An efficient method to improve simultaneously the water resistance, flame retardancy and mechanical properties of POE intumescent flame-retardant systems
Rui-Min Li,
Cong Deng*,
Cheng-Liang Deng,
Liang-Ping Dong,
Hong-Wei Di and
Yu-Zhong Wang*
Center for Degradable and Flame-Retardant Polymeric Materials, College of Chemistry, State Key Laboratory of Polymer Materials Engineering, National Engineering Laboratory of Eco-Friendly Polymeric Materials (Sichuan), Analytical and Testing Center, Sichuan University, Chengdu 610064, China. E-mail: dengcong@scu.edu.cn; yzwang@scu.edu.cn; Fax: +86-28-85410259; Tel: +86-28-85410259
First published on 29th January 2015
Abstract
To improve simultaneously the water resistance, flame retardancy and mechanical properties of an polyethylene-octene elastomer (POE) composite containing ammonium polyphosphate (APP), an effective method was developed, in which the APP was first coated by a silicon compound with a vinyl group, and then introduced into the POE matrix through chemical cross-linking. X-ray protoelectron spectroscopy (XPS) etc. measurements confirmed that the coated APP (CAPP) was prepared successfully. Then, together with a charring agent (CA), CAPP was used to flame retard POE. The combustible performance of the POE composites was investigated by limiting oxygen index (LOI), vertical burning (UL-94), and cone calorimeter (CC) tests. The results showed that the POE composite containing 22.5 wt% CAPP had better flame retardancy than POE containing equal amounts of APP, especially the CAPP system had much lower heat release rate (HRR), total heat release (THR), smoke production rate (SPR), and mass loss rate (MLR) than the APP system with the same amount of flame retardant. Furthermore, the CAPP system passed the UL-94 V-0 rating after water treatment at 70 °C for 7 days; while the APP system did not. Thermal gravimetric analysis (TGA), XPS, etc. measurements demonstrated that the formation of a rich residue containing Si–C, Si–O–C, etc. structures in the residue of the CAPP system should be the most important reason for its better flame retardancy than that of the APP system. Mechanical tests illustrated that the CAPP system had higher tensile strength and elongation at break than the APP system at the same condition, which should be due to the good interfacial adhesion resulting from the existence of silicon compounds at the surfaces of APP and its cross-linking with the POE matrix. All these results illustrate that it should be an efficient method to simultaneously improve the water resistance, flame retardancy and mechanical properties of a POE composite containing APP through functionalizing the APP and then chemically incorporating it into the polymer matrix.
1. Introduction
Due to the inherent bad flame retardancy of POE, some flame-retarded POE composites were developed to expand its application.1–5 At present, the halogen-free flame-retardants are dominant in flame retarding POE. Guo et al.2 used ammonium polyphosphate–pentaerythritol (APP-PER) to flame retard POE with the aid of organo-montmorillonite, and found that significant improvements on thermal stability, flame retardancy and mechanical properties for POE composites were achieved compared with those of neat POE. Hong and co-workers3 applied magnesium hydroxide to flame retard POE blends, and the results showed that the magnesium hydroxide coated with a polymeric material was very efficient to achieve simultaneous improvement of both flame retardation and elongation at break for POE blends.According to the past researches, it can be found that intumescent flame retardants (IFRs) is the most efficient to flame retard POE. For POE/IFR composite, the typical IFR is usually composed of three main components: acid source, carbon source, and gas source.6,7 APP as an acid source and a gas source in IFR has been extensively investigated. However, the APP is moisture-sensitive, and has bad compatibility with many polymer matrices, resulting in the decrease of flame-retardant efficiency and the damage to mechanical properties of polymer composites, Much work has been carried out to improve the water resistance, flame retardancy and mechanical properties of polymer composite containing APP.8,9 At present, the coating through silane coupling agent10,11 or different compatibilzer12,13 at the surface of APP is regarded as the most efficient method to solve the problems mentioned above. The APP prepared through coating has a so-called core–shell structure, which allows the isolation of encapsulated substance from surrounding, and thus protects APP from any degrading factors. Wang et al.14,15 applied two methods to prepare coated APP, in which the first one is that APP was coated by UV-curable epoxy acrylate resin; the second one is that APP was coated by polysiloxane through in situ polymerization. The results showed that both categories of APP products prepared could significantly improve the water resistance and flame retardancy of polypropylene composites.
Although the coating is an very efficient method to improve the water resistance and flame retardancy of polymer composites containing APP, its damage to mechanical properties of polymer is still a big challenge. In order to weaken the damage of APP to polymer matrix, some work has been performed. Hu et al.16,17 reported that the cross-linking through electron beam irradiation could incorporate the coated APP into polymer matrix through the bonding of coating, and the results indicated that excellent mechanical properties were achieved in EVA composites.
Thus, the ideal way to achieve the improvements of water resistance, flame retardancy, and mechanical properties of POE composites containing APP should be that the APP is first functionalized through a material with functional groups, and then the functionalized silica shell are introduced into POE matrix through chemical cross-linking. In this study, a coated APP (CAPP) was prepared by sol–gel process, and then modified with triethoxyvinylsilane, as shown in Scheme 1. The CAPP functionalized was used to prepare flame-retardant POE composites, together with a char-forming agent (CA) and a cross-linker. During the preparation process, CAPP was introduced into POE three-dimensioned network through the radical polymerization proposed by Yin et al.,18 as shown in Scheme 2. The effects of CAPP on the flame retardation, mechanical properties, water resistance, and thermal property of POE were investigated with the aid of different measurements. Finally, the flame retardant mechanism was also discussed in detail.
2. Experimental section
2.1. Materials
The ethylene-octene elastomer (POE, Engage 8150, 25% octene) was supplied by DOW Co. (USA); ammonium polyphosphate (APP, form II, n > 1000) was purchased from Changfeng Chemical Co. (Shifang, China); dicumyl peroxide (bis (1-methyl-1-phenylethyl)) (DCP), tetrathoxysilane (TEOS), alkylphenol polyoxyethylene (10) ether (OP-10) surfactant, ammonia solution (NH3·H2O), triethoxyvinylsilane (VTES), and ethanol, were provided by Kelong Chemical Reagent Co. (Chengdu, China); the nitrogen-containing carbonization agent (poly 1,3,5-triazin-2-aminoethnol) was bought from Weili Flame Retardant Chemicals Co. (Chengdu. China).2.2. Preparation of cross-linked CAPP
The mixture consisting of 50 g APP, 1 g OP-10, 150 mL ethanol, and 50 mL deionized water was fed into 500 mL three-neck flask equipped with a stirrer, and its pH value was adjusted to 9–11 by NH3·H2O, then it was stirred for 10 min at 50 °C, followed by the incorporation of 10 g TEOS. The resulting mixture was kept at 50 °C for 4 h, and then cooled to room temperature. Next, the mixture was filtered, washed with ethanol, and dried at 80 °C in a vacuum oven. Both the dried product and 10 g VTES were put into high-speed mixing machine at 70 °C for 1 h, then cooled to room temperature, finally dried in a vaccum oven at 80 °C to a constant weight. The resulting powder was CAPP.2.3. Preparation of the flame retardant cross-linked POE composites
POE composites containing flame retardant and DCP were prepared through a rheometer (XSS-300, Shanghai Kechuang Plastic Machinery Co., Ltd., China) at a temperature of 170 °C for 10 min. After mixing, the sheet samples for different measurements were obtained through hot pressing at 170 °C under 10 MPa.2.4. Measurement
Fourier transform infrared (FTIR) spectra were recorded by a Nicolet FTIR 170 SX spectrometer (Nicolet, America) using the KBr disk, and the wavenumber range is from 4000 to 400 cm−1.The XPS measurement was carried out on a XSAM80 (Kratos Co., UK) using Al Kα excitation radiation (hν = 1486.6 eV).
Scanning electron microscopy (SEM) was performed on a JEOL JSM 5900LV scanning electron microscope (JEOL, Japan), and the accelerated voltage was 5 kV. The specimens of POE composites were cryogenically fractured in liquid nitrogen, and then sputter-coated with a conductive layer before being examined.
The water contact angle (WCA) of the samples was measured on a JC2000D contact angle measurement instrument (Shanghai Zhongchen Digital Technic Apparatus, China). Before being detected, all samples were pressed into disks. In a measurement, 3 mL deionized water was used, and the observation time was 30 s.
The particle size distributions of APP and CAPP were characterized by a laser diffraction particle analyzer (Master Sizer 2000, Malvern Instruments Ltd., UK). Before the measurement, the samples were dispersed in distilled water and sonicated for 30 min.
To measure the solubility in water. 10 g APP or CAPP was put into 100 mL of distilled water at different temperatures, and stirred for 1 h. Next, the suspension was filtered, and 50 mL filtrate was taken out, then dried to a constant weight at 100 °C. Finally, the solubility of samples in water could be obtained.
The LOI value of all samples with the dimension of 130 mm × 6.5 mm × 3.2 mm was measured using an HC-2C oxygen index instrument (Jiangning, China) according to ASTM D2863-97.
The UL-94 vertical burning level was tested on a CZF-2 instrument (Jiangning, China) according to ASTM D3801. The dimension of all samples is 130 mm × 13 mm × 3.2 mm.
To determine the combustible performance of POE composite after water resistance measurement. The specimens were put in 500 mL of distilled water at 70 °C, and kept at this temperature for 168 h. Then, these specimens were taken out, and dried in a vacuum oven for burning tests.
The TGA was performed on a thermogravimetric analyzer instrument (209 F1, NETZSCH, Germany) at a heating rate of 10 °C min−1 under N2 atmosphere at a flow rate of 50 mL min−1 in the temperature range from 40 to 700 °C.
The flammability of POE composites was measured by a CC device (Fire Testing Technology, UK). The samples with dimension of 100 mm × 100 mm × 3 mm were exposed to a radiant cone at a heat flux of 50 kW m−2.
The TG-FTIR analysis was performed on a TG 209 F1 apparatus (NETZSCH, Germany) coupled with a 170 SX FTIR spectrometer (Nicolet, America). The sample (about 6 mg) was heated at a rate of 10 °C min−1 in the temperature range from 40 to 700 °C under nitrogen atmosphere.
3. Results and discussion
3.1. Characterization of CAPP
The FTIR spectra of APP and CAPP are shown in Fig. 1. The typical absorption peaks of APP include 3200 cm−1 (N–H), 1254 cm−1 (P–O), 1078 cm−1 (P–O symmetric stretching vibration), 1017 cm−1 (symmetric vibration of PO2 and PO3), 886 cm−1 (P–O asymmetric stretching vibration), and 802 cm−1 (P–O–P).19 The spectra of CAPP show new absorption bands at 3060 cm−1, 1620 cm−1, and 1110 cm−1, which correspond to the stretching vibration of unsaturated C–H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and asymmetric Si–O–Si stretching,16 respectively, indicating the existence of silicon compound in CAPP.20
C and asymmetric Si–O–Si stretching,16 respectively, indicating the existence of silicon compound in CAPP.20
The XPS spectra of APP and CAPP are shown in Fig. 2 and the related elemental compositions of APP and CAPP are presented in Table 1. For CAPP, the P, N, and O contents are 0.77 wt%, 0.32 wt%, and 33.58 wt%, respectively, which are much lower than the corresponding values of APP. However, the C content of CAPP is 33.45 wt%, much higher than that of APP. Generally, the detected depth for XPS measurement is less than 10 nm, so there might be no the peaks corresponding to P and N in the XPS spectrum of CAPP due to the lack of P and N at the surface of CAPP. Two new peaks locating at 103.7 eV and 153.7 eV can be observed, which are attributed to Si2p and Si2s of silicon compound, respectively. Both the change of elemental contents and the appearance of new peaks demonstrated that APP was well coated by the cross-linking silicon compound.
| Sample | C (wt%) | O (wt%) | N (wt%) | P (wt%) | Si (wt%) |
|---|---|---|---|---|---|
| APP | 10.53 | 45.46 | 15.33 | 28.68 | 0 |
| CAPP | 33.45 | 33.58 | 0.32 | 0.77 | 37.85 |
The surface morphologies of APP and CAPP particles are shown in Fig. 3. It is clear that the surfaces of APP particles are very smooth. After being coated, CAPP particles present quite rough surfaces. It seems that a lot of little particles were stuck on the surfaces of APP, which should be the silicon compound.
 | ||
| Fig. 3 SEM photographs of (a) APP and (b) CAPP particles. The inset at top right corner is the corresponding WCA result. | ||
3.2. Granularity, morphology, and water solubility for APP and CAPP
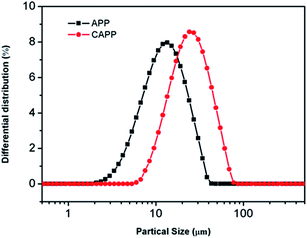
The hydrophilicity of CAPP has been evaluated by WCA, as shown in Fig. 3. The WCA of neat APP is 12.5°. However, after being modified by VTES, the WCA increases to about 110.5°, indicating that the incorporation of hydrophobic organic group on the surface of APP resulted in its transformation from hydrophilicity to hydrophobicity. The results presented above also demonstrate that APP was coated by the hydrophobic silicon compound.Fig. 4 shows the particle size distributions of APP and CAPP. The mean size of CAPP is about 25.5 μm, much larger than 10.34 μm of APP. Obviously, after being coated, CAPP has a larger particle size due to the existence of silicon compound shell. Meanwhile, the larger mean size of CAPP also illustrates that APP was coated by silicon compound successfully.
The water solubility of APP and CAPP are shown in Fig. 5. Obviously, the solubility of APP increases with increasing the water temperature, and the solubilities of APP are 0.253 g/100 mL H2O and 3.521 g/100 mL H2O, respectively, at corresponding 25 °C and 80 °C, indicating that APP was easily attacked by water, especially at high temperature. After the coating, the solubility of CAPP decreases sharply compared that of APP, especially at high temperature. The solubility of CAPP is lower than 0.500 g/100 mL H2O even though the water temperature reaches 80 °C, which is much lower than that of APP under the same condition, proving that the silicon compound on the surface of CAPP effectively protected APP from water, and enhanced its hydrophobicity via the functionalized organic group.
3.3. Flame retardancy
The flame retardancy of neat POE and its composites were evaluated by LOI and UL-94 test, and the results are shown in Table 2.| Samples | POE (%) | APP (%) | CAPP (%) | CA (%) | UL-94 | LOI (%) |
|---|---|---|---|---|---|---|
| POE1 | 100 | 0 | 0 | 0 | NR | 17.2 |
| POE2 | 70 | 20.0 | 0 | 10.0 | V-0 | 28.5 |
| POE3 | 70 | 22.5 | 0 | 7.5 | V-0 | 29.5 |
| POE4 | 70 | 24.0 | 0 | 6.0 | V-2 | 27.5 |
| POE5 | 70 | 0 | 20.0 | 10.0 | V-0 | 31.0 |
| POE6 | 70 | 0 | 22.5 | 7.5 | V-0 | 33.5 |
| POE7 | 70 | 0 | 24.0 | 6.0 | V-0 | 30.0 |
The vertical burning test showed that the UL-94 rating of POE/CAPP/CA was V-0 at 30 wt% CAPP/CA with the weight ratio of 4/1, 3/1 or 2/1. When the weight ratio of CAPP/CA is 3/1, the POE composites have the maximum LOI value, reaching 33.5%. Although the UL-94 rating of POE/APP/CA system was V-0 rating at 30 wt% APP/CA with the weight ratio of 2/1 or 3/1, it decreased to V-2 rating at the weight ratio of 4/1. In addition, each POE/CAPP/CA system has higher LOI value compared with that of POE/APP/CA system with equal amount of flame retardant. Obviously, POE/CAPP/CA system has better flame retardancy than that of POE/APP/CA at the same loading of flame retardant.
3.4. Effect of DCP concentration on mechanical properties
Based on the results of flame retardancy of POE composites, the POE system with the weight ratio of 3/1 (APP/CA or CAPP/CA) was chosen to study on the effect of DCP concentration on the mechanical properties of POE composites.Both tensile strength and elongation at break for flame-retarded POE composites with different DCP concentrations are shown in Fig. 6. The tensile strength of flame-retarded POE composites drastically increases in the DCP range from 0 to 0.05 wt%. With further increasing DCP concentration, the tensile property of POE composites decreases. Here, the increase of tensile strength may be attributed to both the change of interfacial adhesion between POE and flame retardant, and the formation of a three-dimensional network shown in Scheme 2. In order to investigate the interfacial adhesion between POE and flame retardant, the SEM measurement of fracture sections for POE/APP/CA/DCP and POE/CAPP/CA/DCP composites were performed. As shown in Fig. 7. For POE/APP/CA system, some cavities can be seen on its surface, and there is clear interfaces between flame retardant particles and polymeric matrix, indicating that the compatibility between POE and flame retardant is not good, which should be due to the different polarity between flame retardant and POE, causing a weak interfacial adhesion. After being coated, almost no obvious interface can be observed between fillers and the matrix, as shown in Fig. 7(b), Obviously, the interfacial adhesion between POE and flame retardant was improved compared that between POE and APP, and the reason for the improvement should be from the similar polarity between POE and silicon compound existing on the surface of APP, and the formation of a three-dimensional network. For the contribution of the similar polarity to the tensile strength of POE composite, it can be confirmed by the difference of tensile strengths of POE composites at 0 wt% DCP. As for the formation of a three-dimensional network after cross-linking, as proposed by Yin et al.,18 the silicon compound shell including the functionalized organic group double bond (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) allowed the CAPP to be incorporated into POE three-dimensional network, which could greatly enhance the interfacial adhesion between APP and polymer matrix, consequently led to the improvement of tensile strength. At higher than 0.05 wt% DCP, the tensile strength decreases dramatically with increasing the content of DCP, which might be ascribed to the excess degree of cross-linking.21 The results presented above proves that the suitable content of DCP to achieve the excellent tensile strength of POE composites should be about 0.05 wt%. In the range of 0–0.09 wt%, the elongation at break for POE composites always decreases with increasing the flame retardant. However, the CAPP system always has higher elongation at break than that of APP system containing equal amount of flame retardant.
C) allowed the CAPP to be incorporated into POE three-dimensional network, which could greatly enhance the interfacial adhesion between APP and polymer matrix, consequently led to the improvement of tensile strength. At higher than 0.05 wt% DCP, the tensile strength decreases dramatically with increasing the content of DCP, which might be ascribed to the excess degree of cross-linking.21 The results presented above proves that the suitable content of DCP to achieve the excellent tensile strength of POE composites should be about 0.05 wt%. In the range of 0–0.09 wt%, the elongation at break for POE composites always decreases with increasing the flame retardant. However, the CAPP system always has higher elongation at break than that of APP system containing equal amount of flame retardant.
From the above results, it can be found that, in the range of 0–0.09 wt% DCP, POE/CAPP/CA has the best tensile strength and high elongation at break at about 0.05 wt% DCP, therefore, in the following work, the investigations on water resistance, combustion performance, and thermal stability are based on the POE system containing 0.05 wt% DCP.
3.5. Water resistance and flame retardancy of cross-linked POE composites after water treatment
Fig. 8 shows the SEM micrographs of POE composites after being treated by water at 70 °C for 7 days. As shown in Fig. 8(a), some pores are exposed on the surface of APP system after being treated by water for 168 h at 70 °C, which should be due to the corrosion of APP particles by water. Fig. 8(b), shows that the surface of CAPP system is smooth, and has almost no hole after the treatment, which might be due to the protection of the silicon compound shell. To further study the effect of CAPP on the water resistance of POE composite, the SEM test of fracture surfaces for flame-retarded POE composites was performed. Fig. 8(c), shows that there are many little holes on the fracture surface of APP system, which is attributed to the hydrolyzed APP particles; while Fig. 8(d), presents that many CAPP particles still exist on the fracture surface of CAPP system after the water treatment, which is ascribed to the hydrophobicity of silicon compound. SEM results directly prove that the water resistance of POE/APP/CA was greatly improved by the incorporation of silicon compound shell on the surfaces of APP.Table 3 shows the LOI and UL-94 tests results of POE composites after water treatment. After being treated by water at 70 °C for 168 h, POE/APP/CA composite burnt rapidly in UL-94 test, showing no rating, and its LOI value decreased compared with POE/APP/CA untreated. For POE/CAPP/CA treated, the sample still passed the UL-94 V-0 rating. Although its LOI value also reduced, it is still higher than that of APP system treated. These results demonstrate that the POE/CAPP/CA system had better flame retardancy than POE/APP/CA system after water treatment. Here, it should be noted that the burning time of POE/CAPP/CA treated is longer than that of POE/CAPP/CA untreated in UL-94 test, although its burning rating passed the V-0 rating. Obviously, both UL-94 and LOI results were affected by the water treatment for POE/CAPP/CA. According to the water solubility test results shown in Fig. 5 and 8, it can be found that only a little CAPP was attacked by water during the treatment for POE/CAPP/CA system. Generally, a little loss of CAPP is not enough to greatly deteriorate the flame retardant performance of POE/CAPP/CA composite, but a little damage to the flame retardation of the POE composite might be caused in this case. So the POE/CAPP/CA treated by water passed the UL-94 V-0 rating, but had longer burning time than POE/CAPP/CA untreated, and its LOI also decreased to 29.0% from 33.5% of the CAPP system untreated.
| Sample | Untreated | After water treatment | ||
|---|---|---|---|---|
| LOI (%) | UL-94 | LOI (%) | UL-94 | |
| POE/APP/CA | 29.5 | V-0 | 25.0 | No rating |
| POE/CAPP/CA | 33.5 | V-0 | 29.0 | V-0 |
3.6. Combustion performance
The CC is an effective method to study the flammability of materials.22,23 The HRR, THR, SPR, and MLR curves of neat POE and POE composites are shown in Fig. 9 and the corresponding data are presented in Table 4. | ||
| Fig. 9 HRR (a), THR (b), SPR (c), and ML (d) curves of neat POE and POE composites as a function of time in CC test. | ||
| Sample | TTI (s) | PHRR (kW m−2) | THR (MJ m−2) | FPI | Peak SPR (m2 s−1) | TSP (m2 m−2) | Char residue mass (wt%) |
|---|---|---|---|---|---|---|---|
| POE | 38 | 718 | 111 | 0.05 | 0.074 | 10.90 | 5.24 |
| POE/APP/CA | 28 | 229 | 96 | 0.12 | 0.044 | 9.80 | 22.96 |
| POE/CAPP/CA | 18 | 94 | 24 | 0.20 | 0.008 | 1.66 | 77.97 |
Generally, when the heat release rate (HRR) is lower than a relatively low value (40 kW m−2 in our experiment) in cone calorimeter test, it is thought that the burning ends. In the CC test, there are three systems with different components. Neat POE burnt out completely rapidly due to its terrible flame retardancy, and its HRR fell down to 40 kW m−2 quickly. POE/CAPP/CA could extinguish within short time, owing to its good flame retardancy resulting from the formation of a compact intumescent char layer during the combustion process, thus the burning time for POE/CAPP/CA was very short. However, for POE/APP/CA the intumescent char layer formed during the combustible process was not compact, and had bad protection for the material underneath, leading the continuous burning of the substrate, so the burning time for POE/APP/CA is longer than that for POE/CAPP/CA or neat POE.
Table 4 shows that the TTI of POE/CAPP/CA is far lower than that of POE/APP/CA. Meanwhile, the residue of the former is far more than that of the latter. In CC test, POE/APP/CA system could decompose in advance compared with POE/CAPP/CA system due to the better thermal stability of CAPP, and release the NH3 etc. gases, which might dilute the concentration of the combustible gases, leading to the delay of TTI for POE/APP/CA system.
The HRR curve of flame-retardant POE composites exhibits two peaks. The first peak is assigned to the ignition and the formation of an expanded protective shield; the second peak is ascribed to the destruction of the intumescent shield and the formation of a carbonaceous residue.24 From Fig. 9(a), it can be found that neat POE burnt out within 450 s. A very sharp HRR peak appeared at about 178 s, and the corresponding value for the peak of HRR (pHRR) is about 718 kW m−2. However, the flame-retardant POE composites show a dramatic decline in pHRR. The pHRR value for POE/APP/CA is 229 kW m−2, much lower than that of neat POE. Moreover, the pHRR for POE/CAPP/CA further decreases to 94 kW m−2.
In order to judge the fire hazard more clearly, the fire performance index (FPI) was calculated. The FPI is defined as the proportion of TTI and the pHRR.25,26 Generally, when the value of FPI reduces, the time to flashover will be advanced. Namely, if the value of FPI of a material is lower, its fire risk is higher. Table 4 shows that the FPI of POE/CAPP/CA composite is 0.20, lower than that of POE/APP/CA system, indicating that the silicon compound locating on the surfaces of APP can reduce the risk of POE/APP/CA in a fire hazard.
The THR is also an important parameter to evaluate the fire safety of a material. An obvious decrease for THR can be observed for POE composites compared with neat POE. At the end of the test, neat POE released a total heat of 111 MJ m−2; while POE/APP/CA and POE/CAPP/CA released total heat of 94 and 24 MJ m−2, respectively, indicating that the silicon compound coated on the surfaces of APP could greatly reduce the THR of cross-linked POE/APP/CA composite.
The SPR and MLR rate curves of neat POE and POE composites are presented in Fig. 9(c) and (d), respectively. The SPR peak values of both POE/APP/CA and POE/CAPP/CA composites were significantly reduced compared with that of neat POE. The SPR peak of neat POE is 0.074 m2 s−1. Compared with neat POE, the SPR peak of POE/APP/CA composite was decreased to 0.044 m2 s−1, and the value of POE/CAPP/CA composite was further reduced to 0.008 m2 s−1. These results indicate that the smoke suppression effect of POE/CAPP/CA composite is better than that of POE/APP/CA composite. For POE/APP/CA composite, the average MLR was decreased to 0.028 g s−1 from 0.05 g s−1 of neat POE, and it was further reduced to 0.014 g s−1 for PP/CAPP/CA composite. Obviously, POE/CAPP/CA system has a much lower MLR, which might be due to the formation of a dense protective carbon layer on the surface of POE/CAPP/CA during the combustion process, thus prevented the further degradation and combustion of the underlying material.
The digital photographs of the residual chars after CC test are shown in Fig. 10. At the end of CC test, there was no residue left for neat POE, while an intumescent char layer was formed for POE/APP/CA or POE/CAPP/CA. In order to obtain more detailed information on the residue chars, SEM measurement was performed after CC tests. Fig. 11(a1) and (a2) show that the residue of POE/APP/CA are quite loose, and there are some big holes at the outer and inner surfaces of the residue. Therefore, it could not provide good flame shield for the underlying material during burning. However, as can be seen from Fig. 11(b1) and (b2), compact structures at the outer and inner surfaces were formed for POE/CAPP/CA, and no big hole can be observed. Furthermore, there are some folds on the surface, which could act as a skeleton to strengthen the surface layer.27,28 The digital photos and SEM micrographs illustrate that the formation of a compact and intumescent char layer for the cross-linked POE/CAPP/CA system should be an important reason for its better flame retardancy than that of POE/APP/CA in CC test.
 | ||
| Fig. 10 Digital photographs of the residues after CC test: (a), neat POE; (b), POE/APP/CA; (c), POE/CAPP/CA. | ||
 | ||
| Fig. 11 SEM micrographs of POE/APP/CA (a1, outer; a2, inner) and POE/CAPP/CA (b1, outer; b2, inner). | ||
3.7. Thermal stability
TGA and DTG curves of POE and flame-retardant POE composites under N2 atmosphere are shown in Fig. 12 and the related TGA data are listed in Table 5. It can be found that the thermal degradation of neat POE is composed of one step. The maximum weight loss occurred at 401 °C, which is defined as Tmax. Compared with neat POE, the initial degradation temperature (Tonset) of flame-retardant POE composites decreases, owing to the thermal degradation of intumescent flame retardant. Here, Tonset is defined as the temperature at 5 wt% of weight loss. Obviously, the Tonset of POE/CAPP/CA is higher than that of POE/APP/CA, which should be due to the existence of silicon compound on the surface of CAPP. Generally, the silicon compound can play a role in delaying the decomposition of APP due to its good thermal stability. The flame-retardant POE composite exhibited more stable thermal behavior at the temperature range from 465 to 700 °C, and both POE/CAPP/CA and POE/APP/CA had more residue than neat POE. For POE/CAPP/CA system, more residue and better thermal stability than those of POE/APP/CA were achieved at high temperature, indicating that silicon compound facilitated the formation of more residue with good thermal stability than that of POE/APP/CA system at high temperature, which could protect POE from further decomposing during a fire. Therefore, the good flame retardancy of POE/CAPP/CA system should be related to its charring during burning.| Sample | T5% (°C) | Tmax (°C) | Residues at 700 °C |
|---|---|---|---|
| POE | 401.1 | 465.0 | 0.5 |
| POE/APP/CA | 382.5 | 465.8 | 12.39 |
| POE/CAPP/CA | 393.7 | 466.8 | 20.07 |
3.8. Fame-retardant mechanism of CAPP/CA system
To illustrate the effect of silicon compound on the flame-retardant efficiency of APP, the FTIR test of gas phases and condensed phases for CAPP/CA system at different temperatures were performed. Fig. 13 shows the FTIR-TG spectra of the gaseous phases for CAPP/CA and APP/CA during the thermal degradation. The main evolved gases from the decomposition of two systems include carbon dioxide (CO2, 2335 and 2360 cm−1), ammonia (NH3, 926, 964, 1520 ∼ 1670 cm−1), and water vapor (H2O, 3920 ∼ 3520 cm−1).29,30 Further, the release of NH3 should be from NH4+ of APP, while the CO2 and H2O might be produced during the carbonization process of IFR system. Interestingly, the intensity of the NH3 absorbing peak increases sharply at 320 °C for the two systems, and then decreases with increasing the temperature, suggesting that the two systems have similar thermal stability. According to the results shown above, it can be found that the contribution of the gaseous phase for APP has not been changed obviously after being coated through silicon compound. | ||
| Fig. 13 FTIR spectra of the gaseous products of APP/CA (a) and CAPP/CA (b) during the thermal degradation. | ||
In order to further demonstrate the flame-retardant mechanism of CAPP/CA system, the condensed phases of CAPP/CA were investigated by FTIR test. Fig. 14 shows the FTIR spectra of the condensed phases at different temperatures. The strong and broad absorbing peak at 3250 cm−1 is assigned to the stretching vibration of N–H bond of NH4+; while the peak gradually decreases at high temperature, suggesting the release of ammonia from the APP component and the formation of ultra-, pyro- or poly-phosphoric acid with free acidic hydroxyl groups during thermal dehydration.31,32 The peak at about 1637 cm−1 appeared at 320 °C, indicating the existence of species containing C–H and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond. At 560 °C, the peak at 1000 cm−1 is ascribed to the stretching vibration of P–O–C group.33 It should be noted that the absorption bands at 1091 cm−1 and 1226 cm−1 suggest that the cross-linked structure consisting of Si–O and P
C bond. At 560 °C, the peak at 1000 cm−1 is ascribed to the stretching vibration of P–O–C group.33 It should be noted that the absorption bands at 1091 cm−1 and 1226 cm−1 suggest that the cross-linked structure consisting of Si–O and P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O aromatic structure was formed, which was probably bridged by P–O–P group, resulting in the formation of the residue with complex structure, which could efficiently prohibit the evolution of combustible gases and the decomposition of the underlying materials, as well as prevent the heat and oxygen from transferring to the matrix interior, and thus improve the flame-retardant efficiency of APP.
O aromatic structure was formed, which was probably bridged by P–O–P group, resulting in the formation of the residue with complex structure, which could efficiently prohibit the evolution of combustible gases and the decomposition of the underlying materials, as well as prevent the heat and oxygen from transferring to the matrix interior, and thus improve the flame-retardant efficiency of APP.
XPS data for the condensed products of CAPP/CA at different temperatures can be used to further demonstrate the silicon compound on the flame-retardant efficiency of APP. Fig. 15 shows the C1s, N1s, O1s, P2p and Si2p XPS spectra of CAPP/CA at 170 °C and 560 °C. The C1s spectra of CAPP/CA are shown in Fig. 15(a1 and a2), The bands at around 284.4 eV could be assigned to C–H and C–C in aliphatic and aromatic species. The peak at 286.0 eV can be ascribed to C–O or C–N in cyclized compounds. At 560 °C there is a new peak at 287.8 eV which could be C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C
O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, and C
C, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N groups,34 indicating that more steady cross-links were further formed by aromatization at high temperature. Fig. 15(b1 and b2), shows the N1s spectra of CAPP/CA, one peak around 399.5 eV is from C
N groups,34 indicating that more steady cross-links were further formed by aromatization at high temperature. Fig. 15(b1 and b2), shows the N1s spectra of CAPP/CA, one peak around 399.5 eV is from C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N; the other peak around 401.4 eV is assigned to the N–H bond of NH4+.35 The O1s spectra are shown in Fig. 15(c1 and c2), At 170 °C and 560 °C, the binding energy in the vicinity of 532.3 eV can be ascribed to the C–O or P–O groups.36 A new peak at 533.5 eV can be attributed to the P–O–P and/or P–O–C groups at 560 °C. The P2p spectra of CAPP/CA are shown in Fig. 15(d1 and d2), The peaks between 134.0 eV and 135.0 eV can be assigned to P–O–C and/or PO3− groups in the phosphorus-rich crosslinks.37 The Si2p spectra of CAPP/CA are shown in Fig. 15(e1 and e2), The Si2p peak locates at 101.9 eV at 170 °C, but the peak moved to 104 eV at 560 °C. The shift of Si2p might be due to the fact that the Si–C or Si–O–C bonds at low temperatures were converted into Si–O–Si bonds at high temperatures.
N; the other peak around 401.4 eV is assigned to the N–H bond of NH4+.35 The O1s spectra are shown in Fig. 15(c1 and c2), At 170 °C and 560 °C, the binding energy in the vicinity of 532.3 eV can be ascribed to the C–O or P–O groups.36 A new peak at 533.5 eV can be attributed to the P–O–P and/or P–O–C groups at 560 °C. The P2p spectra of CAPP/CA are shown in Fig. 15(d1 and d2), The peaks between 134.0 eV and 135.0 eV can be assigned to P–O–C and/or PO3− groups in the phosphorus-rich crosslinks.37 The Si2p spectra of CAPP/CA are shown in Fig. 15(e1 and e2), The Si2p peak locates at 101.9 eV at 170 °C, but the peak moved to 104 eV at 560 °C. The shift of Si2p might be due to the fact that the Si–C or Si–O–C bonds at low temperatures were converted into Si–O–Si bonds at high temperatures.
The elemental contents obtained in XPS test are shown in Table 6, including C, N, O, P, and Si. In detail, the content of C decreased from 46.26 wt% to 18.03 wt% because of the oxidization of unsteady C and the formation of steady C, such as C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C structure in aromatic moieties; the content of Si also decreased from 16.98 wt% to 8.62 wt%, which should be due to the formation of P–O–Si and C–O–Si. Obviously, the content of N was reduced due to the volatilization of NH4+/NH3 during heating. By contrast, the content of O and P increased due to the formation of the P–O–P and P–O–C structure. The change tendencies of elemental contents are consistent with the FTIR results at different temperatures, demonstrating that the charring process in which rich P and Si were deposited should be the most important reason for the better flame-retardant efficiency of CAPP than that of APP.
C structure in aromatic moieties; the content of Si also decreased from 16.98 wt% to 8.62 wt%, which should be due to the formation of P–O–Si and C–O–Si. Obviously, the content of N was reduced due to the volatilization of NH4+/NH3 during heating. By contrast, the content of O and P increased due to the formation of the P–O–P and P–O–C structure. The change tendencies of elemental contents are consistent with the FTIR results at different temperatures, demonstrating that the charring process in which rich P and Si were deposited should be the most important reason for the better flame-retardant efficiency of CAPP than that of APP.
| Temperature | C (wt%) | N (wt%) | O (wt%) | P (wt%) | Si (wt%) |
|---|---|---|---|---|---|
| 170 °C | 46.26 | 11.60 | 24.24 | 0.91 | 16.98 |
| 560 °C | 18.03 | 2.44 | 47.67 | 23.24 | 8.62 |
XPS results demonstrate that the silicon compound was engaged in charring process during the decomposing process, leading to the formation of a stable and compact char layer, which resulted in the better flame-retardant efficiency of CAPP/CA than that of APP/CA. On the basis of the above experimental data, we can propose a potential char-forming mechanism of CAPP/CA was shown in Scheme 3.
4. Conclusion
In this paper, APP coated by silicon compound containing triethoxyvinylsilane with a vinyl group at its surface was prepared successfully, and the CAPP was used to flame-retarded POE together with CA in the case of DCP. The results demonstrated that CAPP system had better water resistance, flame retardation, and mechanical properties than APP system with equal amount of flame retardant. TG, FTIR, and XPS demonstrated that the improvement of flame retardancy for POE composite containing APP after being coated by silicon compound should be due to the formation of rich residue containing Si–C, Si–O–C etc. structures; The SEM observation confirmed that the increases of tensile strength and elongation at break for CAPP system should be due to the improvement of interfacial adhesion between POE and flame retardant. Obviously, it should be an efficient method to simultaneously improve the water resistance, flame retardancy and mechanical properties of POE composite containing APP through functionalizing the APP and then chemically incorporating it into the polymer matrix.Acknowledgements
Financial support by the National Natural Science Foundation of China (Grant no. 51121001) and the Program for Changjiang Scholars and Innovative Research Team in University (IRT1026) would be sincerely acknowledged.References
- Q. Ren, C. Wan, Y. Zhang and J. Li, Polym. Adv. Technol., 2011, 22, 1624 CrossRef.
- J. Guo, M. He, Q. Li, J. Yu and S. Qin, J. Appl. Polym. Sci., 2013, 129, 2063 CrossRef CAS.
- C. H. Hong, Y. B. Lee and J. W. Bae, J. Appl. Polym. Sci., 2005, 97, 2311 CrossRef CAS.
- H. Horacek and S. Pieh, Polym. Int., 2000, 49, 1106 CrossRef CAS.
- S. Bourbigot, M. Lebras and S. Duquesne, Macromol. Mater. Eng., 2004, 289, 499 CrossRef CAS.
- K. Wu, Z. Wang and Y. Hu, Polym. Adv. Technol., 2008, 19, 1118 CrossRef CAS.
- D. Y. Wang, Y. Liu, X. G. Ge, Y. Z. Wang, A. Stec and B. Biswas, Polym. Degrad. Stab., 2008, 93, 1024 CrossRef CAS PubMed.
- H. Q. Qu, W. H. Wu, J. W. Hao, C. Z. Wang and J. Z. Xu, Fire Mater., 2014, 38, 312 CrossRef CAS.
- L. Liu, Y. Zhang and L. Li, Polym. Adv. Technol., 2011, 22, 2403–2408 CrossRef CAS.
- C. Chiang and R. Chang, Compos. Sci. Technol., 2008, 68, 2849 CrossRef CAS PubMed.
- W. Y. Chiang and C. H. Hu, Composites, Part A, 2001, 32, 517 CrossRef.
- P. Song, Y. Shen and Z. P. Fang, ACS Appl. Mater. Interfaces, 2009, 1, 452 CAS.
- S. P. Liu, J. R. Ying, X. P. Zhou, X. L. Xie and Y. W. Mai, Compos. Sci. Technol., 2009, 69, 1873 CrossRef CAS PubMed.
- C. L. Deng, C. Deng, J. Zhao and Y. Z. Wang, Polym. Adv. Technol., 2014, 25, 861 CrossRef CAS.
- C. L. Deng, S. L. Du, C. Deng and Y. Z. Wang, Polym. Degrad. Stab., 2014, 108, 97 CrossRef CAS PubMed.
- B. Wang, X. F. Wang, L. Song and Y. Hu, Compos. Sci. Technol., 2012, 72, 1042 CrossRef CAS PubMed.
- B. Wang, Q. Tai, L. Song and Y. Hu, Ind. Eng. Chem. Res., 2011, 50, 5596 CrossRef CAS.
- J. Ge, Y. Hu, T. Zhang and Y. Yin, J. Am. Chem. Soc., 2007, 129, 8974 CrossRef CAS PubMed.
- M. Bugajny, S. Bourbigot, M. Lebras and R. Delobel, Polym. Int., 1999, 48, 264 CrossRef CAS.
- M. Barsbay, H. K. Can, A. Güner and Z. M. Rzaev, Polym. Adv. Technol., 2005, 16, 32 CrossRef CAS.
- P. Jansen, A. S. Gomes and B. G. Soares, J. Appl. Polym. Sci., 1996, 61, 591 CrossRef CAS.
- K. Wu, Z. Wang and Y. Hu, Polym. Adv. Technol., 2008, 19, 1118 CrossRef CAS.
- M. L. Bras, M. Bugajny, J. M. Lefebvre and S. Bourbigot, Polym. Int., 2000, 49, 1115 CrossRef.
- B. Wang, S. Hu, L. Song and Y. Hu, Ind. Eng. Chem. Res., 2011, 50, 11476 CrossRef CAS.
- B. Wang, Q. Tang, L. Song and Y. Hu, ACS Appl. Mater. Interfaces, 2011, 3, 3754 CAS.
- B. N. Jang and C. A. Wilkie, Polymer, 2005, 46, 3264 CrossRef CAS PubMed.
- B. Du, Z. Guo, P. Song and Y. Wu, Appl. Clay Sci., 2009, 45, 178 CrossRef CAS PubMed.
- X. Su, Y. Yi, J. Tao and H. Qi, Polym. Degrad. Stab., 2012, 97, 2128 CrossRef CAS PubMed.
- N. B. Colthup, L. H. Daly and S. E. Wiberley, Introduction to Infrared and Raman Spectroscopy, Academic Press, San Diego, CA, 1990 Search PubMed.
- H. Zou, C. Yi, L. Wang, H. Liu and W. Xu, J. Therm. Anal. Calorim., 2009, 97, 929 CrossRef CAS PubMed.
- Y. W. Yan, L. Chen, R. K. Jian, S. Kong and Y. Z. Wang, Polym. Degrad. Stab., 2012, 97, 1423 CrossRef CAS PubMed.
- G. Camino, L. Costa and L. Trossarelli, Polym. Degrad. Stab., 1984, 7, 25–31 CrossRef CAS.
- W. Xing, L. Song, Y. Hu, S. Zhou and K. Wu, Polym. Degrad. Stab., 2009, 94, 1503 CrossRef CAS PubMed.
- S. Gaan, G. Sun, K. Hutches and M. H. Engelhard, Polym. Degrad. Stab., 2008, 93, 99 CrossRef CAS PubMed.
- S. Bourbigot, M. Lebras, L. Gengembre and R. Delobel, Appl. Surf. Sci., 1994, 81, 299 CrossRef CAS.
- S. Delpeux, F. Beguin and R. Benoit, Eur. Polym. J., 1998, 34, 905 CrossRef CAS.
- S. Bourbigot, M. Lebras, R. Delobel and L. Gengembre, Appl. Surf. Sci., 1997, 120, 15 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |