Electrochemical performance of an asymmetric supercapacitor based on graphene and cobalt molybdate electrodes
Abstract
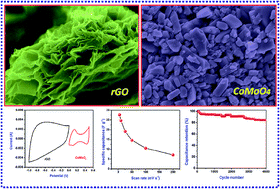
In this article, we report the fabrication and electrochemical performance of asymmetric supercapacitors (ASCs) based on a reduced graphene oxide (rGO) negative electrode and a cobalt molybdate (CoMoO4) positive electrode. The rGO and CoMoO4 electrode materials were synthesized by hydrothermal and sonochemical methods, respectively. Physico-chemical characterization techniques such as X-ray diffraction, field-emission scanning electron microscopy, Fourier-transform infrared spectroscopy, Raman spectroscopy, and nitrogen adsorption–desorption isotherm analysis were used to characterize the electrode materials. The rGO nanosheets and CoMoO4 nanostructures delivered a specific capacitance of about 168.8 and 98.34 F g−1, respectively measured in a three electrode system. The rGO‖CoMoO4 ASC device demonstrated a maximum specific capacitance of 26.16 F g−1 (at a current density of 0.5 mA cm−2), an energy density of 8.17 W h kg−1, and a maximum working voltage of 1.5 V. The fabricated device possessed excellent capacitance retention of about 85% after 4000 cycles (at a current density of 1.0 mA cm−2) suggesting the superior cyclic stability of the fabricated ASC device.

 Please wait while we load your content...
Please wait while we load your content...