.87 (brs, 1H, N–H), 6.37–6.38 (m, 1H, H-3), 7.27–7.43 (m, 5H, Ar–H). 13C NMR (CDCl3, 400 MHz): δ = 28.9, 42.0, 46.4, 67.7, 81.5, 127.9, 128.8, 128.9, 129.1, 129.3, 129.4, 132.5, 133.0, 152.0, 155.4.
A white solid (n-hexane–EtOAc), mp 177–179 °C, yield 62%. 1H NMR (DMSO, 400 MHz): δ = 1.51 (s, 9H, t-Bu), 1.92–1.99 (m, 1H, CH2), 2.14–2.22 (m, 1H, CH2), 3.30–3.38 (m, 1H, H-1), 3.70–3.77 (m, 1H, H-2), 4.59–4.66 (m, 1H, H-5), 7.30–7.41 (m, 5H, Ar–H), 8.92–8.99 (brs, 1H, N–H). 13C NMR (DMSO, 400 MHz): δ = 28.5, 28.9, 41.1, 46.0, 80.2, 81.1, 105.8, 127.9, 129.5, 134.6, 140.8, 153.5, 175.9.
Methine carbon (C4) and the phenyl group attached to it are disordered over two positions by symmetry operation (mirror plane) hence the occupation ratio of the two distinct parts is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. In addition, ethyl group attached to carboxylate functionality is similarly disordered. The general planar structure (reinforced with strong intramolecular NH⋯O hydrogen bond) containing only few bendable groups generates voids in the crystal lattice which (with the lack of solvent of crystallization) most likely causes the observed disorders.
1. In addition, ethyl group attached to carboxylate functionality is similarly disordered. The general planar structure (reinforced with strong intramolecular NH⋯O hydrogen bond) containing only few bendable groups generates voids in the crystal lattice which (with the lack of solvent of crystallization) most likely causes the observed disorders.
The deposition number CCDC 1038709 contains the supplementary crystallographic data for this paper.†
Crystal data for C19H25NO4 (M = 331.40 g mol−1): orthorhombic, space group Pnma (no. 62), a = 14.778(3) Å, b = 6.9181(14) Å, c = 17.853(4) Å, V = 1825.2(6) Å3, Z = 4, T = 123 K, μ(Mo Kα) = 0.084 mm−1, Dcalc = 1.206 g cm−3, 3465 reflections measured (5.332° ≤ 2Θ ≤ 51.994°), 1951 unique (Rint = 0.0224, Rsigma = 0.0232) which were used in all calculations. The final R1 was 0.0549 (I > 2σ(I)) and wR2 was 0.1478 (all data).
Acknowledgements
We are grateful to the Hungarian Research Foundation (OTKA nos K100530 and NK81371) and TÁMOP-4.2.2.A-11/1/KONV-2012-0035 for financial support. This work was supported by the János Bolyai Research Scholarship to L.K. of the Hungarian Academy of Sciences. One of the authors (M.N.) is grateful for the support of the European Union and the State of Hungary, co-financed by the European Social Fund in the framework of TÁMOP 4.2.4. A/2/11-1-2012-0001 “National Excellence Program”.References
- (a) J. Tsuji, in Palladium in Organic Synthesis, Topics in Organometallic Chemistry, Springer, 2005, vol. 14 Search PubMed; (b) L. F. Tietze, H. Ila and H. P. Bell, Chem. Rev., 2004, 104, 3453 CrossRef CAS PubMed; (c) K. C. Majumdar and B. Sinha, Synthesis, 2013, 45, 1271 CrossRef CAS PubMed; (d) T. W. Lyons and M. S. Sanford, Chem. Rev., 2010, 110, 1147 CrossRef CAS PubMed; (e) K. C. Majumdar, S. Samanta and B. Sinha, Synthesis, 2012, 44, 817 CrossRef CAS PubMed; (f) K. C. Nicolaou, P. G. Bulger and D. Sarlah, Angew. Chem., Int. Ed., 2005, 44, 4442 CrossRef CAS PubMed; (g) I. Nakamura and Y. Yamamoto, Chem. Rev., 2004, 104, 2127 CrossRef CAS PubMed; (h) B. Magano and J. R. Dunetz, Chem. Rev., 2011, 111, 2177 CrossRef PubMed; (i) G. P. McGlacken and I. J. S. Fairlamb, Eur. J. Org. Chem., 2009, 4011 CrossRef CAS; (j) X. Chen, K. M. Engle, D.-H. Wang and J.-Q. Yu, Angew. Chem., Int. Ed., 2009, 48, 5094 CrossRef CAS PubMed; (k) A. de Meijere and F. Diederich, in Metal-Catalyzed Cross Coupling Reactions, Wiley-VCH, Weinheim, 2nd edn, 2004 Search PubMed; (l) N. Miyaura, in Cross-Coupling Reaction, Topics in Current Chemistry, Springer, 2002, vol. 219 Search PubMed; (m) Á. Molnár, Chem. Rev., 2011, 111, 2251 CrossRef PubMed; (n) V. P. Mehta and E. V. Van der Eycken, Chem. Soc. Rev., 2011, 40, 4925 RSC; (o) N. Kambe, T. Iwasaki and J. Terao, Chem. Soc. Rev., 2011, 40, 4937 RSC; (p) C. M. So and F. Y. Kwong, Chem. Soc. Rev., 2011, 40, 4963 RSC.
- (a) L. Kiss and F. Fülöp, Chem. Rev., 2014, 114, 1116 CrossRef CAS PubMed; (b) N. Griebenov, in Medicinal chemistry of alicyclic beta-amino acids. Amino acids, peptides and proteins in organic chemistry, ed. Hughes A. B., Wiley, Weinheim, 2011, vol. 4 Search PubMed; (c) F. Kudo, A. Miyanaga and T. Eguchic, Nat. Prod. Rep., 2014, 31, 1056 RSC; (d) E. Juaristi and V. A. Soloshonok, in Enantioselective Synthesis of β-Amino Acids, Wiley-VCH, New York, 2nd edn, 2005 Search PubMed; (e) S. H. Choi, M. Ivancic, I. A. Guzei and S. H. Gellman, Eur. J. Org. Chem., 2013, 3464 CrossRef CAS; (f) E. Mayans, A. Gargallo, A. Álvarez-Larena, O. Illa and R. M. Ortuño, Eur. J. Org. Chem., 2013, 1425 CrossRef CAS; (g) L. Berlicki, M. Kaske, R. Gutieérrez-Abad, G. Bernhardt, O. Illa, R. M. Ortuño, C. Cabrele, A. Buschauer and O. Reiser, J. Med. Chem., 2013, 56, 8422 CrossRef CAS PubMed; (h) L. Berlicki, L. Pilsl, E. Wéber, I. M. Mándity, C. Cabrele, T. Martinek, F. Fülöp and O. Reiser, Angew. Chem., Int. Ed., 2012, 51, 2208 CrossRef CAS PubMed; (i) D. Fernandez, E. Torres, F. X. Aviles, R. M. Ortuno and J. Vendrell, Bioorg. Med. Chem., 2009, 17, 3824 CrossRef CAS PubMed; (j) C. Fernandes, S. Faure, E. Pereira, V. V. Declerck, R. Guillot and D. J. Aitken, Org. Lett., 2010, 12, 3606 CrossRef CAS PubMed.
- (a) T. A. Martinek and F. Fülöp, Chem. Soc. Rev., 2012, 41, 687 RSC; (b) I. M. Mándity, A. Monsignori, L. Fülöp, E. Forró and F. Fülöp, Chem.–Eur. J., 2014, 20, 4591 CrossRef PubMed; (c) D.-W. Zhang, X. Zhao, J.-L. Hou and Z.-T. Li, Chem. Rev., 2012, 112, 5271 CrossRef CAS PubMed; (d) J. M. Rodriguez and A. D. Hamilton, Angew. Chem., Int. Ed., 2007, 46, 8614 CrossRef CAS PubMed; (e) H. Jiang, J. M. Léger and I. Huc, J. Am. Chem. Soc., 2003, 125, 3448 CrossRef CAS PubMed; (f) T. Steiner, Angew. Chem., Int. Ed., 2002, 41, 48 CrossRef CAS; (g) A. Ruffoni, N. Ferri, S. K. Bernini, C. Ricci, A. Corsini, I. Maffucci, F. Clerici and A. Contini, J. Med. Chem., 2014, 57, 2953 CrossRef CAS PubMed; (h) A. Brik, Adv. Synth. Catal., 2008, 350, 1661 CrossRef CAS; (i) A. S. Kamlet, C. Préville, K. A. Farley and D. W. Piotrowski, Angew. Chem., Int. Ed., 2013, 52, 10607 CrossRef CAS PubMed; (j) T. J. Ritchie and S. J. F. Macdonald, Drug Discovery Today, 2009, 14, 1011 CrossRef CAS PubMed; (k) T. J. Ritchie, S. J. F. Macdonald, R. J. Young and S. D. Pickett, Drug Discovery Today, 2011, 16, 164 CrossRef CAS PubMed; (l) F. Lovering, J. Bikker and C. Humblet, J. Med. Chem., 2009, 52, 6752 CrossRef CAS PubMed.
- (a) M. Risseeuw, M. Overhand, G. W. J. Fleet and M. I. Simone, Amino Acids, 2013, 45, 613 CrossRef CAS PubMed; (b) L. Kiss and F. Fülöp, Synlett, 2010, 1302 CAS; (c) J. Mittendorf, F. Kunisch, M. Matzke, H.-C. Militzer, A. Schmidt and W. Schönfeld, Bioorg. Med. Chem. Lett., 2003, 13, 433 CrossRef CAS; (d) M. Palkó, L. Kiss and F. Fülöp, Curr. Med. Chem., 2005, 12, 3063 CrossRef.
- M. C. Whisler and P. Beak, J. Org. Chem., 2003, 68, 1207 CrossRef CAS PubMed.
- T. Miura, T. Harumashi and M. Murakami, Org. Lett., 2007, 9, 741 CrossRef CAS PubMed.
- V. Sridharan and J. C. Menendez, Org. Lett., 2008, 10, 4303 CrossRef CAS PubMed.
- (a) S. G. Davies, M. J. Durbin, S. J. Hartman, A. Matsuno, P. M. Roberts, A. J. Russell, A. D. Smith, J. E. Thomson and S. M. Toms, Tetrahedron: Asymmetry, 2008, 19, 2870 CrossRef CAS PubMed; (b) E. Abraham, S. G. Davies, A. J. Docherty, K. B. Ling, P. M. Roberts, A. J. Russell, J. E. Thomson and S. M. Toms, Tetrahedron: Asymmetry, 2008, 19, 1356 CrossRef CAS PubMed.
- (a) D. Pan and N. Joao, Synlett, 2010, 1577 CAS; (b) J. Muzart, Tetrahedron, 2013, 69, 6735 CrossRef CAS PubMed; (c) B. Karimi, H. Behzadnia, D. Elhamifar, P. F. Akhavan, F. K. Esfahani and A. Zamani, Synthesis, 2010, 1399 CrossRef CAS PubMed; (d) D. McCartney and P. J. Guiry, Chem. Soc. Rev., 2011, 40, 5122 RSC; (e) D. W. Piotrowski and J. Polivkova, Tetrahedron Lett., 2010, 51, 17 CrossRef CAS PubMed; (f) C. C. Oliveira, E. A. D. Santos, J. H. B. Nunes and C. R. D. Correira, J. Org. Chem., 2012, 77, 8182 CrossRef CAS PubMed; (g) C. G. Hartung, K. Köhler and M. Beller, Org. Lett., 1999, 1, 709 CrossRef CAS; (h) Z. Yang and J. Zhou, J. Am. Chem. Soc., 2012, 134, 11833 CrossRef CAS PubMed; (i) X. Wu and J. Zhou, Chem. Commun., 2013, 49, 4794 RSC; (j) A. B. Dounay and L. E. Overman, The Mizoroki–Heck Reaction, ed. M. Oestreich, Wiley, 2009 Search PubMed; (k) L. E. Overman and D. J. Poon, Angew. Chem., Int. Ed., 1997, 36, 518 CrossRef CAS; (l) A. Ashimori, B. Bachand, M. A. Calter, S. P. Govek, L. E. Overman and D. J. Poon, J. Am. Chem. Soc., 1998, 120, 6488 CrossRef CAS; (m) A. B. Dounay, P. G. Humphreys, L. E. Overman and A. D. Wrobleski, J. Am. Chem. Soc., 2008, 130, 5368 CrossRef CAS PubMed; (n) M. Shibasaki, E. M. Vogl and T. Ohshima, Adv. Synth. Catal., 2004, 346, 1533 CrossRef CAS; (o) M. Shibasaki, C. D. J. Boden and A. Kojima, Tetrahedron, 1997, 53, 7371 CrossRef CAS.
- (a) L. Kiss, E. Forró, R. Sillanpää and F. Fülöp, J. Org. Chem., 2007, 72, 8786 CrossRef CAS PubMed; (b) L. Kiss, M. Nonn, E. Forró, R. Sillanpää and F. Fülöp, Tetrahedron Lett., 2009, 50, 2605 CrossRef CAS PubMed.
- L. Kiss, E. Forró and F. Fülöp, Tetrahedron, 2012, 68, 4438 CrossRef CAS PubMed.
- (a) M. Nonn, L. Kiss, R. Sillanpää and F. Fülöp, Tetrahedron, 2012, 68, 9942 CrossRef CAS PubMed; (b) L. Kiss, E. Forró, S. Fustero and F. Fülöp, Eur. J. Org. Chem., 2011, 4993 CrossRef CAS; (c) E. Forró, L. Schönstein, L. Kiss, A. Vega-Peñaloza, E. Juaristi and F. Fülöp, Molecules, 2010, 15, 3998 CrossRef PubMed.
- Z. Otwinowski and W. Minor, in Methods in Enzymology, Macromolecular Crystallography, Part A, ed. C. W. Carter Jr and R. M. Sweet, Academic Press, New York, 1997, vol. 276, pp. 307–326 Search PubMed.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64, 112 CrossRef CAS PubMed.
- O. V. Dolomanov, J. Bourhis, R. J. Gildea, J. A. K. Howard and H. Puschmann, J. Appl. Crystallogr., 2009, 42, 339 CrossRef CAS.
- Diamond-Crystal and Molecular Structure Visualization, Crystal Impact – Dr H. Putz & Dr K. Brandenburg GbR, Kreuzherrenstr. 102, 53227 Bonn, Germany, http://www.crystalimpact.com/diamond.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1038709. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4ra16196c |
| This journal is © The Royal Society of Chemistry 2015 |