Nano-caged shikimate as a multi-site cross-linker of collagen for biomedical applications†
Abstract
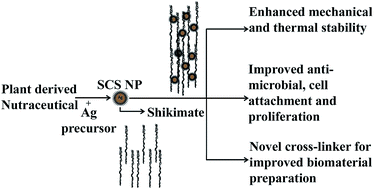
The present study evaluated the application of a nano-biotechnological intervention of nutraceutical shikimate for the development of a potential multi-site cross-linker with enhanced cross linking, anti-microbial and cell proliferative activities. The cross-linking and therapeutic properties of the nutraceutical shikimate were simultaneously utilized by caging them onto silver nanoparticles. The caging of shikimate on silver nanoparticles resulted in the cumulative expression of the physico-chemical properties of both silver nanoparticles and shikimate. We observed that in shikimic acid caged silver nanoparticle (SCS nanoparticle) cross-linked collagen, the viscosity and self-assembly process of collagen, along with its mechanical and thermal properties, were significantly improved when compared with native collagen. The three dimensional conformation of collagen was also retained after cross-linking with SCS nanoparticles. Cell viability with SCS nanoparticle cross-linked collagen was found to be enhanced, in comparison to collagen films cross-linked with shikimic acid and native collagen. SCS nanoparticle-stabilized collagen possessed both cell proliferative and anti-microbial properties, which would make SCS nanoparticles a superior cross-linker of collagen. The results suggested a new strategy for cross-linking collagen and provide scope for alternative biocompatible interventions in the development of biomaterials.

 Please wait while we load your content...
Please wait while we load your content...