A DFT-D study of hydrogen adsorption on functionalized graphene
Mahamadou Seydou*a,
Karima Lassouedabc,
Frederik Tielensd,
François Maurela,
Faycal Raouafib and
Boubakar Diawarac
aUniversité Paris Diderot, Sorbonne Paris Cité, ITODYS, UMR 7086 CNRS, 15 rue J.-A. de Baïf, 75205 Paris cedex 13, France. E-mail: mahamadou.seydou@univ-paris-diderot.fr
bLaboratoire de physico-chimie des microstructures et microsystèmes, IPEST La Marsa, Tunisie
cÉcole Nationale Supérieure de Chimie de Paris, CNRS-ENSCP, IRCP, 11 rue Pierre et Marie Curie, 75005 Paris, France
dSorbonne Université, UPMC, Univ Paris 06, UMR 7574, Laboratoire Chimie de la Matière Condensée, Collège de France, 11 place Marcelin Berthelot, 75231 Paris Cedex 05, France
First published on 21st January 2015
Abstract
In this paper, we use density functional theory with dispersion correction functional (DFT-D) as implemented in the Vienna ab initio simulation package in order to investigate hydrogen adsorption on graphene (GH) and fluorographene (GF). The adsorption sites at different surface coverage rates were studied to determine the most stable configurations. The comparison between the results obtained using standard pure DFT functionals and dispersion corrected ones highlight the role of the dispersion effect in the adsorption energies and the orientation of the molecules relative to the surface. The coverage rate is found to increase up to 75% on the two sides, making these nanoporous materials promising candidates for hydrogen storage. Electronic properties such as density of states and band structures were calculated on both GH and GF systems. It is observed that after H2 adsorption the band gap of GH is only slightly modified, whereas the opposite trend is observed on GF.
Introduction
The search for new functionalized porous materials for storage applications, capture and purification of pure gases and mixtures, is a very active research field with considerable potential for industrial applications.1–4 Among these new materials graphene is particularly attractive because of the possibility to be functionalized with hydrogen, or halogens like fluorine e.g. giving the possibility to tune its chemical and adsorption properties. Ever since its discovery, significant efforts have been made to boost its electronic and storage properties.5,6At one hand, graphene and halogen functionalized graphene have been widely studied in order to take advantage of their small electronic band gap, and their relatively small dimensions, which is expected to be a potential substituent to silicon in electronic circuits. Fluorographene (GF) and graphene (GH) show bands gaps of 3.0 eV and 3.8 eV, respectively, and become very promising for practical applications.7 It was shown that these band gaps are modified after adsorption or doping with molecules or atoms.8–10
At the other hand, beside their outstanding electronic and mechanical properties, the fluorographene/graphene system has important storage properties.11–13 Indeed it is possible to functionalize the graphene with hydrogen using a gas stream, yielding graphene.5 The bonded hydrogen can simply be released by heating.9,10 This process enables to store a very large mount of hydrogen in a small volume. To enhance the storage properties, graphene and fluorographene were doped by alkaline metals.11,12
Even if, since their discovery in 2004, a growth of experimental and theoretical studies are carried out on their electronic or mechanical properties at the atomic scale, the experimental development of new materials based on graphene and their handling remains particularly difficult, and not yet routinely used. Quantum chemical calculations are particularly relevant to progress in the knowledge of these materials and to be able to predict their properties. Theoretically, Sofo et al.14 predicted two geometrical configurations for graphene, completed recently with a third conformation discovered by Bhattacharya et al.15 Among them the armchair one was found to be the most stable conformation. Concerning fluorographene two configurations are highlighted.7,16
Mesoscopic simulations using Monte Carlo or classical molecular dynamics, based on empiric potentials using Lennard-Jones parameters, were carried out on pristine and doped GH and GF.14 Among these studies, Lamari and Levesque17 used Monte Carlo simulations based on the Lennard-Jones potential14,18 showing a relationship between the adsorption rate and temperature.
To our knowledge there is no ab initio based potential to parameterize the Monte Carlo code for GH and GF. Nevertheless, different ab initio calculations were performed on the pristine–GF and pristine–GH systems, such as: scanning the potential energy surface to investigate the different equilibrium geometries,7,10,16,19 the study of the functionalization reaction,8,17,19–24 the calculation of their electronic properties,18–21 and the effect of adsorption of metallic atoms such as Mn25 and Li.11,26 In the latter case, the effect on molecular hydrogen storage was also investigated.11
Despite the large number of studies on the topic, no specific study was carried out on molecular hydrogen adsorption on GH and GF. In the present work, we investigate molecular hydrogen adsorption on a (2 × 2) GH, and GF supercell, by DFT-D. In particular, binding sites and recovery rates on the both sides of these nano-porous structures were investigated.
Computational method
Calculations were performed in the frame of periodic DFT by means of the Vienna Ab initio Simulation Package (VASP 5.2.11).27,28 The electron–ion interactions were described by the projector augmented wave (PAW)29,30 method, representing the valence electrons, as provided in the code libraries. The convergence of the plane-wave expansion was obtained with a cut off of 500 eV. The generalized gradient approximation (GGA) was used with the functional of Perdew–Burke–Ernzerhof (PBE).31,32 The sampling in the Brillouin zone was performed on a grid of k-points separated by 0.5 Å−1 for the geometry optimizations and 0.1 Å−1 for the DOS calculations.The standard functionals in DFT calculations lack the important dispersion effects which are essential to the modeling of weak interactions. To improve the accuracy of DFT calculations Grimme et al.33 proposed to introduce an empirical correction of dispersion contribution to the standard density functionals (denoted as DFT-D); using this strategy, the estimation of non-covalent interactions can be computed very accurately at the DFT level. All the computations reported in this paper are performed using the dispersion-including DFT Grimme D2 method, as implemented in VASP 5.2.11. DFT-D2 Grimme (G, D2). This method describes the dispersion interactions between a particle and its neighbors in a given radius, via a simple pair-wise force field summed to the pure DFT energy.
The substrate is modeled as a slab, where a unit cell is periodically reproduced in the 3D space (see Fig. 1). In this approach the surface is infinite in two dimensions (in x and y directions), with a vacuum space (22 Å) in the z axis direction. This vacuum space should be large enough to enable the hydrogen adsorption and disable its interaction with the consecutive repetition of the system slabs. In the present case, the slabs representing an 2 × 2 supercell of armchair GH and GF were built from optimized unit cell (see Fig 1.), using Modelview software.34 The primitive unit cell parameters of GH are: a = 2.53 Å, b = 2.53 Å, c = 20 Å, α = β = 90° and γ = 120°, being in agreement with experimental35 and previous theoretical results.7,25,36,37 For GF, the unit cell parameters are: a = 2.59 Å, b = 2.59 Å, α = β = 90° and γ = 120°, being in a good agreement with previous experiment and theoretical results.6,7,9,16
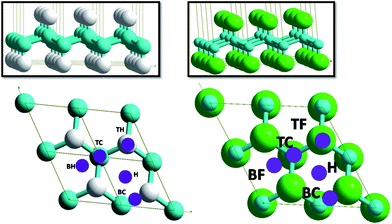 | ||
| Fig. 1 Armchair conformations and adsorption site labeling of GH (left) and GF (right). Carbon atoms are in blue, hydrogen in white and fluorine in green. | ||
Molecular hydrogen was deposited on the different sites on GH and GF, shown in Fig. 1. The energies of adsorption (ΔEads) are calculated as follows:
| ΔEads = E(GX − nH2) − E(GX) − E(H2) | (1) |
In order to measure the effect of adsorption on hydrogen molecules stretching modes, vibrational spectra have been calculated within the harmonic approximation. All atoms are considered in the Hessian matrix. This matrix is computed by the finite difference method followed by a diagonalization procedure. The eigenvalues of the resulting matrix lead to the frequency values. The assignment of the vibrational modes is done by inspection of the corresponding eigenvectors.
Results and discussion
Adsorption sites
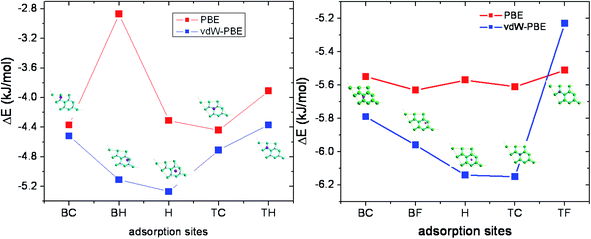
In order to investigate the most stable adsorption site, a 2 × 2 supercell was used, in which one hydrogen molecule is placed, leading to a coverage rate of 12.5%. The lattice constant is 5.09 Å, the C–C and C–H bond length are 1.54 and 1.11 Å, respectively. The vertical spacing between sp3 carbon planes was set to 0.47 Å. The parameters are in agreement with previous theoretical calculations by Sofo et al.35 (1.52 and 1.11 Å for C–C and C–H, respectively) and Alzharani25 (1.49, 1.11 and 0.49 for C–C, C–H and the vertical spacing, respectively) and others.11,13,38In order to determine the favorable site, a hydrogen molecule was placed on five different sites named bridge C (BC), bridge H (BH), hollow (H), top C (TC), and top H (TH) (Fig. 1). Adsorption energy is calculated by full optimization of complex systems and isolated graphene, fluorographene and the hydrogen molecule, separately. Results show that the most stables sites are the hollow and top C sites for graphene and fluorographene, respectively (Fig. 2). Calculations were performed with and without van der Waals corrections in order to highlight the effect of this latter on structures and adsorption energies. For GH and GF, the adsorption energies for all proposed sites and optimized structures are shown in Fig. 1a, with or without inclusion of van der Waals corrections.
In the case of GH, in absence of van der Waals terms, adsorptions energies are −4.37, −2.87, −4.31, −4.44, −3.91 kJ mol−1, for BC, BH, H, TC and TH sites, respectively (see Table 1). The adsorption energy is weakly exothermic, nevertheless it is larger than the thermal energy at ambient conditions. The most stable configuration corresponds to 1.5 kT at 300 K. The H–H bond length is found between 0.751 and 0.753 Å. For comparison, in the ref. 11, the adsorption energies of hydrogen molecule on alkaline doped graphene were found to −0.06 eV (−5.79 kJ mol−1) for lithium and sodium and −0.04 eV (−3.89 kJ mol−1) for potassium. Also, the binding energy C13H22 dimer is found to −536 meV that to say −24 meV (−2.35 kJ mol−1) per H⋯H bond.39,40 The vertical spacing between the hydrogen molecule and the surface depends on the site. For the most favorable sites, such as the TC and BC, the vertical spacing is found to be 2.233 and 2.461 Å, respectively. In the other cases, the vertical spacing varies between 2.14 and 2.23 Å.
| Adsorption sites | PBE | vdW–PBE | PBE | EvdW–PBE | PBE | vdW–PBE |
|---|---|---|---|---|---|---|
| ΔEadsa (kJ mol−1) | ΔEadsa (kJ mol−1) | dH–H | dH–H | dH–S | dH–S | |
| a For comparison two decimals in the energy values are considered, which fall out of the expected accuracy limits. | ||||||
| Bridge CC | −4.37 | −4.52 | 0.746 | 0.752 | 2.461 | 2.46 |
| Bridge HH | −2.87 | −5.11 | 0.752 | 0.752 | 2.139 | 1.85 |
| Hollow | −4.31 | −5.27 | 0.751 | 0.752 | 2.216 | 1.821 |
| Top C | −4.44 | −4.71 | 0.751 | 0.751 | 2.233 | 2.233 |
| Top H | −3.91 | −4.37 | 0.752 | 0.752 | 2.233 | 2.229 |
By taking into account vdW interactions, a gain of 1 kJ mol−1 in adsorption energies is obtained. Consequently, the stabilization trend is altered and the hollow and bridge H–H become the most stable sites. H–C bond lengths do not change, but the vertical distance decreases considerably to 1.821 and 1.850 Å, for the two most stable sites.
One can observe that the geometries do not change significantly, after taking into account vdW interactions in the self consist field optimization. Nevertheless, it changes considerably the vertical spacing and increases slightly the adsorption energies (see Table 2). Also the intrinsic geometry of GH is not significantly modified. The lattice parameters, the C–C and C–H bond lengths stay unchanged after adsorption.
| Adsorption sites | PBE | vdW–PBE | EPBE | vdW–PBE | PBE | vdW–PBE |
|---|---|---|---|---|---|---|
| ΔEadsa (kJ mol−1) | ΔEadsa (kJ mol−1) | dH–H | dH–H | dH–S | dH–S | |
| a For comparison two decimals in the energy values are considered, which fall out of the expected accuracy limits. | ||||||
| Bridge CC | −5.55 | −5.79 | 0.750 | 0.750 | 2.607 | 2.288 |
| Bridge FF | −5.63 | −5.96 | 0.750 | 0.750 | 2.67 | 2.231 |
| Hollow | −5.57 | −6.14 | 0.750 | 0.750 | 2.546 | 2.193 |
| Top C | −5.61 | −6.15 | 0.750 | 0.750 | 2.665 | 2.186 |
| Top F | −5.53 | −5.29 | 0.750 | 0.750 | 2.778 | 2.457 |
For GF, the 2 × 2 supercell was optimized, and the lattice constant was found to be equal to 5.18 Å. The calculated C–C and CH bond lengths are found to be equal to 1.57 Å and 1.38 Å, respectively in a good agreement with previous works.14,37,41
In absence of van der Waals correction, adsorptions energies are found to be −4.55, −5.63, −5.57, −5.61, −5.53 kJ mol−1, for BC, BF, H, TC and TF sites, respectively (Table 2). These value are in line of previous CF⋯H binding energies found at MP2 (ref. 42) level (−2.9 kJ mol−1) and PBE-D39 level (−2.26 kJ mol−1). The H–H bond length stay equal to 0.750 Å, and the vertical spacing varies between 2.546 (hollow site) and 2.778 Å (TF site) in a good agreement with the ref. 39. This range is slightly less important than in the case of graphene, probably due to the polarization effect induced by the fluorine atoms. Dispersion allows gaining up to 0.6 kJ mol−1 per molecule in adsorption energy, and decreases the vertical spacing up to 2.186 Å for the most stable vdW site (TC). The lateral position of hydrogen was unchanged after optimization, and the hydrogen molecule remains perpendicular to the surface. Adsorption is exothermic and does not change significantly the surface parameters.
In summary, dispersion plays an important role and affects the geometry as well as the adsorption energy. Inclusion of dispersion, increases the adsorption energies between 10 to 25%, and reduces the molecule-surface spacing between 2 to 14% in the case of graphene, depending on the adsorption site. For fluorographene, the effect is less important with an increase between 4 to 10% in adsorption energy, and between 9 to 17% in vertical spacing between molecules and surface, for the different adsorption sites studied. Our results are in a good agreement of previous calculations39 on GH and GF bilayers binding energies those are found to −84 meV (−8.11 kJ mol−1) for CF⋯HH bond and −66 meV (−5.89 kJ mol−1) for GH/GH bilayer (CH⋯HH bond). They are in the same magnitude of values obtained for hydrogen molecule adsorption on different Zn(II) active sites of zeolites43 and higher than small carbon nanotubes adhesive energies on graphene.4,44
Coverage rate
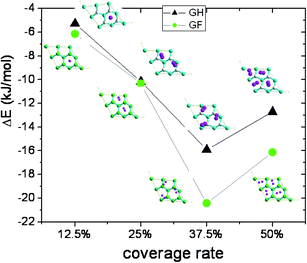
In the 2 × 2 supercell four identical sites for hydrogen molecule are available on each side. So, one, two, three, and four hydrogen molecules were considered in the models, corresponding to a total coverage of 12.5, 25, 37.5 and 50%, respectively.
The adsorption energy as function of the coverage is investigated for the most stable sites such as the H site for GH and TC for GF, by increasing the number of hydrogen molecules which were placed initially on the (H) or TC sites for GH and GF, respectively.
In Table 3, we present adsorption energies, H2 bond lengths and minimum distances between molecule and surface. One can observe a minimum of adsorption energy in both GH and GF cases at 37.5% of coverage corresponding to three adsorbed hydrogen molecules.
| Recovery rate (%) | GH supercell 2 × 2 | GF supercell 2 × 2 | ||||
|---|---|---|---|---|---|---|
| ΔEa (kJ mol−1) | dH–H (Å) | dH–Smin (Å) | ΔE (kJ mol−1) | dH–H (Å) | dH–Smin (Å) | |
| a For comparison two decimals in the energy values are considered, which fall out of the expected accuracy limits. | ||||||
| 12.5 | −5.27 | 0.752 | 1.821 | −6.15 | 0.75 | 2.186 |
| 25 | −10.17 | 0.751 | 2.025 | −12.76 | 0.749 | 2.532 |
| 37.5 | −15.91 | 0.751 | 2.086 | −20.57 | 0.749 | 2.456 |
| 50 | −12.71 | 0.749 | 1.81 | −16.16 | 0.748 | 2.5 |
For GH, the adsorption energy increases with the number of adsorbed hydrogen molecules, up to three molecules in the supercell (θ = 37.5%) for which it becomes higher than 2 kT (−5.30 kJ mol−1 per molecule). The geometry of surface does not change significantly. But the hydrogen molecules move and form a quasi-equilateral triangle on the surface with the vertices length equal to about 2.4 Å. These kinds of hydrogen molecules assembly were previously observed on graphene doped by lithium.11,12 All molecules are tilted with an angle of about 60° relative to the surface normal vector. The distance between molecules and surface varies from 1.81 to 2.09 Å for 37.5% coverage. The average H–H bond length is found to be equal to 0.751 Å.
The same is observed in the case of GF for which a maximum coverage rate of 37.5% and maximum adsorption energy per molecule of −6.86 kJ mol−1 is found (Fig. 3). At this coverage rate, all hydrogen molecules are planes and form a scalene triangle on the surface with vertices equal to 2.48, 2.68 and 2.86 Å. The average H–H bond length is calculated to be equal to 0.749 Å. The vertical spacing of the molecules varies between 2.63 and 2.67 Å.
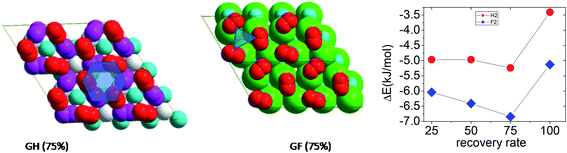
In the case of GH, molecules are placed initially on the most stable adsorption site (H) and a full optimization of the molecules and surface was performed. The results show an increase of adsorption energy with the coverage rate, up to six molecules (three per side), and a sudden fall (2 kJ mol−1 per molecule) after (see Fig. 4). The maximum coverage rate is found for three molecules per side (θ = 75%) corresponds to an adsorption energy of −5.78 kJ mol−1 per molecule.
The projection of the positions of the hydrogen molecules on both side of the surface generates a hexagonal pattern (see Fig. 4). The vertical spacing to the surface varies from 1.99 Å for the tilted molecules to 2.52 Å for the parallel ones. The distances between the hydrogen molecules on one side of the surface vary from 2.60 to 2.78 Å and on the other side it varies from 2.30 to 2.78 Å.
In the case of GF, the adsorption energies per molecule are −6.04, −6.78, −7.70, and 0.01 kJ mol−1, for one, two, three, and four adsorbed molecules per side of the surface, respectively. The maximum coverage rate is found to three molecules per side (6 molecules), and the corresponding adsorption energy is lower than for GH of about 2 kJ mol−1. The geometry is also different, the molecules above and under the surface occupy the same position relative to the surface and form a triangle design, in contrast with the GH system. The vertical distance varies between 2.51 and 2.70 Å in agreement with ref. 39.
In conclusion, we found an optimal rate of 75%, for both graphene and fluorographene. The position of the molecules on the surface is very dependent on the coverage rate. At 25%, the hydrogen molecules are tilted by about 10°, relative to the surface normal vector. The augment of the coverage rate increases the tilting angle of the molecule in order to take full advantage of intermolecular interactions. Molecular hydrogen adsorption energy on GF is 20% higher than on GH.
Electronic properties
Density of states
Global and projected density of states are computed for different sites and coverage rates by increasing the Brillouin zone partition k-points to 15 × 15 × 1. Fig. 4 presents density of states of GH, GH with hydrogen adsorbed on the most stable site (H) and the less stable one (TH). In this study, the calculated band gaps with PBE are 3.6 and 3.0 eV for GH and GF respectively. The results are in a good agreement with previous calculations35,39 and slightly smaller than experiences (fluorescence and NEXAFS) measurements16,19,22,45 which indicate a band gap higher than 3.8 eV for GF.Concerning the adsorption sites, the most stable configuration (hollow site) shows a slight increase of the band gap of about 90 meV for GH. The projection on the atomic orbitals presented in Fig. 5 shows a shift of the LUMO due to the molecular hydrogen interaction with the surface forming a sigma orbital. For the other sites, as well as for the other coverage rate the band gap is not significantly affected.
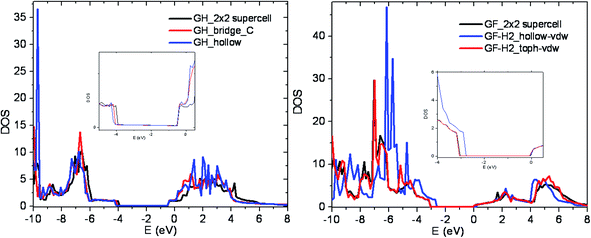 | ||
| Fig. 5 Density of states of pristine, most and less stable adsorption sites for GH (left) and GF (right). The inserts are the zoom near Fermi level. | ||
In the case of GF, significant band gap increase (300 meV) is observed for the most stable configuration (TC). Previous theoretical calculations show that estimation of fluorographene band gap was found to 3.1 eV for GGA functional or 4.9, 8.1 and 8.3 eV for hybrid (HSE06), GW on top of PBE and on top of HSE06 respectively.37 In the same reference, they have calculated the optical transition by Bethe–Salpeter equation (BSE) and found 5.1 eV. We have not observed a variation of the band gap as a function of the coverage rate.
Infrared vibration of H2
The wave number of the H–H stretch (νH–H) was found experimentally to 4401 cm−1 by Dickenson et al.46 The harmonic frequency47 was reported to 4400 cm−1 and it was shown that the anharmonic effect48 lower this values up to 4161 cm−1. Previous ab initio calculations gave values in the range 4100 to 4500 cm−1, depending on the type of basis set used pseudo potentials49,50 or localized basis sets.51,52 It was shown that the νH–H stretch shifts up to 174 cm−1 when molecular hydrogen is adsorbed on zeolite43,53 surfaces or on transition metal.54,55 Our calculations show that the νH–H stretch (4292 cm−1), shifts 20 and 50 cm−1 for the molecule adsorbed on the GH and GF surface, respectively. The shift of νH–H increases with the coverage rate up to 80 cm−1 for the best coverage rate (75%).Conclusion
In this paper hydrogen adsorption on functionalized graphene is investigated by means of DFT-D calculations. Although, the results are subjected to the accuracy of the calculation level, and that some energies are almost identical, one can summarize our results as following: results show that the hollow and top C are the most stable sites for graphene and fluorographene, respectively. Regarding the recovery rate for hollow site, we found a maximum rate of 75%, for both graphene and fluorographene. The position of molecules on surface is heavily dependent on the recovery rate. At 25%, hydrogen molecule is tilted by about ten degrees relative to the surface normal vector. The rise of the coverage rate increases the inclination of molecule in order to take full advantage of intermolecular interactions. Dispersion plays a very important role in the adsorption energies as shown by the results obtained using DFT-D calculations. It changes significantly adsorption energies from 10 to 25%, and reduces the molecule surface spacing from 2 to 14% in case of graphene. For fluorographene, the effect is less pronounced with 4 to 10% in adsorption energy changes and 9 to 17% in vertical spacing between molecules and surface.Projected density of states were computed for different sites and recovery rates and show a slight (90 meV) and significant (300 meV) band gap dependences of band gap for the most stable configuration of hydrogen adsorbed on graphene and fluorographene respectively. Infrared calculations show a significant shift of hydrogen molecules stretch mode by about 20 and 50 cm−1 in the case of graphene and fluorographene, respectively.
The current findings add to a growing body of literature on energetic and structure data on hydrogen adsorption on to GH and GF surfaces and suggest that these nanomaterials are promising candidates for the storage of hydrogen. The current investigation was limited by the size of systems. More research is required to determine the yield of adsorption at mesoscopic scale as well as temperature dependence. Further experimental investigations are needed to confirm these results.
Acknowledgements
This work was performed using HPC resources from GENCI-[CCRT/CINES/IDRIS] (Grant 2014-[c2014097006]). We acknowledge the financial support from the French Government's Investissementsd'Avenir program: Laboratoired'Excellence ‘Sciences and Engineering for Advanced Materials and devices – SEAMs’ (grant no. ANR-10-LABX-96).References
- R. Coontz and B. Hanson, Science, 2004, 305, 957 CrossRef CAS.
- C. Liu and Z. Zeng, Appl. Phys. Lett., 2010, 96, 123101 CrossRef PubMed.
- A. K. Geim, Science, 2009, 324, 1530 CrossRef CAS PubMed.
- M. Seydou, Y. J. Dappe, S. Marsaudon, J. P. Aimé, X. Bouju and A. M. Bonnot, Phys. Rev. B: Condens. Matter Mater. Phys., 2011, 83, 045410 CrossRef.
- D. C. Elias, R. R. Nair, T. M. G. Mohiuddin, S. V. Morozov, P. Blake, M. P. Halsall, A. C. Ferrari, D. W. Boukhvalov, M. I. Katsnelson, A. K. Geim and K. S. Novoselov, Science, 2009, 232, 610–613 CrossRef PubMed.
- J. T. Robinson, J. S. Burgess, C. E. Junkermeier, S. C. Badescu, T. L. Reinecke, F. K. Perkins, M. K. Zalalutdniov, J. W. Baldwin, J. C. Culbertson, P. E. Sheehan and E. S. Snow, Nano Lett., 2010, 10, 3001–3005 CrossRef CAS PubMed.
- O. Leenaerts, H. Peelaers, A. D. Hernández-Nieves, B. Partoens and F. M. Peeters, Phys. Rev. B: Condens. Matter Mater. Phys., 2010, 82, 195436 CrossRef.
- S. Tang and S. Zhang, J. Phys. Chem. C, 2011, 115, 16644–16651 CAS.
- F. Withers, M. Dubois and A. K. Savchenko, Phys. Rev. B: Condens. Matter Mater. Phys., 2010, 82, 073403 CrossRef.
- R. Zboril, F. Karlicky, A. B. Bourlinos, T. A. Steriotis, A. K. Stubos, V. Georgakilas, K. Safarova, D. Jancik, C. Trapalis and M. Otyepka, Small, 2010, 6, 2885–2891 CrossRef CAS PubMed.
- L. Y. Antipina, P. V. Avramov, S. Sakai, H. Naramoto, M. Ohtomo, S. Entani, Y. Matsumoto and P. B. Sorokin, Phys. Rev. B: Condens. Matter Mater. Phys., 2012, 86, 085435 CrossRef.
- T. Hussain, B. Pathak, T. A. Maark, C. M. Araujo, R. H. Scheicher and R. Ahuja, EPL, 2011, 96, 27013 CrossRef.
- X. Wang, Z. Zeng, H. Ahn and G. Wang, Appl. Phys. Lett., 2009, 95, 183103 CrossRef PubMed.
- L. Reatto, M. Nava, D. E. Galli, C. Billman, J. O. Sofo and M. W. Cole, cond-mat.mes-hall arXiv:1204.3061v1.
- A. Bhattacharya, S. Bhattacharya, C. Majumdar and G. P. Das, Phys. Rev. B: Condens. Matter Mater. Phys., 2011, 83, 033404 CrossRef.
- R. R. Nair, W. Ren, R. Jalil, I. Riaz, V. G. Kravets, L. Britnell, P. Blake, F. Schedin, A. S. Mayorov, S. Yuan, M. I. Katsnelson, H.-M. Cheng, W. Strupinsk, L. G. Bulusheva, A. V. Okotrub, I. V. Grigorieva, A. N. Grigorenko, K. S. Novoselov and A. K. Geim, Small, 2010, 6, 2877–2884 CrossRef CAS PubMed.
- F. D. Lamari and D. Levesque, Carbon, 2011, 49, 5196–5200 CrossRef PubMed.
- M. Nava, D. E. Galli, M. W. Cole and L. Reatto, Phys. Rev. B: Condens. Matter Mater. Phys., 2012, 86, 174509 CrossRef.
- F. Karlicky, R. Zboril and M. Otyepka, J. Chem. Phys., 2012, 137, 034709 CrossRef PubMed.
- S.-H. Cheng, K. Zou, F. Okino, H. R. Gutierrez, A. Gupta, N. Shen, P. C. Eklund, J. O. Sofo and J. Zhu, Phys. Rev. B: Condens. Matter Mater. Phys., 2010, 81, 205435 CrossRef.
- A. M. Ilyin, Physica E, 2011, 43, 1262–1265 CrossRef CAS PubMed.
- Z. F. Wang, J. Q. Wang, Z. P. Li, P. W. Gong, X. H. Liu, L. B. Zhang, J. F. Ren, H. G. Wang and S. R. Yang, Carbon, 2012, 50, 5403–5410 CrossRef CAS PubMed.
- Z. Ao, S. Li, Hydrogenation of Graphene and Hydrogen Diffusion Behavior on Graphene/Graphane Interface, in Graphene Simulation, ed. J. R. Gong, Croatia, 2011, pp. 53–74 Search PubMed.
- L. Tsetseris and S. T. Pantelides, J. Mater. Sci., 2012, 47, 7571–7579 CrossRef CAS PubMed.
- A. Z. AlZahrani, Phys. B, 2012, 407, 992–1002 CrossRef CAS PubMed.
- P. V. C. Medeiros, F. d. BritoMota, A. J. S. Mascarenhas and C. M. C. d. Castilho, Nanotechnology, 2010, 21, 115701 CrossRef PubMed.
- G. Kresse and J. Hafner, Phys. Rev. B: Condens. Matter Mater. Phys., 1993, 47, 558–561 CrossRef CAS.
- G. Kresse and J. Hafner, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 49, 14251–14269 CrossRef CAS.
- P. E. Blochl, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 50, 17953–17979 CrossRef.
- G. Kresse and D. Joubert, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 59, 1758–1775 CrossRef CAS.
- B. Hammer, L. B. Hansen and J. K. Norskov, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 59, 7413–7421 CrossRef.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1997, 78, 1396 CrossRef CAS.
- S. Grimme, J. Comput. Chem., 2006, 27, 1787–1799 CrossRef CAS PubMed.
- http://www.enscp.fr/labos/LPCS/MRS/Modelview.
- J. O. Sofo, A. S. Chaudhari and G. D. Barber, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 75, 153401–153404 CrossRef.
- M. Garay-Tapia, A. H. Romero and V. Barone, J. Chem. Theory Comput., 2012, 8, 1064–1071 CrossRef.
- F. Karlicky and M. Otyepka, J. Chem. Theory Comput., 2013, 9, 2115–2125 CrossRef.
- D. S. L. Abergel, V. Apalkov, J. Berashevich, K. Ziegler and T. Chakraborty, Adv. Phys., 2010, 59, 261–482 CrossRef CAS.
- Y. Li, F. Li and Z. Chen, J. Am. Chem. Soc., 2012, 134, 11269–11275 CrossRef CAS PubMed.
- A. A. Fokin, D. Gerbig and P. R. Schriner, J. Am. Chem. Soc., 2011, 133, 20036–20039 CrossRef CAS PubMed.
- F. Karlický, K. K. R. Datta, M. Otyepka and R. Zbořil, ACS Nano, 2013, 7, 6434–6464 CrossRef PubMed.
- K. Reichenbächer, H. I. Süss and J. Hulliger, Chem. Soc. Rev., 2005, 34, 22–30 RSC.
- L. A. M. M. Barbosa, G. M. Zhidomirov, R. A. v. Santen and D. S. L. Abergel, Catal. Lett., 2001, 77, 1–3 CrossRef.
- M. Seydou, S. Marsaudon, J. Buchoux, J. P. Aimé and A. M. Bonnot, Phys. Rev. B: Condens. Matter Mater. Phys., 2009, 80, 245421 CrossRef.
- K. Jeon, Z. J. Lee, E. Pollak, L. Moreschini, A. Bostwick, C. M. Park, R. Mendelsberg, V. Radmilovic, R. Kostecki, T. J. Richardson and E. Rotenberg, ACS Nano, 2011, 5, 1042–1046 CrossRef CAS PubMed.
- G. D. Dickenson, M. L. Niu, E. J. Salumbides, J. Komasa, K. S. E. Eikema, K. Pachucki and W. Ubachs, Phys. Rev. Lett., 2013, 110, 193601 CrossRef CAS.
- K. P. Huber and G. Herzberg, Van Nostrand Reinhold, Molecular spectra and molecular structure: IV constants of diatomic molecules, New York, 1979 Search PubMed.
- B. P. Stoicheff, Can. J. Phys., 1957, 35, 730 CrossRef CAS.
- C. G. V. d. Walle, Phys. Rev. Lett., 1998, 80, 2177–2180 CrossRef.
- O. A. Syzgantseva, M. Calatayud and C. Minot, J. Phys. Chem. C, 2012, 116, 6636–6644 CAS.
- W. Kolos and L. Wolniewicz, J. Chem. Phys., 1968, 49, 404 CrossRef CAS PubMed.
- D. E. Bernholdt, S. Liu and C. E. Dykstra, J. Chem. Phys., 1986, 85, 5120 CrossRef CAS PubMed.
- V. B. Kazansky, V. Y. Borovkov, A. I. Serykh, R. A. v. Santen and P. J. Stobbelaar, Phys. Chem. Chem. Phys., 1999, 1, 2881 RSC.
- G. J. Kubas, R. R. Ryan, B. I. Swanson, P. J. Vergamini and H. J. Wasserman, J. Am. Chem. Soc., 1984, 106, 451 CrossRef CAS.
- W. D. Harman and H. Taube, J. Am. Chem. Soc., 1990, 112, 2261 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |