Photocatalytic performance of Ag2S under irradiation with visible and near-infrared light and its mechanism of degradation†
Wei Jiang*,
Zhaomei Wu,
Xiaoning Yue,
Shaojun Yuan,
Houfang Lu and
Bin Liang
Multi-phases Mass Transfer and Reaction Engineering Laboratory, College of Chemical Engineering, Sichuan University, No. 24 South Section 1, Yihuan Road, Chengdu, Sichuan, China 610065. E-mail: weijiang@scu.edu.cn; Tel: +86-28-85990133
First published on 11th February 2015
Abstract
Ag2S has only rarely been investigated as a photocatalyst on its own, although it has been widely used as an important component of composite photocatalysts. We synthesized Ag2S by a facile ion-exchange method at room temperature and used it directly as an effective photocatalyst. Our results confirmed the excellent performance of Ag2S in completely photodegrading methyl orange within 30 min under irradiation with visible light and within 70 min under irradiation with near-infrared light. This good performance is ascribed to the narrow band gap (1.078 eV) of Ag2S and the lower recombination efficiency of the photogenerated electron–hole pairs of Ag2S in the photocatalytic process. The active species in the photo-oxidation process was identified as anioic ozone radicals. This simple preparation method and high photocatalytic performance increases the possible future applications of Ag2S.
1. Introduction
TiO2 is often used as a stable and inexpensive photocatalyst active under ultraviolet (UV) radiation;1–3 however, its application is restricted as it cannot be used with visible light. Photocatalysts that work with visible light, which makes up about 48% of the solar spectrum, or even infrared light (44% of solar spectrum)4 have therefore been the focus of much research. To achieve a high efficiency over a wide range of the solar spectrum, various photocatalysts, such as non-metals5 or metals6 doped with TiO2, composite semiconductor photocatalysts7 and new photocatalysts with a small band gap,8 have been investigated. Photocatalysts that work under visible and near-infrared (NIR) irradiation include Cu2(OH)PO4,9 YF3:Yb3+Tm3+/TiO2,10 Bi2WO6/TiO2,11 CQDs/Cu2O,12 BiVO4/CaF2:Er3+, Tm3+, Yb3+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 and NaYF4:Yb, Tm/CdS/TiO2.14 The preparation of most of these photocatalysts is too complex for mass production and the time taken to photodegrade organic compounds under NIR irradiation can be as long as 2, 4 or even 50 hours. It is therefore desirable to develop an efficient photocatalytic material with a simple method of preparation and higher activity in both the visible and NIR regions of the solar spectrum.
13 and NaYF4:Yb, Tm/CdS/TiO2.14 The preparation of most of these photocatalysts is too complex for mass production and the time taken to photodegrade organic compounds under NIR irradiation can be as long as 2, 4 or even 50 hours. It is therefore desirable to develop an efficient photocatalytic material with a simple method of preparation and higher activity in both the visible and NIR regions of the solar spectrum.
Ag2S is an important chalcogenide compound and has been investigated for use in photoconductors, photovoltaic cells and superionic conductors as a result of its good chemical stability and excellent optical limiting properties.15 Ag2S has often been combined with other semiconductor catalysts to form a composite photocatalyst, improving the performance of the bulk photocatalyst. Xie et al.16 reported that TiO2 nanocomposites coupled with Ag2S absorbed visible light. Subash et al.17 proved that the photocatalytic efficiency of ZnO was enhanced by loading with Ag2S and Shen et al.18 confirmed that CdS nanostructures with highly dispersed Ag2S had the highest photocatalytic activity for hydrogen evolution when the concentration of Ag2S was 5% by weight. Other composite photocatalysts containing Ag2S, such as Ag2S/ZnIn2S4,19 ZnS–In2S3–Ag2S,20 Ag2S/MCM-41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21 and Ag2S-coupled ZnO/ZnS,22 all exhibit good photocatalytic activity.
21 and Ag2S-coupled ZnO/ZnS,22 all exhibit good photocatalytic activity.
These successful examples of increased photocatalytic performance with the addition of Ag2S suggested that Ag2S may be an effective photocatalyst. However, there have only been a few reports of the performance of pure Ag2S as a photocatalyst. Yang et al.23 reported that only 7.8% of methyl orange (MO) was degraded by pure Ag2S after 30 min of irradiation with visible light, whereas Pourahmad21 reported 41% degradation of methylene blue by pure Ag2S within 60 min under UV light. Reddy et al.24 reported the photodegradation of rhodamine B with Ag2S with a 21.78% rate of conversion after 120 min of irradiation with sunlight.
These results suggest that the photocatalytic performance of pure Ag2S is unsatisfactory. However, the low band gap energy (0.9–1.05 eV)25 of Ag2S with a conduction band (CB) at about 0 eV vs. NHE26 means that it absorbs in the UV, visible and NIR regions, making it a possible semiconductor material for photocatalytic applications under visible and NIR irradiation. Thus we thought that it was worth systematically investigating the photocatalytic ability of pure Ag2S for potential application to the degradation of persistent organic pollutants.
We prepared a pure Ag2S photocatalyst by a facile ion-exchange method and used it to decompose MO under visible and NIR irradiation. The morphology and structure of the as-prepared Ag2S were characterized and its photocatalytic performance was investigated in detail. The working mechanism of Ag2S was determined by simulation, electron spin resonance (ESR) analysis and photodegradation experiments.
2. Experimental
2.1 Chemicals
All the reagents used were of analytical-reagent grade and were used without further purification. All the reagents were obtained from Chengdu Ke Long Chemical of China.2.2 Preparation of Ag2S photocatalyst
The Ag2S photocatalyst was prepared by a facile ion-exchange method at room temperature. In a typical process, 30 mL of Na2S·9H2O (0.1 M) was dropped into 40 mL of AgNO3 (0.2 M) solution using an automatic titrator at a speed of 3.2 mL min−1. After 30 min of magnetic stirring, the black products were filtered and washed with deionized water at least five times to remove the unreacted components and the NaNO3 produced. Finally, the black powders were vacuum-dried overnight at 60 °C in the dark. All these operations were carried out as far away from light as possible.2.3 Characterization
X-ray diffraction (XRD) patterns for Ag2S powder samples were obtained by glancing-angle X-ray diffraction (X'Pert proMPD, the Netherlands) with a Cu Kα 40 kV/40 mA X-ray source (λ = 0.15406 nm). Scanning electron microscopy (SEM) images and energy-dispersive X-ray spectrometry (EDS) analysis were carried out using a JSM-7500F field-emission scanning electron microscope (JEOL). The transmission electron microscopy (TEM) and high-resolution TEM (HRTEM) images were obtained using a Tecnai G2 F20 S-TWIN microscope.X-ray photoelectron spectrometry (XPS) measurements were performed on a VG ESCALAB MKII XPS system with an Al Kα source and a charge neutralizer. UV-visible diffusion reflection spectrometry (UV-visible DRS) was performed using a UV-visible spectrophotometer (TU-1901, Ge Beijing Spectrometer, China) and a fine-grained BaSO4 powder was used as the standard. Photoluminescence (PL) spectra were recorded with a fluorescence spectrometer (F-7000, Hitachi, Japan). The absorption spectrum of the MO solution was obtained using a UV-visible spectrophotometer (TU-1901, Ge Beijing Spectrometer, China). The particle size distribution of the prepared Ag2S samples was determined with a JL-1198 laser particle size analyser (Chengdu Jingxin Powder Analyser Instruments Co. Ltd, China). The surface areas of the photocatalysts were determined by the nitrogen adsorption method at −196 °C with a BET analyser (SSA-3500, China). The FTIR spectra of the samples were recorded with KBr pellets in the range 4000–400 cm−1 on an FTIR spectrometer (Spectrum II L1600300, PerkinElmer) at room temperature. The active species were investigated with an ER200-SRC ESR analyser.
2.4 Measurement of photocatalytic activity
The photocatalytic activities of the samples were evaluated by the degradation of MO under visible and NIR light irradiation in an OCRS-IV photoreactor system, which included a 500 W Xe lamp and a 500 W Hg lamp, a cut-off filter with a specified light wavelength and a water filter. About 0.1 g of the Ag2S photocatalyst was placed in a quartz reactor tube with 10 mL of aqueous MO solution (16 mg L−1). The suspension was stirred for 120 min in the dark to reach adsorption–desorption equilibrium. Visible light (λ > 380 nm) or NIR light (λ > 850 nm) irradiation was switched on to start the photodegradation reaction. The photoreaction was terminated after the scheduled time and the suspension was sampled and centrifuged to remove the solid particles. The concentration of the MO solution was determined by UV-visible spectrophotometry. Fig. S3† shows the corresponding standard absorption curve of the MO solution.2.5 Theoretical calculations
Theoretical calculations were carried out using the plane wave pseudopotential density functional theory (DFT) method. The ion–electron interaction was modelled by the ultrasoft pseudopotential in the Vanderbilt form. The energy cut-off for a plane wave basis set was 400 eV and a Monkhorst–Pack k-mesh of 6 × 6 × 6 was used. Geometry optimizations were performed before the single-point energy calculation and self-consistent convergence accuracy was set at 1 × 10−6 eV per atom. The convergence criterion for the maximum force between atoms was 0.01 eV. The maximum displacement was 5 × 10−4 Å and the stress was 60 GPa. The Up parameter for S was 7 eV and the Ud parameter for Ag was 13.2 eV. As a result of the complete symmetry between the spin-up and spin-down states, we only report the spin-up results here.In general, Ud + Up increases the splitting between the occupied and empty energy levels because the correction depends on the occupancy of the orbitals. The energy band structure using GGA + U, where US,p = 7 eV and UAg,d = 13.2 eV, was used.
3. Results and discussion
3.1 Characterization of photocatalyst
The as-prepared Ag2S samples were black or dark brown powders. The surface morphology, structure and band structure were characterized using XRD, XPS, SEM, EDS and UV-visible DRS.where D is the crystallite size for the catalyst, k is a dimensionless constant, λ is the wavelength of the X-rays (0.15406 nm), β is the full-width at half-maximum of the diffraction peak and θ is the diffraction angle.
The peaks at 368.21 and 374.26 eV in Fig. 1c can be assigned to the Ag3d5/2 and Ag3d3/2 of Ag+ ions in the Ag2S photocatalyst.27 The peaks at 161.3 and 162.5 eV of the S2p photoelectron spectrum in Fig. 1d were in perfect agreement with the binding energy of S2p3/2 and S2p1/2 of S2−.28 It was concluded that the as-prepared sample consisted of pure Ag2S without any Ag and S atoms of other valencies.
Fig. 2a is a typical SEM image of the Ag2S samples showing that the Ag2S particles were an aggregation of crystal grains with diameters of 30–80 nm, consistent with the XRD results. The TEM image in Fig. 2b confirms that the size of the Ag2S aggregates was about 0.2–0.7 μm, consistent with the results obtained with the laser particle size analyser (Fig. S2†) and BET analysis; the average particle size was 0.7226 μm and the specific surface area was 6.062 m2 g−1. Fig. 2c shows the HRTEM image of an Ag2S sample in which the lattice fringes can be clearly seen. The clear fringes with intervals of 0.34 and 0.38 nm can be indexed to the (012) and (002) lattice planes of Ag2S, respectively.
Fig. 3b showed the results of UV-visible DRS, which is used to estimate the band gap of semiconductors. By plotting the graph of (ahv)2 versus hv, where a is the absorption coefficient and hv is the photon energy,29 the band gap of the as-prepared Ag2S samples was estimated to be 1.078 eV, which was in agreement with previously reported values.30
The PL emission spectra of pure Ag2S and pure TiO2 were examined in the wavelength range 300–700 nm (Fig. 4). The intensity of the PL spectrum of pure TiO2 was much higher than that of the Ag2S samples. It can be reasonably inferred that pure Ag2S should have a higher photocatalytic performance than TiO2.
3.2 Photocatalytic activity
A typical persistent organic dye, MO, was selected as the target compound in the photodecomposition experiment to evaluate the photo-oxidation ability of Ag2S. The adsorption and photodegradation of MO were determined with Ag2S as a photocatalyst under irradiation with visible and NIR light.The fast rate of degradation of MO with Ag2S under irradiation with NIR light can be attributed to the huge surface area resulting from the aggregated structure of the Ag2S crystal grains and the strong absorption in visible and NIR regions. However, the relatively slow decomposition rate of MO under irradiation with NIR light compared with visible light can be ascribed to the weak illumination of the NIR light from the same Xe lamp, which supplied a visible light illumination of 18–21 klx and an NIR light illumination of 0.09–0.12 klx.
These excellent properties confirmed the proposal that Ag2S could work under NIR irradiation with a high degradation rate because of the narrow band gap (1.078 eV) and high quantum efficiency. This outstanding photocatalytic activity and wide working spectrum of Ag2S is attractive for further development.
The results in Fig. 6 confirm the complete decomposition of the MO adsorbed on the surface of the Ag2S. The peaks at 1601 and 814 cm−1 of sample 2 can be assigned to the framework vibrations of the benzene ring and the two substituted phenyl groups, respectively. These two characteristic peaks of MO were not observed in the FTIR spectrum of sample 1.
 | ||
| Fig. 6 FTIR spectra of Ag2S with adsorbed MO: after (sample 1) and before (sample 2) light illumination. | ||
Samples 1 and 2 were both soaked in deionized water. This phenomenon (Fig. S4†) and the results of FTIR analysis proved that MO can be completely photodegraded with Ag2S under light irradiation in both the adsorbed and dissolved states.
3.3 Photocatalytic mechanism of Ag2S photocatalyst
˙OH radicals and photogenerated holes are both possible active species in the photocatalytic degradation of organic pollutants. However, as the band gap of Ag2S is only 1.078 eV and ˙OH has a large electrode potential of 2.80 eV, ˙OH may not play a part in the working mechanism for Ag2S. It was therefore necessary to determine the active species and work out the photocatalytic mechanism for Ag2S under light irradiation.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was observed, consistent with the nitroxide DMPO–˙O3−. The determined free radical should therefore be the ozone anion radical.34 As the unique signal of ozone anion radicals was the only signal observed in solution with Ag2S under light irradiation, we concluded that this free radical was photogenerated by Ag2S in aqueous solution.
1 was observed, consistent with the nitroxide DMPO–˙O3−. The determined free radical should therefore be the ozone anion radical.34 As the unique signal of ozone anion radicals was the only signal observed in solution with Ag2S under light irradiation, we concluded that this free radical was photogenerated by Ag2S in aqueous solution.
The calculated band structure (Fig. 10) confirmed that Ag2S was a semiconductor with a direct band gap of 1.063 eV at the G point. This calculated value for the Ag2S band gap was slightly lower than the determined value of 1.078 eV, but closer to the previously reported value of 1.05 eV.30 This slight discrepancy may be ascribed to the underestimation of the DFT, which considered only the excited states in the calculation.
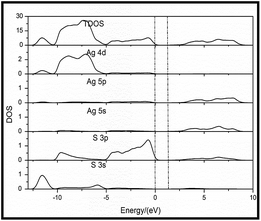
Fig. 11 plots the calculated total density of states (TDOS) and the partial density of states (PDOS) for Ag2S. The valence atomic configurations were 3s23p6 for S and 4d10 for Ag, respectively. The valence band of Ag2S is clearly split into two regions. The lower region probably has S3p features, whereas the upper region is dominated by the Ag4d states. There were hybridized S2p states near the Fermi level, with little practical contribution from the Ag5p states that have a dominant weight at the bottom of the CB. The width of the valence band for Ag2S was 10 eV. The S3p states had an intensity peak at 0.68 eV. In the upper region, two Ag4d maxima were found at −9.2 and −7.18 eV.
| EVB = X − Ee + 0.5Eg |
| ECB = EVB − Eg |
The value of X for Ag2S is about 4.968 eV.37 Based on these equations, the top of the VB and the bottom of the CB of Ag2S were calculated to be 0.992 and −0.072 eV, respectively. This result agrees with previously published data.38
Based on this analysis, the photo-oxidation mechanism of Ag2S driven by light can be established and described as follows. (1) Under light irradiation, Ag2S is transformed into the excited state, generating excited electrons and holes on the surface or in the interior simultaneously. (2) The electrons and holes react with the oxygen dissolved in aqueous solution and the water molecules absorbed on the surface of Ag2S particles, producing ozone anion radicals. (3) The ozone anion radicals, extremely reactive species, oxidize the organic molecules. (4) The photogenerated holes can also oxidize the organic molecules directly.
Fig. 12 is a schematic diagram of the charge-transfer process in Ag2S.
| Ag2S + hv → e− + h+ | (1) |
| O2 + 2OH− + 2h+ + e− → O3− + H2O | (2) |
| O3− + dye → oxidation products | (3) |
| h+ + dye → oxidation products | (4) |
4. Conclusion
An Ag2S photocatalyst has been successfully synthesized by a facile ion-exchange method. The as-prepared Ag2S was an aggregation of crystal grains with diameters of 30–80 nm and was an excellent photocatalyst for the decomposition of MO under light irradiation. The complete degradation of MO with Ag2S was reached within 30 min under visible light illumination and within 70 min under NIR light illumination. MO was completely photodegraded by Ag2S under light irradiation whether it was in the adsorbed or dissolved state. Such an attractive performance is ascribed to the narrow band gap of Ag2S (1.078 eV) obtained both by theoretical calculations and by experiment. The PL spectrum of pure Ag2S confirmed its high quantum efficiency. The combined function of the photogenerated holes and the ozone anion radicals of Ag2S in aqueous solution under light irradiation dominated the reaction process of the oxidative degradation of MO. The simple preparation method, excellent photocatalytic performance and ability to work under NIR light irradiation broadens the possible applications of Ag2S as a promising photocatalyst.Acknowledgements
We appreciate the financial support from the National Natural Science Foundation of China Project (no. 21176157 and 21476146).References
- A. L. Linsebigler, G. Lu and J. T. Yates Jr, Chem. Rev., 1995, 95, 735–758 CrossRef CAS.
- D. B. Asay and S. H. Kim, J. Phys. Chem. B, 2005, 109, 16760–16763 CrossRef CAS PubMed.
- G. K. Zhang, X. M. Ding, F. S. He, X. Y. Yu, J. Zhou, Y. J. Hu and J. W. Xie, Langmuir, 2008, 24, 1026–1030 CrossRef CAS PubMed.
- M. R. Hoffmann, S. T. Martin, W. Choi and D. W. Bahnemann, Chem. Rev., 1995, 95, 69–96 CrossRef CAS.
- T. A. Vu, C. D. Dao, T. T. Hoang, G. H. Le, K. T. Nguyen, P. T. Dang, H. T. Tran and T. V. Nguyen, Internet J. Nanotechnol., 2013, 10, 235–246 CrossRef CAS.
- P. M. Albrecht, D. Chen, D. R. Mullins, S. Tenney, R. Galhenage, H. Yan, N. Park and G. Henkelman, J. Phys. Chem., 2013, 117, 7191–7201 Search PubMed.
- L. Song, S. Zhang, X. Wu, S. Zhang, H. Tian and J. Ye, Chem. Eng. J., 2013, 214, 336–342 CrossRef CAS PubMed.
- T. Omata, H. Nagatani, I. Suzuki, M. Kita, H. Yanagi and N. Ohashi, J. Am. Chem. Soc., 2014, 136, 3378–3381 CrossRef CAS PubMed.
- G. Wang, B. Huang, X. Ma, Z. Wang, X. Qin, X. Zhang, Y. Dai and M. H. Whangbo, Angew. Chem., 2013, 125, 4910–4913 CrossRef.
- W. P. Qin, D. S. Zhang, D. Zhao, L. L. Wang and K. Z. Zheng, Chem. Commun., 2010, 46, 2304–2306 RSC.
- J. Tian, Y. Sang, G. Yu, H. Jiang, X. Mu and H. Liu, Adv. Mater., 2013, 25, 5075–5080 CrossRef CAS PubMed.
- H. Li, R. Liu, Y. Liu, H. Huang, H. Yu, H. Ming, S. Lian, S.-T. Lee and Z. Kang, J. Mater. Chem., 2012, 22, 17470–17475 RSC.
- S. Huang, N. Zhu, Z. Lou, L. Gu, C. Miao, H. Yuan and A. Shan, Nanoscale, 2014, 6, 1362–1368 RSC.
- X. Guo, W. Di, C. Chen, C. Liu, X. Wang and W. Qin, Dalton Trans., 2014, 43, 1048–1054 RSC.
- E. Aazam, J. Ind. Eng. Chem., 2014, 20, 4033–4038 CrossRef CAS PubMed.
- Y. Xie, S. H. Heo, Y. N. Kim, S. H. Yoo and S. O. Cho, Nanotechnology, 2010, 21, 015703 CrossRef PubMed.
- B. Subash, B. Krishnakumar, M. Swaminathan and M. Shanthi, Spectrochim. Acta, Part A, 2013, 105, 314–319 CrossRef CAS PubMed.
- S. Shen, L. Guo, X. Chen, F. Ren and S. S. Mao, Int. J. Hydrogen Energy, 2010, 35, 7110–7115 CrossRef CAS PubMed.
- S. Shen, X. Chen, F. Ren, C. X. Kronawitter, S. S. Mao and L. Guo, Nanoscale Res. Lett., 2011, 6, 1–6 Search PubMed.
- Y. Li, G. Chen, C. Zhou and J. Sun, Chem. Commun., 2009, 2020–2022 RSC.
- A. Pourahmad, Superlattices Microstruct., 2012, 52, 276–287 CrossRef CAS PubMed.
- S. Liu, X. Wang, W. Zhao, K. Wang, H. Sang and Z. He, J. Alloys Compd., 2013, 568, 84–91 CrossRef CAS PubMed.
- W. Yang, L. Zhang, Y. Hu, Y. Zhong, H. B. Wu and X. W. D. Lou, Angew. Chem., Int. Ed., 2012, 51, 11501–11504 CrossRef CAS PubMed.
- D. A. Reddy, R. Ma, M. Y. Choi and T. K. Kim, Appl. Surf. Sci., 2015, 324, 725–735 CrossRef PubMed.
- P. Kumari, P. Chandran and S. S. Khan, J. Photochem. Photobiol., B, 2014, 141, 235–240 CrossRef CAS PubMed.
- S. Liu, X. Wang, W. Zhao, K. Wang, H. Sang and Z. He, J. Alloys Compd., 2013, 568, 84–91 CrossRef CAS PubMed.
- C. Xing, Y. Zhang, Z. Wu, D. Jiang and M. Chen, Dalton Trans., 2014, 43, 2772–2780 RSC.
- L. Armelao, P. Colombo, M. Fabrizio, S. Gross and E. Tondello, J. Mater. Chem., 1999, 9, 2893–2898 RSC.
- J. Xu, X. Yang, Q.-D. Yang, T.-L. Wong and C.-S. Lee, J. Phys. Chem. C, 2012, 116, 19718–19723 CAS.
- M. A. Ehsan, H. Khaledi, A. A. Tahir, H. N. Ming, K. Wijayantha and M. Mazhar, Thin Solid Films, 2013, 536, 124–129 CrossRef CAS PubMed.
- J.-G. Yu, H.-G. Yu, B. Cheng, X.-J. Zhao, J. C. Yu and W.-K. Ho, J. Phys. Chem. B, 2003, 107, 13871–13879 CrossRef CAS.
- X. Li, F. Li, C. Yang and W. Ge, J. Photochem. Photobiol., A, 2001, 141, 209–217 CrossRef CAS.
- S. Boributh, A. Chanachai and R. Jiraratananon, J. Membr. Sci., 2009, 342, 97–104 CrossRef CAS PubMed.
- C. Yao, X. Li, K. G. Neoh, Z. Shi and E. T. Kang, Appl. Surf. Sci., 2009, 255, 3854–3858 CrossRef CAS PubMed.
- G. Dai, J. Yu and G. Liu, J. Phys. Chem. C, 2012, 116, 15519–15524 CAS.
- R. G. Pearson, Proc. Natl. Acad. Sci. U. S. A., 1986, 83, 8440–8441 CrossRef CAS.
- Y. Xu and M. A. Schoonen, Am. Mineral., 2000, 85, 543–556 CAS.
- H. Zhang, B. Wei, L. Zhu, J. Yu, W. Sun and L. Xu, Appl. Surf. Sci., 2013, 270, 133–138 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: EDS and the particle size distribution of Ag2S; spectrum of methyl orange solution at different concentrations; extraction of adsorbed MO on Ag2S with DI water; repeatability experiments of MO degradation with Ag2S under light irradiation. See DOI: 10.1039/c4ra15774e |
| This journal is © The Royal Society of Chemistry 2015 |