Citrate modified β-cyclodextrin functionalized magnetite nanoparticles: a biocompatible platform for hydrophobic drug delivery
Kunnoth N. Jayaprabha and
Pattayil A. Joy*
Physical and Materials Chemistry Division, CSIR-National Chemical laboratory, Pune, India 411008. E-mail: pa.joy@ncl.res.in
First published on 17th February 2015
Abstract
Water-dispersible magnetite nanoparticles functionalized with citric acid (CIT) modified β-cyclodextrin (CD) are prepared and curcumin (CUR) is loaded inside the cavity of CD. The CUR loading capacity of CD–CIT functionalized magnetite nanoparticles is found to be much larger than that of CD alone as well as the CD–CIT conjugate. The release profile of curcumin is found to follow zero order kinetics at the physiological pH, and thus, can release CUR at a constant rate, after the initial burst release. Relaxivity studies using NMR showed that the functionalized nanoparticles are suitable for contrast enhancement in MRI. Thus, the water-dispersible, CIT modified β-CD functionalized magnetite nanofluid is an efficient carrier for water insoluble curcumin, and can be used for magnetic drug targeting/delivery as well as for contrast enhancement in MRI due to the superparamagnetic magnetite core.
I. Introduction
Drug targeting and release, especially hydrophobic drugs, is an area of intense research using nanomaterials with an emphasis on their multifunctionality. Continuous efforts are being made to develop systems for controlled release of drugs because the appropriate dosage decides the therapeutic efficiency of the drugs. The main objectives of the current drug delivery research using appropriate nanoparticles are to develop specific targeting and delivery of drugs, reduction in toxicity while maintaining the therapeutic effects, greater safety and biocompatibility.1 Drug delivery systems being developed based on nanotechnology include polymeric micelles, polymeric nanoparticles, magnetic nanoparticles, liposomes and dendrimers.2–4 Of all these, magnetic nanoparticles are of specific interest in drug delivery due to the benefit of targeting the carrier using an external magnetic field,5 and therefore, the use of magnetic nanoparticles for site-specific drug release are also an important area of research.6 Nanoparticles with magnetite core and organic surface coating are being developed for theragnostic applications, which can be simultaneously used for imaging, targeting and drug delivery.7Superparamagnetic iron oxide nanoparticles (SPIONs) have gained the attention of researchers due to their biocompatibility and unique magnetic properties at the nanoregime where these nanoparticles exhibit magnetism only in the presence of a magnetic field.8 Modified SPIONs are widely marketed as MRI contrast agents in different names such as feridex, ferumoxtran, resovist, etc.9 Iron oxide (Fe3O4, magnetite) nanoparticles coated with suitable molecules also act as a multifunctional platform which can be simultaneously used as contrast agents in magnetic resonance imaging (MRI), magnetic hyperthermia and drug delivery.10 Even though magnetite nanoparticles are studied during the past many decades, stabilization of these nanoparticles in aqueous medium is still a challenge. The nanoparticles undergo aggregation by van der Waal's interaction, apart from the aggregation due to the magnetic dipole–dipole interactions.11 Hence, it is necessary to coat the surface of the magnetite nanoparticles with suitable surfactants to avoid aggregation and the coated nanoparticles should be biocompatible and biodegradable to be used for biomedical applications.12
Even though there are various reports on the biomedical applications of magnetite nanoparticles, delivery of hydrophobic drugs using these particles, without losing the therapeutic efficacy of the drug is of great importance. The delivery of the hydrophobic drugs to the target site is suggested through different carriers like polymeric micelles, silica nanoparticles, cyclodextrin derivatives etc.13–15 Of these, cyclodextrins, which have a hydrophobic cavity, can be an efficient candidate for entrapment of hydrophobic drugs. Cyclodextrins are cyclic oligosaccharides of a glucopyranose, with a hydrophobic inner cavity and hydrophilic outer surface.16 These molecules easily form inclusion complexes both in solution and solid state.17 The widely used natural cyclodextrins are α-, β-, and γ-cyclodextrins, consisting of six, seven, and eight D-glucopyranose residues, respectively, linked by α-1,4-glycosidic bonds into a macrocycle. They are known to have the ability to form inclusion complexes with guest molecules, which are being widely applied in food, cosmetics and pharmaceutical industries and also for analytical purpose. β-cyclodextrin, the most common natural cyclodextrin, has 21 hydroxyl groups with a cavity diameter of 7.8 Å.18 It is also used as a drug carrier to increase the stability, solubility and bioavailability of drug molecules.19
Curcumin is well-known for its anti-cancer activity and there is a renewed interest in the recent past on the studies on the delivery of curcumin at the target site using a carrier, due to its poor water solubility.20–22 Citric acid modified β-cyclodextrin is reported to be an efficient carrier of water insoluble drugs,23 and cyclodextrin derivative has been studied for the delivery of potential anti-cancer agents like curcumin.24 Cyclodextrin forms a stable inclusion complex with curcumin by supramolecular host–guest interaction25 and the inner cavity of the β form is more appropriate for curcumin loading than the α and γ forms.26 The use of magnetite nanoparticles with a cyclodextrin shell can play a multifunctional role in biomedical applications.27 Water based magnetic fluids, functionalized with cyclodextrin, can be an efficient carrier for hydrophobic drugs and therefore can be used for delivery of the water insoluble drugs at specific sites. The presence of the superparamagnetic magnetite core makes them efficient candidates to be used as a negative contrast agent in magnetic resonance imaging (MRI). Here we report the synthesis, characterization, and studies on curcumin loaded, citric acid modified β-cyclodextrin (CD) capped magnetite nanoparticles. CD has been treated with citric acid (CIT) to form a CD–citric acid conjugate (CD–CIT) to increase the water solubility of CD. It is found that the curcumin loading capacity of CD is increased after functionalizing on the nanoparticles. Thus, the magnetic core and the cyclodextrin coating make the water dispersible fluid an efficient platform for simultaneous imaging (by MRI), drug targeting and delivery.
II. Experimental
Materials
Ferric chloride hexahydrate (≥98%), ferrous chloride tetrahydrate (99%), citric acid monohydrate, curcumin and β-cyclodextrin were purchased from Sigma Aldrich. Ammonium hydroxide (25%), dimethyl sulphoxide (DMSO), nitric acid and 2-propanol were procured from Merck. All the chemicals were used without further purification and double distilled water was used throughout this work.Preparation of CD–CIT complex
The CD–CIT complex was prepared by modifying the procedure reported by El-Tahlawy et al.28 3 g of β-cyclodextrin and 1 g of citric acid was dissolved in 10 ml of water and the mixture was stirred at 80 °C for three hours. The transparent solution obtained was treated with 2-propanol, which gave a white precipitate. The product was washed thoroughly to remove unreacted components and further dried at 60 °C for 24 hours to get the white CD–CIT complex.Preparation of surface functionalized magnetite nanoparticles
Magnetite nanoparticles were prepared by the reverse co-precipitation method.29 A mixed solution of 2 mmol of FeCl3·6H2O and 1 mmol of FeCl2·4H2O in water was added to 100 ml of 19% ammonium hydroxide solution under argon atmosphere. The mixture was stirred well for complete formation and growth of magnetite nanoparticles. The nanoparticles were washed with distilled water to remove excess base. Then the pH was brought down to 7 by washing with water and the resultant nanoparticles were re-dispersed in 100 ml distilled water. 2 g of the CD–CIT complex dissolved in water was added drop-wise to the dispersion and stirred for 4 hours at 80 °C. The stable dispersion obtained was then dialyzed against water for three days to remove excess CD–CIT complex. The dispersion was then dried at 70 °C to get solid nanoparticles. The coated nanoparticles were well dispersible in water and at the physiological pH to form a nanofluid. The sample was labeled as CDmf. Citric acid coated magnetite nanoparticles were also synthesized following the same procedure for comparison. The nanoparticles coated with citric acid also formed stable dispersion in aqueous media and was labeled as CITmf. Uncoated nanoparticles were also prepared under the same reaction conditions and labeled as Unmf (Table 1).Magnetite nanoparticles directly coated with curcumin was synthesized by the procedure reported earlier.30 Briefly, a mixture of ferric chloride hexahydrate and ferrous chloride tetrahydrate, taken in the molar ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, was added to ammonia solution to form magnetite nanoparticles. After stirring for 30 minutes, dilute nitric acid was added to bring down the pH to ∼8–9. Curcumin solution at the same pH was added and the dispersion was stirred for the effective coating of curcumin on the magnetite nanoparticles. The final dispersion was dialyzed against water to remove unreacted excess curcumin and ammonia. The dispersion was dried to get a powder which forms stable dispersion in dimethyl sulfoxide. The curcumin encapsulated sample was labeled as CURmf (Table 1).
1, was added to ammonia solution to form magnetite nanoparticles. After stirring for 30 minutes, dilute nitric acid was added to bring down the pH to ∼8–9. Curcumin solution at the same pH was added and the dispersion was stirred for the effective coating of curcumin on the magnetite nanoparticles. The final dispersion was dialyzed against water to remove unreacted excess curcumin and ammonia. The dispersion was dried to get a powder which forms stable dispersion in dimethyl sulfoxide. The curcumin encapsulated sample was labeled as CURmf (Table 1).
Preparation of CUR inclusion complex
The inclusion complexes were prepared by modifying the procedure reported by Yallapu et al.31 20 mg of the CD–CIT coated sample (CDmf) was dispersed in 30 ml water in a 50 ml vial. To this dispersion, varying amounts of curcumin (10 mg, 20 mg and 30 mg), dissolved in 1 ml acetone, were added while stirring gently. The mixture was stirred for 6 hours to evaporate acetone. The dispersion was then stirred overnight and centrifuged at 5000 rpm for 5 minutes. The supernatant liquid which contains highly water dispersed inclusion complex was dried and stored at 5 °C for further use. The resultant inclusion complexes were labeled as CDmf10, CDmf20 and CDmf30. Inclusion complexes were also prepared using CD alone and the CD–CIT conjugate using 20 mg curcumin and 20 mg of the compound. They were designated as CD20 and CD–CIT20, respectively (details in Table 1).Curcumin loading studies
1 mg of the solid curcumin inclusion complex was dispersed in 10 ml dimethyl sulfoxide (DMSO) to extract the curcumin to the solvent. This dispersion was shaken on a vortex shaker for 24 hours at room temperature. The vial containing the dispersion was covered with an aluminium foil to prevent exposure to light. The dispersion was then centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm to remove the curcumin-free CD–CIT coated sample and the clear yellow supernatant solution of curcumin in DMSO was collected and used for estimation. The amount of curcumin released was estimated from the absorbance measured at 425 nm using a standard graph of absorbance of curcumin dissolved in DMSO.
000 rpm to remove the curcumin-free CD–CIT coated sample and the clear yellow supernatant solution of curcumin in DMSO was collected and used for estimation. The amount of curcumin released was estimated from the absorbance measured at 425 nm using a standard graph of absorbance of curcumin dissolved in DMSO.
The curcumin entrapment efficiency (EE) is calculated using the formula:
In vitro release
The release of curcumin from the CD–CIT coated sample was done at pH 7.4 and pH 5.5, by the dialysis bag method. The CDmf20 sample which showed maximum curcumin loading was dispersed in the phosphate buffer (pH = 7.4) at a concentration of 1 mg ml−1, sonicated to form a stable dispersion and was transferred to a dialysis bag. The dialysis bag tied at both ends was immersed in 50 ml buffer solution and stirred gently. 2 ml of the buffer was withdrawn at particular intervals and replenished with the same amount of fresh buffer. The absorbance was measured at 425 nm, the λmax for curcumin. The amount of curcumin released was then plotted against time. Release rate of curcumin was also determined using acetate buffer (pH = 5.5) using the same procedure.Characterization
Phase purity of the iron oxide nanoparticles was determined by powder X-ray diffraction (XRD) using a PANalytical X'PERT PRO model X-ray diffractometer, in the 2θ range of 10 to 80 degrees, using Cu Kα radiation. TEM analysis was performed on a FEI, TECNAI G2 TF30 instrument. Samples were prepared by placing a drop of dilute dispersion on a carbon coated 200 mesh copper grid and imaged at an accelerating voltage of 300 kV. Zeta potential and hydrodynamic particle size were measured using the dynamic light scattering (DLS) technique using a Brookhaven instruments 90Plus Particle Size Analyzer equipped with a 632.8 nm laser. Infrared spectra were recorded on a Tensor 27 Bruker FT-IR spectrometer, using KBr pellets, in the frequency range of 4000–400 cm−1. Thermogravimetric analysis (TGA) of the synthesized samples, in air, was performed on a Perkin-Elmer TGA7 analyzer. Magnetic measurements were carried out on a Quantum Design MPMS 7TSQUID-VSM. Zero field cooled (ZFC) and field cooled (FC) magnetization measurements were carried out in an applied field of 5 mT (50 Oe) and magnetization versus field measurements, at room temperature, were carried out from −3 T to +3 T. UV-visible spectra were recorded using a Cary 5000 UV-vis-NIR spectrophotometer and the measurements were carried out in a Quartz cell of 10 mm path length. The absorbance measurements for the study of curcumin release were also done on the same instrument. Fluorescence measurements were performed using a Photon Technology International fluorescence QM40 spectrophotometer with a Quartz cell of 10 mm path length. The T1 and T2 relaxation studies were done on a Bruker AV400 NMR spectrometer at a magnetic field of 9.4 Tesla and 400 MHz frequency.III. Results and discussion
Water soluble CD–CIT complex is formed by the esterification between the primary hydroxyl group of CD and the –COOH group of citric acid. The possible products after the reaction are shown in Scheme 1. Evidence for the formation of the CD–CIT complex as well as information on the effectiveness of coating of the CD–CIT conjugate on the surface of the magnetite nanoparticles is confirmed from infrared spectroscopic studies. The IR spectra of CD, citric acid, CD–CIT complex and CDmf20 are shown in Fig. 1. The spectra of the CD–CIT complex resemble the spectra of CD. The major bands at 3350 cm−1, 2925 cm−1, 1158 cm−1 and 1029 cm−1 of CD correspond to the stretching vibrations of –OH, –CH2, –C–C and bending vibration of –OH groups, respectively. The band at 1645 cm−1 corresponds to the H–O–H deformation band of water present in the cavity of CD. The band at 1750 cm−1 in the spectra of citric acid is due to the vibration of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group of the carboxylic acid and this band is shifted to 1730 cm−1 in CD–CIT due to the formation of ester.28 The band at 1730 cm−1, which is due to the C
O group of the carboxylic acid and this band is shifted to 1730 cm−1 in CD–CIT due to the formation of ester.28 The band at 1730 cm−1, which is due to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching of the ester group, is a clear indication for the formation of the CD–CIT conjugate. The intensity of this band of CD–CIT is reduced in CDmf, after coating on the magnetite nanoparticles, indicating that the CD–CIT conjugate binds to the nanoparticle via the C
O stretching of the ester group, is a clear indication for the formation of the CD–CIT conjugate. The intensity of this band of CD–CIT is reduced in CDmf, after coating on the magnetite nanoparticles, indicating that the CD–CIT conjugate binds to the nanoparticle via the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group of the citric acid. The bands in the IR spectra of the coated nanoparticles resemble that of the CD–CIT conjugate indicating the bonding of the conjugate to nanoparticles surface. The band at 1645 cm−1 corresponding to the vibrations of water in the CD cavity is found to be retained in the coated nanoparticles also, indicating that the iron oxide nanoparticles are not occupied inside the cavity and binds to the CD–CIT complex without disturbing the cavity.32,33
O group of the citric acid. The bands in the IR spectra of the coated nanoparticles resemble that of the CD–CIT conjugate indicating the bonding of the conjugate to nanoparticles surface. The band at 1645 cm−1 corresponding to the vibrations of water in the CD cavity is found to be retained in the coated nanoparticles also, indicating that the iron oxide nanoparticles are not occupied inside the cavity and binds to the CD–CIT complex without disturbing the cavity.32,33
 | ||
| Fig. 1 IR spectra of citric acid (CIT), citric acid–cyclodextrin complex (CD–CIT), β-cyclodextrin (CD), citric acid (CIT) and the CD–CIT coated magnetite nanoparticles (CDmf). | ||
| Sample label | Description |
|---|---|
| CIT | Citric acid |
| CD | β-Cyclodextrin |
| CUR | Curcumin |
| CD–CIT | Cyclodextrin–citrate complex |
| Unmf | Uncoated magnetite nanoparticles |
| CITmf | Citric acid coated magnetite nanoparticles/nanofluids |
| CURmf | Curcumin coated magnetite nanoparticles/nanofluids |
| CDmf | CD–CIT coated magnetite nanoparticles/nanofluids |
| CDmf10, CDmf20, CDmf30 | Curcumin loaded CDmf nanofluids by using 20 mg of CDmf and 10, 20 and 30 mg of curcumin, respectively |
| CD20 | Curcumin loaded CD using 20 mg of curcumin |
| CD–CIT20 | Curcumin loaded CD–CIT using 20 mg of curcumin |
The average crystallite size of the CDmf nanoparticles is calculated as 5 nm from the XRD pattern using the Scherrer equation.34 The TEM image in Fig. 2(a) shows isolated particles with average particle size of 5 nm, comparable to the crystallite size. Average particle size of 7.7 nm, with a polydispersity of 0.261, is obtained from DLS measurements as shown in Fig. 2(b).
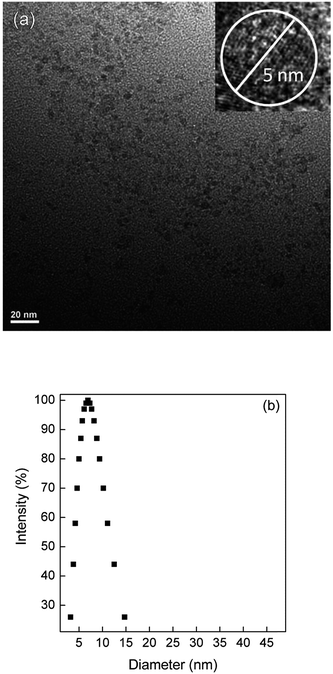 | ||
| Fig. 2 (a) TEM image of CDmf with the inset showing a single particle of size 5 nm, and (b) the log-normal size distribution from DLS measurement showing a mean particle size of 7.7 nm. | ||
TGA curve of CDmf is compared with that of CD and the CD–CIT conjugate in Fig. 3. The total weight loss for CDmf is about 60% and the weight loss path resembles that of bare CD–CIT conjugate, except for a shift in the third weight loss to higher temperatures. The differential thermograms (DTG) of both curcumin treated and untreated sample are used to calculate the amount of water expelled from the cavity of CD (host) to accommodate the curcumin molecule (guest). Dehydration of CDmf and CD–CIT caused a total mass loss of 8.2% and 8.5% (first weight loss below 100 °C), and this corresponds to loss of 6.1 and 6.3 water molecules, respectively, from the cavity of CD. The dehydration of CDmf20 results in a mass loss of 6.1%, indicating the removal of 4.5 water molecules from the CD cavity. Thus, the TGA/DTG results indicate that a fraction of the water molecules has escaped from the CD cavity to accommodate the guest molecule (CUR). It is known that, in aqueous solution, the slightly apolar cyclodextrin cavity is occupied by water molecules which are energetically unfavored (polar–apolar interaction), and therefore can be readily substituted by appropriate guest molecules which are less polar than water. The driving force for the complex formation is the substitution of the high enthalpy water molecules by an appropriate guest molecule.35
 | ||
| Fig. 3 TGA curves of the CD–CIT complex, CDmf and curcumin loaded CDmf (CDmf20). The inset shows the corresponding differential thermograms (DTG). | ||
The M vs. H curves of the iron oxide samples measured at room temperature, before and after surface modifications, are shown in Fig. 4. The lower values of the magnetization (emu per g of sample) of the surface modified samples are due to the non-magnetic organic molecules present. Continuous increase in the magnetization at higher fields and the absence of magnetic hysteresis (zero coercivity) confirm that the iron oxide nanoparticles are superparamagnetic. This is further confirmed from temperature dependent magnetization measurements. The zero field cooled (ZFC) and field cooled (FC) magnetization curves of the uncoated and the different coated nanoparticles are compared in Fig. 5. The superparamagnetic blocking temperature (TB), corresponding to the temperature at which a maximum is observed in the zero field cooled (ZFC) magnetization curve, for the uncoated (Unmf) and citric acid coated (CITmf) samples are obtained as 110 K and 40 K, respectively. Inter-particle magnetic interactions (dipolar and exchange) are known to be reduced or suppressed after coating the magnetic nanoparticles using suitable surfactants due to the decreasing magnetic anisotropy contributed by these interactions and this is evidenced by the decrease in the value of TB after capping. CDmf and the inclusion complex CDmf20 show almost comparable values of TB as 20 K. The lower value of TB for CDmf and CDmf20, compared to the value for CITmf, is due to the further decrease in the anisotropy due to the suppression of magnetic dipolar interactions due to the larger molecules separating the nanoparticles. The FC magnetization of CITmf, CDmf and CDmf20 increases continuously below the blocking temperature which is not observed for Unmf. This behavior shows that the particles are well separated in the case of the coated nanoparticles, where the coatings suppress the magnetic dipolar and exchange interactions between the particles.36 The FC curve of Cdmf shows a saturating trend at very low temperatures (inset of Fig. 5(c)) whereas this trend is not observed for CDmf20 (inset of Fig. 5(d)). This explains the further suppression of the dipolar interactions due to the increasing separation between the nanoparticles after inclusion of curcumin in the CD cavities.
 | ||
| Fig. 4 Magnetization curves of the uncoated and different coated iron oxide nanoparticles, measured at room temperature. | ||
The possible mode of interaction between the cyclodextrin cavity and curcumin is the encapsulation of the benzene ring of curcumin in the cavity of CD. An inclusion complex is formed by increasing the distance between two magnetite nanoparticles. Based on the results, a schematic representation of the formation of a possible supramolecular self-assembly of the nanoparticles of the curcumin inclusion complex can be represented as shown in Scheme 2. CD is represented as a truncated cone with large and small openings at two ends. The hydroxyl groups are at the two rims of the cone and the interior has hydrocarbon chains, making it hydrophobic. The CH3 protons interact with the aromatic ring of the curcumin via CH–π interaction.37
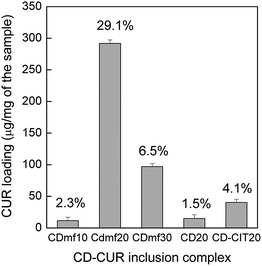
The CD–CIT coated magnetite nanoparticles (CDmf) formed stable water dispersion on re-dispersing the powder obtained from the synthesis procedure described. The CD–CIT coated nanoparticles dispersed in water are treated with curcumin at different weight ratios. The curcumin inclusion complexes, CDmf10, CDmf20 and CDmf30, are analyzed for their curcumin loading capacity (Fig. 6). The encapsulation efficiency was found to be higher when 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 weight ratio of the sample and curcumin (CDmf20) is used (20 g each). The encapsulation efficiency of the coated nanoparticles is also compared with that of bare CD as well as the CD–CIT complex (Fig. 6). Higher efficiency is observed for CD–CIT (4.1%) compared to CD (1.5%). The higher inclusion complexation of CD–CIT (host) to curcumin (guest), compared to that of CD as the host, can be due to the higher solubility CD–CIT due to the binding of the citrate group. The solubility of bare β-cyclodextrin in water is found to be ∼18 mg ml−1, comparable to that reported in the literature,18 whereas the solubility of CD–CIT complex is obtained as ∼60 mg ml−1. The curcumin loading efficiency of CDmf is found to be much larger than that obtained by using polymer nanoparticles.31 Polymeric nanoparticles are studied as a carrier of hydrophobic drugs of which poly(lactic-co-glycolic acid) nanoparticles (PLGA nanoparticles) are the most studied. PLGA nanoparticles show a maximum curcumin loading of about 5–10%.22,38 The amount of curcumin loaded in the case of CD–CIT bound on magnetite nanoparticles is larger than that of the bare CD–CIT complex. In the case of CD–CIT coated nanoparticles, the cyclodextrin cavities are probably more ordered (see Scheme 2) which results in higher encapsulation efficiency. Hence, the coating of the magnetite nanoparticles with the CD–CIT complex increases the curcumin encapsulation efficiency of β-cyclodextrin. Natural therapeutic agents like curcumin needs to be supplied at a higher dose at the affected site,28 and hence the enhanced drug loading efficiency on the CD–CIT modified coated nanoparticles may be highly effective for drug delivery applications.
1 weight ratio of the sample and curcumin (CDmf20) is used (20 g each). The encapsulation efficiency of the coated nanoparticles is also compared with that of bare CD as well as the CD–CIT complex (Fig. 6). Higher efficiency is observed for CD–CIT (4.1%) compared to CD (1.5%). The higher inclusion complexation of CD–CIT (host) to curcumin (guest), compared to that of CD as the host, can be due to the higher solubility CD–CIT due to the binding of the citrate group. The solubility of bare β-cyclodextrin in water is found to be ∼18 mg ml−1, comparable to that reported in the literature,18 whereas the solubility of CD–CIT complex is obtained as ∼60 mg ml−1. The curcumin loading efficiency of CDmf is found to be much larger than that obtained by using polymer nanoparticles.31 Polymeric nanoparticles are studied as a carrier of hydrophobic drugs of which poly(lactic-co-glycolic acid) nanoparticles (PLGA nanoparticles) are the most studied. PLGA nanoparticles show a maximum curcumin loading of about 5–10%.22,38 The amount of curcumin loaded in the case of CD–CIT bound on magnetite nanoparticles is larger than that of the bare CD–CIT complex. In the case of CD–CIT coated nanoparticles, the cyclodextrin cavities are probably more ordered (see Scheme 2) which results in higher encapsulation efficiency. Hence, the coating of the magnetite nanoparticles with the CD–CIT complex increases the curcumin encapsulation efficiency of β-cyclodextrin. Natural therapeutic agents like curcumin needs to be supplied at a higher dose at the affected site,28 and hence the enhanced drug loading efficiency on the CD–CIT modified coated nanoparticles may be highly effective for drug delivery applications.
The zeta potential of the different formulations is measured by dispersing them in water. The zeta potential of CDmf is measured as −19.2 mV. CITmf also gave stable water dispersion with a zeta potential of −21.8 mV. The negative zeta potential values help repel the particles in the suspension resulting in long term stability by avoiding aggregation of the particles.27 The zeta potential for CDmf10, CDmf20 and CDmf30 are obtained as −33.2, −30.3 and −35.8 mV, respectively, indicating the high stability of the dispersions. The relatively lower zeta potential of the highly loaded CDmf20 is due to the blocking of the –OH groups by the curcumin molecule. The negative charge indicates that the unsubstituted –OH groups are pointing towards the aqueous surrounding thereby rendering the hydrophilicity.25
The curcumin loaded inclusion complex (CDmf20) did not show the peaks of curcumin in the FT-IR spectra (spectra not shown), especially the peaks of aromatic ring, since the aromatic group is effectively bound inside the cavity of CD. Moreover, it has been reported that the curcumin bands are masked in the IR spectra when CD is present along with curcumin due to the overlapping of the bands of CD and curcumin.39 Similarly, the UV-visible spectra also do not show any sharp peak at 425 nm which is the characteristic absorption maximum of curcumin. However, the inclusion complex once treated with dimethyl sulfoxide (DMSO) gives the characteristic peak of curcumin, as shown in Fig. 7, where the UV spectra of CDmf dispersed in different media are compared. The UV-vis spectrum of the magnetic fluid with curcumin loaded in the CD cavity is comparable with that of the nanofluid without curcumin. In aqueous media, the CDmf20 sample with the highest encapsulation efficiency does not show the characteristic peak of CUR whereas once the sample is dispersed in DMSO, the characteristic peak at 425 nm is observed, due to the release of CUR from the CD cavity in the solvent DMSO. The higher solubility of CUR in DMSO compared to that in water renders CUR to overcome its interaction with the –OH group of the CD cavity.
The release profile of CUR from CDmf20 sample was analyzed at the physiological pH 7.4 and that of the diseased cells pH 5.5, as reported in the literature.40,41 The release profiles are compared with nanoparticles directly coated with CUR (CURmf). As shown in Fig. 8, the amount of CUR released from the CD cavity is very low at pH 5.5, initially, compared to that released at the physiological pH 7.4. In the case of CDmf there is an initial burst release whereas the curcumin directly coated on the magnetite nanoparticles shows a pulsatile release at the initial stage itself. The amount of curcumin released from CURmf is very low compared to that from CDmf. Stella et al. have reported the mechanism of drug release from CD cavities, where dissociation being the major release mechanism.42 The amount of CUR released is plotted against time and fitted to a straight line using the equation
| Q = Q0 + k0t |
 | ||
| Fig. 8 The drug (curcumin) release profile of (a) CDmf and (b) CURmf at pH7.4 and 5.5. The inset of (a) shows the zero order fitting curves of the release profile. | ||
The relaxivity of cyclodextrin coated magnetite nanoparticles is measured on an NMR spectrophotometer at a magnetic field of 9.4 T and frequency of 400 MHz. The CDmf sample was dispersed in water at different concentrations and the spin-lattice relaxation time T1 and spin–spin relaxation time T2 are measured. The reciprocals of the relaxation times are plotted against concentration (Fig. 9) to obtain the corresponding relaxivity values r1 and r2 which describe the ability to shorten the relaxation times per millimole of the concentration of contrast agent.44 The relaxivity values, r1 and r2, calculated from the slopes of the plots are 0.0082 mM−1 s−1 and 6.875 mM−1 s−1, respectively. The r2/r1 ratio is obtained as 838. The r2 and r1 values are calculated by considering the particle diameter as 5 nm as obtained from TEM, which will have approximately 880 magnetic iron ions.45 The relaxivity values are known to depend on the frequency as well as the applied magnetic field and the relaxivity ratio, r2/r1, decides whether a material can be used as a contrast agent or not.46,47 The r2/r1 ratio is larger than the minimum threshold (= 2) value required to be used as an effective contrast agent.48 Hence the modified cyclodextrin coated nanoparticles can be used as a negative contrast agent in MRI, along with the capability of the delivery of curcumin.
 | ||
| Fig. 9 The reciprocals of (a) spin lattice (T1) and (b) spin–spin (T2) relaxation times plotted against concentration. | ||
IV. Conclusions
Magnetite nanoparticles coated with citrate modified β-cyclodextrin are synthesized and characterized using TEM, DLS, XRD, FT-IR and UV-visible spectroscopy. The magnetic characteristics are studied using a SQUID-VSM. The cavity of CD on the coated nanoparticles forms an inclusion complex with curcumin and the curcumin loading efficiency is found to be larger than that on free CD. The in vitro release profile shows higher amount of CUR release as compared to CUR directly coated on the magnetite nanoparticles. CUR is released in the initial stage at a faster rate known as the “burst release” followed by release at a constant rate following the zero order kinetics at both pH = 7.4 and pH = 5.5. Hence, the as-synthesized nanoparticles, which form a stable fluid in water, can be effectively used for targeting and delivery of hydrophobic drugs to the affected site. The magnetic core adds the benefit of targeting via an external magnetic field. The relaxivity of the nanofluid was studied at a frequency of 400 MHz. The calculated relaxivity ratio is well above the minimum threshold value to be used as a negative contrast agent in MRI. Hence, the synthesized nanoparticles which form a stable water suspension can be simultaneously used for imaging, targeting and delivery of hydrophobic drugs to the affected site.Acknowledgements
K. N. Jayaprabha is grateful to Council of Scientific and Industrial Research (CSIR), India, for financial assistance in the form of a research fellowship.References
- W. H. D. Jong and J. P. Borm, Int. J. Nanomed., 2008, 3, 133–149 CrossRef.
- M. Jones and J. C. Leroux, Eur. J. Pharm. Biopharm., 1999, 48, 101–111 CrossRef CAS.
- V. P. Torchilin, J. Controlled Release, 2001, 73, 137–172 CrossRef CAS.
- M. Colombo, S. C. Romero, M. F. Casula, L. Gutièrrez, M. P. Morales, I. B. Böhm, J. T. Heverhagen, D. Prosperi and W. J. Parak, Chem. Soc. Rev., 2012, 41, 4306–4334 RSC.
- S. Kayal and R. V. Ramanujan, Mater. Sci. Eng., C, 2010, 30, 484–490 CrossRef CAS PubMed.
- C. Xu and S. Sun, Adv. Drug Delivery Rev., 2013, 65, 732–743 CrossRef CAS PubMed.
- M. Liong, J. Lu, M. Kovochich, T. Xia, S. G. Ruehm, A. E. Nel, F. Tamanoi and J. I. Zink, ACS Nano, 2008, 2, 889–896 CrossRef CAS PubMed.
- M. Mahmoudi, S. Sant, B. Wang, S. Laurent and T. Sen, Adv. Drug Delivery Rev., 2011, 63, 24–46 CrossRef CAS PubMed.
- Y. J. Wang, Quantitative Imaging in Medicine and Surgery, 2011, 1, 35–40 Search PubMed.
- T. K. Jain, J. Richey, M. Strand, D. L. Leslie-Pelecky, C. A. Flask and V. Labhasetwar, Biomaterials, 2008, 29, 4012–4021 CrossRef CAS PubMed.
- S. Laurent, D. Forge, M. Port, A. Roch, C. Robic, L. V. Elst and R. N. Muller, Chem. Rev., 2008, 108, 2064–2110 CrossRef CAS PubMed.
- A. K. Gupta and M. Gupta, Biomaterials, 2005, 26, 3995–4021 CrossRef CAS PubMed.
- M. Sun, Y. Gao, C. Guo, F. Cao, Z. Song, Y. Xi, A. Yu, A. Li and G. Zhai, J. Nanopart. Res., 2010, 12, 3111–3122 CrossRef CAS.
- J. Lu, M. Liong, J. I. Zink and F. Tamanoi, Small, 2007, 3, 1341–1346 CrossRef CAS PubMed.
- K. Uekama, F. Hirayama and T. Irie, Chem. Rev., 1998, 98, 2045–2076 CrossRef CAS PubMed.
- A. Vyas, S. Saraf and S. Saraf, J. Inclusion Phenom. Macrocyclic Chem., 2008, 62, 23–42 CrossRef CAS.
- F. Hirayama and K. Uekama, Adv. Drug Delivery Rev., 1999, 36, 125–141 CrossRef CAS.
- J. Zhang and P. X. Ma, Adv. Drug Delivery Rev., 2013, 65, 1215–1233 CrossRef CAS PubMed.
- M. E. Brewster and T. Loftsson, Adv. Drug Delivery Rev., 2007, 59, 645–666 CrossRef CAS PubMed.
- T. K. Biji and R. H. Scofield, Trends Pharmacol. Sci., 2009, 30, 334–335 CrossRef PubMed.
- R. Wilken, M. S. Veena, M. B. Wang and E. S. Srivatsan, Mol. Cancer, 2011, 10, 12–31 CrossRef CAS PubMed.
- P. Verderio, P. Bonetti, M. Colombo, L. Pandolfi and D. Prosperi, Biomacromolecules, 2013, 14, 672–682 CrossRef CAS PubMed.
- S. S. Banerjee and D. H. Chen, Chem. Mater., 2007, 19, 6345–6349 CrossRef CAS.
- M. M. Yallapu, M. Jaggi and S. C. Chauhan, Macromol. Biosci., 2010, 10, 1141–1151 CrossRef CAS PubMed.
- H. Rachmawati, C. A. Edityaningrum and R. Mauludin, AAPS PharmSciTech, 2013, 14, 1303–1312 CrossRef CAS PubMed.
- V. R. Yadav, S. Suresh, K. Devi and S. Yadav, AAPS PharmSciTech, 2009, 10, 752–762 CrossRef CAS PubMed.
- M. M. Yallapu, S. F. Othman, E. T. Curtis, B. K. Gupta, M. Jaggi and S. C. Chauhan, Biomaterials, 2011, 32, 1890–1905 CrossRef CAS PubMed.
- K. El-Tahlawy, M. A. Gaffar and S. El-Rafie, Carbohydr. Polym., 2006, 63, 385–392 CrossRef CAS PubMed.
- V. Sreeja, K. N. Jayaprabha and P. A. Joy, Appl. Nanosci., 2014 DOI:10.1007/s13204-014-0335-0.
- K. N. Jayaprabha and P. A. Joy, J. Nanofluids, 2014, 3, 1–7 CrossRef CAS PubMed.
- M. M. Yallapu, M. Jaggi and S. C. Chauhan, Colloids Surf., B, 2010, 79, 113–125 CrossRef CAS PubMed.
- N. V. Roik and L. V. Belyakova, J. Inclusion Phenom. Macrocyclic Chem., 2011, 69, 315–319 CrossRef CAS.
- L. A. C. Cruz, C. A. M. Perez, H. A. M. Romero and P. E. G. Casillas, J. Alloys Compd., 2008, 466, 330–334 CrossRef PubMed.
- B. D. Cullity and S. R. Stock, Elements of X-ray diffraction, Prentice Hall, New Jersey, 3rd edn, 2001 Search PubMed.
- J. Szejtli, Chem. Rev., 1998, 98, 1743–1753 CrossRef CAS PubMed.
- V. Sreeja and P. A. Joy, Int. J. Nanotechnol., 2011, 8, 907–915 CrossRef CAS.
- R. K. Raju, H. Hillier, N. A. Burton, M. A. Vincent, S. Doudou and R. A. Bryce, Phys. Chem. Chem. Phys., 2010, 12, 7959–7967 RSC.
- T. Govender, S. Stolnik, M. C. Garnett, L. Illum and S. S. Davis, J. Controlled Release, 1999, 57, 171–185 CrossRef CAS.
- P. R. Krishna Mohan, G. Sreelakshmi, C. V. Muraleedharan and R. Joseph, Vib. Spectrosc., 2012, 62, 77–84 CrossRef PubMed.
- Y. Tang, Z. Teng, Y. Liu, Y. Tian, J. Sun, S. Wang, C. Wang, J. Wang and G. Lu, J. Mater. Chem. B, 2014, 2, 4356–4362 RSC.
- L.-C. Ho, C.-H. Hsu, C.-M. Ou, C.-W. Wang, T.-P. Liu, L.-P. Hwang, Y.-Y. Lin and H.-T. Chang, Biomaterials, 2015, 37, 436–446 CrossRef CAS PubMed.
- V. J. Stella, V. R. Rao, E. A. Zannou and V. Zia, Adv. Drug Delivery Rev., 1999, 36, 3–16 CrossRef CAS.
- S. Dash, P. N. Murty and L. Nath, Acta Pol. Pharm., 2010, 67, 217–223 CAS.
- U. I. Tromsdorf, O. T. Bruns, S. C. Salmen, U. Beisiegel and H. Weller, Nano Lett., 2009, 9, 4434–4440 CrossRef CAS PubMed.
- J. Bridot, A. Faure, S. Laurent, C. Rivière, C. Billotey, B. Hiba, M. Janier, V. Josserand, J. Coll, L. V. Elst, R. Muller, S. Roux, P. Perriat and O. Tillement, J. Am. Chem. Soc., 2007, 129, 5076–5084 CrossRef CAS PubMed.
- Y. Gossuin, S. Disch, Q. L. Vuong, P. Gillis, R. P. Hermann, J.-H. Park and M. J. Sailor, Contrast Media Mol. Imaging, 2010, 5, 318–322 Search PubMed.
- Y. Gossuin, P. Gillis, A. Hocq, Q. L. Vuong and A. Roch, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2009, 3, 299–310 CrossRef PubMed.
- M. F. Casula, P. Floris, C. Innocenti, A. Lascialfari, M. Marinone, M. Corti, R. A. Sperling, W. J. Parak and C. Sangregorio, Chem. Mater., 2010, 22, 1739–1748 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |