Synthesis of PVC/CNT nanocomposite fibers using a simple deposition technique for the application of Alizarin Red S (ARS) removal†
Mudassir Hasana,
Rajeev Kumar*b,
M. A. Barakatbc and
Moonyong Lee*a
aSchool of Chemical Engineering, Yeungnam University, Gyeongsan-si, Gyeongbuk 712-749, South Korea. E-mail: mynlee@ynu.ac.kr; Fax: +82-53-811-3262; Tel: +82-53-810-2512
bDepartment of Environmental Sciences, Faculty of Meteorology, Environment and Arid Land Agriculture, King Abdulaziz University, Jeddah, 21589, Saudi Arabia. E-mail: olifiaraju@gmail.com
cCentral Metallurgical R & D Institute, Helwan, 11421, Cairo, Egypt
First published on 21st January 2015
Abstract
This paper reports the synthesis of PVC/CNT nanocomposite fibers using a simple deposition technique as well as their application towards removal of Alizarin Red S (ARS). The as-prepared PVC/CNT nanocomposite fibers were characterized by Brunauer, Emmett and Teller surface area measurements, Raman spectroscopy, X-ray photoelectron spectroscopy, X-ray diffraction, transmission electron microscopy, scanning electron microscopy, thermogravimetric analysis, differential scanning calorimetry and diffuse reflectance spectroscopy. PVC/CNT nanocomposite fibers showed a remarkable 81% increase in surface area compared to the PVC fibers. PVC/CNT nanocomposite fibers showed a lower band gap and higher glass transition temperature than the PVC fibers. Both PVC and PVC/CNT nanocomposite fibers were applied to adsorb ARS and the nanocomposite fibers showed almost double the adsorption capacity than PVC fibers. The thermodynamic study revealed that adsorption of ARS was exothermic in nature and physical forces were involved in adsorption of dye onto PVC and PVC/CNT nanocomposite fibers.
Introduction
Recently, polymer/carbon nanotube (CNT) nanocomposites have become an important and interesting area of research worldwide. The obvious reason is because CNTs have unique properties, such as low-weight, high aspect ratio,1 good mechanical properties2 and high electrical3 and thermal conductivity.4 CNTs have been used successfully to enhance the mechanical, thermal, optical and barrier properties of several polymer/CNT nanocomposite structures.5–7 On the other hand, further systematic and optimized study of the physico-chemical properties of these polymer/CNT nanocomposites are necessary to find future applications for practical use.Polyvinyl chloride (PVC) is one of the most versatile and widely used polymers after polyethylene. Moreover, it is chemically stable, biocompatible and relatively inexpensive polymer.8 One of the main problem that limits the practical use of the polymer is its lower thermal stability.9 Considerable work has been carried out over the past decade to enhance the thermal stability, mechanical strength and electrical conductivity of PVC using a range of nanofillers.10–14
Little information is available on the production of nanocomposite fibers of PVC with CNT.15 In industry, PVC fibers are produced using a range of techniques, such as wet-spinning, dry-spinning and melt-spinning. On the other hand, these techniques have a range of limitations such as requirement of chemicals, need for high temperature and higher cost. Therefore, before PVC/CNTs nanocomposite fibers can be used to replace the existing conventional method for the production of PVC fibers, more work will be needed to simplify the production technique and explore various characteristic properties of the PVC/CNTs nanocomposite fibers. Therefore, the aim of the present study was to prepare nanocomposite fibers of PVC with CNTs, and compare the physico-chemical properties with those of PVC/GN nanocomposite fibers. This research may be fruitful to people working in the area of polymer nanocomposites for practical use. In this study, PVC/CNTs nanocomposite fibers were prepared using a simple and facile deposition route. The structure, morphology, thermal stability, optical, and adsorption properties were also examined.
Experimental
Materials
PVC (average molecular weight ∼ 1020) was purchased from Yakuri pure chemicals, Japan and MWCNT (diameter ∼ 10–20 nm and average length ∼ 20 μm with 95% purity) was purchased from Hanwha Nanotech, Korea. Tetrahydrofuran (THF) was supplied by Duksan pure chemicals, Korea, and used as received. Alizarin Red S (ARS) [C.I., 58005, M.F.: C14H7NaO7S] was purchased from BDH chemicals LTD, England.Preparation of the PVC/CNT nanocomposite fibers
The PVC/CNT nanocomposite fibers were prepared in two steps: the synthesis of PVC/CNT nanocomposites by mixing CNT in a THF solution of PVC followed by a deposition technique to prepare the PVC/CNT nanocomposite fibers. In a typical process, PVC was first dissolved in 50 mL of THF with continuous stirring for 24 hours for complete dissolution. The CNTs were mixed with the above solution with continuous stirring and occasional shaking in bath ultrasonic system for 5 minutes to allow proper dispersion of CNTs inside the THF solution of PVC. Subsequently, the mixture was poured into distilled water with vigorous stirring to obtain the PVC/CNT nanocomposite fibers. The fibers were washed with distilled water and an excess of methanol, and later dried at 60 °C in an air oven for 24 hours. Pure PVC fibers were also prepared using a similar route in the absence of CNT. Table S1† lists the preparation conditions.Characterization of PVC, PVC/CNT nanocomposite fibers
Structural analyses of the samples were carried out by X-ray diffraction (XRD, PAN analytical, X'pert PRO-MPD) in the range, 10–90° 2θ, using CuKα radiation (λ = 0.15405 nm). Raman spectroscopy (InVia Reflex UV Raman microscope, Renishaw, UK) was employed to examine the defects created on the lattice of CNTs in the PVC/CNT nanocomposite fibers. X-ray photoelectron spectroscopy (XPS, ESCALAB 250) was performed using a monochromatized AlKα X-ray source (hν = 1486.6 eV) with a 500 μm spot size. Scanning electron microscopy (SEM, Hitachi-4200) was employed to examine surface morphology. The optical properties were obtained by ultraviolet-visible-near infrared (UV-VIS-NIR, VARIAN, Cary 5000, USA) spectrophotometry. The microstructure was analyzed by field emission transmission electron microscopy (FE-TEM, TecnaiG2 F20, FEI, USA) operating at an accelerating voltage of 200 KV. Thermogravimetric analysis (TGA, SDT Q600, USA) was performed by heating the samples from 25 °C to 800 °C at a rate of 10 °C min−1 in a nitrogen atmosphere. Differential scanning calorimetry (DSC, Q 200, USA) was performed by heating the samples from 20 °C to 200 °C. Quantachrome, Asic-7 physisorption analyzer was used to estimate the surface area of fibers while the pore size and pore volume were obtained using the Barrett–Joyner–Halenda (BJH) theory.Adsorption Studies
The adsorption experiments were conducted by taking 20 mL of the dye solution in a series of flasks containing 0.02 g adsorbent fibers at 30 °C and the dye concentration was varied from 5 to 50 mg L−1. For the thermodynamic studies, 0.02 g fibers were added to the flask containing 20 mL of an 20 mg L−1 ARS solution with shaking at 200 rpm at varying temperatures (30, 40 and 50 °C). The amount of ARS remaining in the supernatant solution was determined using HACH LANGE DR 6000 UV-visible spectrophotometer at 423 nm. The adsorption capacity of polymeric fibers for ARS was calculated using following equation:| qe = (C0 − Ce)V/m | (1) |
Results and discussion
Synthesis
A simple facile deposition technique was used to prepare PVC/CNT nanocomposite fibers using two immiscible solvent systems, i.e. THF and water. The PVC/CNT dispersed in THF solvents with vigorous stirring precipitated readily in water because of its insolubility in water. The surface area of the fibers was expected to increase after incorporating the CNTs into the PVC matrix due to the high surface area of nanomaterials, which is desirable for the surface-related properties, such as adsorption and catalysis.X-ray diffraction
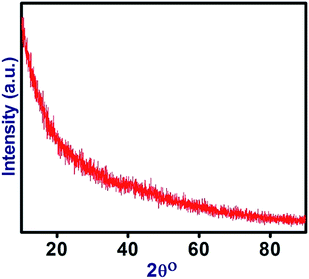
Fig. 1 shows XRD patterns of the PVC/CNT nanocomposite fibers. The XRD patterns of the PVC/CNT nanocomposite fibers (Fig. 1) showed no clear peak of PVC and CNT, which might be due to the encapsulation of CNT by the dense fibrous structure PVC. Hasan et al.16 reported a similar effect of encapsulation by PVC fibers on PVC/Graphene nanocomposite fibers. In this case, the XRD pattern clearly revealed the complete reduction and diffusion of the peak intensities because of the fibrous structure of PVC/CNT nanocomposites.Raman analysis
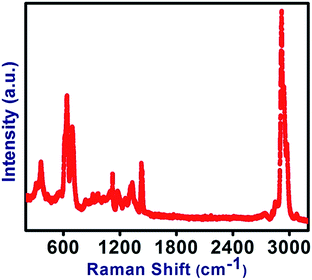
Raman spectroscopy was used for qualitative investigation of the structural defects created on the lattices of CNTs present in the PVC/CNT nanocomposite system. The Raman spectra (Fig. 2) of the PVC/CNT nanocomposite fibers showed two characteristic bands, the D band that can be attributed to the vibration of sp2-bonded carbon atoms in a two-dimensional hexagonal lattice and G band, which can be attributed to scattering from the sp3-hybridized carbon defects in the hexagonal framework of CNTs wall.17 The band intensity ratio (ID/IG) of the PVC/CNT nanocomposite, which is suggestive of the degree of covalent functionalization of the CNTs can be used for a qualitative estimation of the structural defects created on the lattice of CNTs. The intensity ratio between the D- and G-bands (ID/IG) was calculated to be 0.93 and 0.84 for PVC/CNT nanocomposite fibers and pristine CNTs, respectively. The band intensity ratio (ID/IG ≈ 1) indicates considerable lattice defects.13 The observed increase in the ID/IG ratio of the PVC/CNT nanocomposite fibers compared to the CNTs confirmed the enhancement in the structural defects in the lattice of CNTs. Owing to structural defects created in the lattice of CNTs, property of PVC/CNT nanocomposite fibers is expected to be different from the neat PVC fibers.SEM and TEM analysis
Fig. 3a and b present SEM images of PVC and PVC/CNT nanocomposite fibers, respectively. Both PVC and PVC/CNT nanocomposite showed a clear image of the long stranded fibers with loose cotton like morphology. The average diameter of the fibers was measured to be ∼0.23 μm. The loose cotton like morphology would increase the surface area of the PVC and PVC/CNT nanocomposite fibers, which was confirmed by BET surface area measurements, suggesting the suitability of the method for the preparation for the fibrous structures.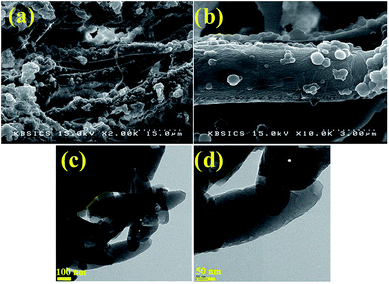 | ||
| Fig. 3 SEM images of (a) PVC/CNT at 15 μm and (b) PVC/CNT at 3 μm. (c) and (d) TEM images of PVC/CNT nanocomposite fibers at 100 and 50 nm. | ||
Fig. 3c presents TEM images of the PVC/CNT nanocomposite fibers. The low-magnification TEM images (Fig. 3c) clearly showed the PVC/CNT nanocomposite fibers attached to the wall of the CNTs. In addition, the mean thickness and length of the PVC/CNT nanocomposite fibers was measured to be 0.3 and 1 μm, respectively (Fig. 3c). The TEM images of the nanocomposite fibers clearly revealed the successful incorporation of CNTs inside PVC, which may give rise to an enhanced glass transition temperature (Fig. 5) and increased surface area (Fig. 7).
Thermogravimetric analysis
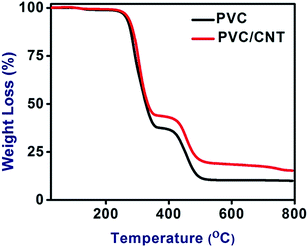
Fig. 4 shows the TGA curves of the PVC and PVC/CNT nanocomposite fibers. The weight loss below 150 °C for both PVC and PVC/CNT nanocomposite fibers can be assigned to the evaporation of volatile components and trapped THF. Both PVC and PVC/CNT nanocomposite fibers degraded in two stages. In the first stage a weight loss of 65.85 and 58.03% was observed for the PVC and PVC/CNT nanocomposite fibers, respectively. The PVC and PVC/CNT nanocomposite fibers showed the first stage weight loss over the temperature range, 270–415 and 260–418 °C, respectively and can be attributed to the loss of HCl.15 In the second stage a weight loss of 16.63 and 17.87% was observed in the case of the PVC and PVC/CNT nanocomposite fibers, respectively, which took place over the temperature ranges, 425–550 and 420–540 °C, respectively. The second stage of weight loss was much shorter than the first stage and could be attributed to thermal degradation of the polyene backbone, resulting in the formation of volatile aromatic compounds and a stable carbonaceous residue.18,19 Finally a residual weight of 16.41 and 21.78% was observed in case of the PVC and PVC/CNT nanocomposite fibers, respectively, confirming the enhanced thermal stability of PVC/CNT nanocomposite fibers.In addition, the CNTs are highly thermal stable might have imparted enhanced thermal stability to the PVC/CNT nanocomposite fibrous system, which might be due to the better thermal stabilization between the CNTs and PVC. Similar work was also reported by Hasan et al.20
Differential scanning calorimetry
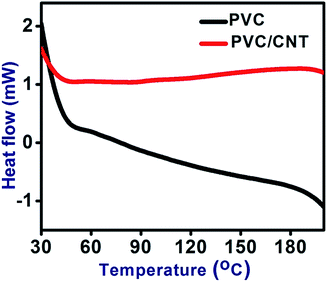
Fig. 5 shows the DSC thermograms of the PVC and PVC/CNT nanocomposite fibers. A hump relaxation, which is the characteristic for glass transition temperature (Tg),at around 79–84 °C for PVC and PVC/CNT nanocomposite fibers was shown by the plot between the heat flow rate versus temperature. Tg of the PVC and PVC/CNT nanocomposite fibers were found to be 81 and 82 °C respectively. Therefore, CNTS although very low in quantity, had some effect on the glass transition temperature of PVC/CNT nanocomposite fibers. The increase in Tg can be interpreted as the reinforcing effect of CNTs, which reduces the chain segmental mobility of PVC backbone.14 Table 1 list the other thermal properties of interest, such as the onset (To), peak temperature (Tp) along with the glass transition temperature (Tg).| Sample name | To,m (°C) | Tp,m (°C) | Tg (°C) |
|---|---|---|---|
| PVC | 234 | 294 | 81 |
| PVC/CNT | 268 | 302 | 82 |
Optical properties
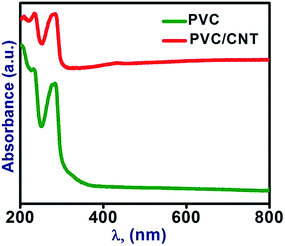
Both PVC and PVC/CNT nanocomposite fibers showed strong absorption in the UV region (Fig. 6). The PVC/CNT nanocomposite fiber showed considerably greater absorption compared to PVC fiber at wavelength λ = 280 nm. The absorbance of PVC/CNT nanocomposite fiber showed an enhancement of 56% with the CNT loading of 3 wt% in PVC. Interestingly, with the same loading, 3 wt% of CNT and GN in PVC the absorption of PVC/CNT nanocomposite fiber was approximately 70% higher compared to the PVC/GN nanocomposite fiber.16 The absorbance spectra showed absorbance peaks at λ = 234 and 280 nm, which can be assigned to the n–π* and π–π* transition, respectively.The optical band gap of the PVC and PVC/CNT nanocomposite fibers was obtained using the approximate relationships among the reflectance (R), absorption coefficient (α), and scattering coefficient (s) based on Kubelka–Munk theory.21
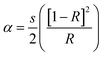 | (2) |
In above equation, α and s are functions of the energy of incident radiation (hν). The assumption was that s compared to α does not depend significantly on the wavelength at the absorption edge. Therefore, s was considered to be constant. The band gap of the PVC and PVC/CNT nanocomposite fibers was obtained from the absorption coefficient (α) using the following equation:22
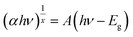 | (3) |
This equation was formularized by the relationship for the parabolic bands at the onset of the absorption edge, where hν is the incident photon energy, A is a material constant, and x is an exponent that depends on the type of transition. The value of x in the case of a direct allowed transition, indirect allowed transition and direct forbidden transition is 1/2, 2, 3/2, respectively. The band gap was determined by a plot of 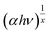 and hν at approximately the fundamental absorption region (Fig. S2 and S3†).
and hν at approximately the fundamental absorption region (Fig. S2 and S3†).
Fig. S2 and S3† show the direct and indirect band gap calculation for the PVC and GN@PVC nanocomposite fibers. The direct and indirect allowed band gaps of all nanocomposite fibers were lower than those of PVC (Table S2†). The estimated direct and indirect band gaps of PVC were 4.76 and 4.13 eV, respectively. Emad et al.23 and Bushra et al.24 reported similar direct and indirect band gaps for the PVC thin films. The decrease in the direct and indirect band gap in the present case for the PVC/CNT nanocomposite fibers were attributed to the formation of polarons in the fibers of the PVC loaded with CNTs. Some modifications in the size of the amorphous domains in PVC due to the incorporation of CNTs can also explain the optical band gap reduction for the nanocomposite fibers.25
Nitrogen adsorption isotherms
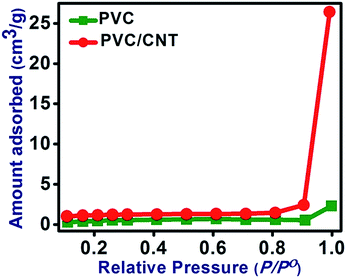
The nitrogen adsorption isotherms of the PVC and PVC/CNT nanocomposite fibers were obtained (Fig. 7). Interestingly, both isotherms showed a hysteresis loop, confirming the characteristic feature of the type IV isotherms.26 The isotherms of PVC fibers showed a very slow ascending section up to p/p0 = 0.61. Afterwards it showed a slow descending section, which extended up to p/p0 = 0.91. Finally, the isotherm showed an up-ward jump at saturation pressure p/p0 = 0.99. On the other hand, the PVC/CNT nanocomposite fibers did not exhibit a descending section, showing a continues slow ascending section up to p/p0 = 0.91. A similar but an enhanced jump at a saturation pressure p/p0 = 0.99 was observed. Nitrogen adsorption at high p/p0 values was greater for the PVC/CNT nanocomposite fibers. The BET measurement clearly showed that the pores of the PVC/CNT nanocomposite fibers are micro porous. In addition, the BET surface area and pore volume of PVC/CNT nanocomposite fibers increased greatly (Table 2). Compared to PVC/GN nanocomposite fibers reported previously,16 this materials showed an increase in the surface area and pore volume (Table 2).| Samples | Surface area (m2 g−1) | Average pore volume (cm3 g−1) | Average pore diameter (nm) |
|---|---|---|---|
| PVC | 2.018 | 5.467 × 10−3 | 26.74 |
| PVC/CNT | 3.66 | 4.378 × 10−2 | 16.40 |
Dye adsorption studies
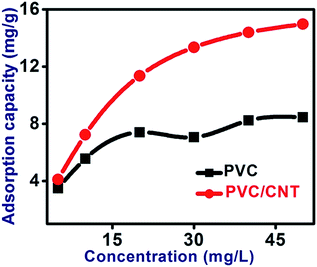
The efficiency of the PVC and PVC/CNT nanocomposite fibers for the removal of ARS was tested. The removal of ARS by using PVC and PVC–CNT fibers were increased from 3.5 to 9.5 mg g−1 and 4.1 to 14.9 mg g−1, respectively, with increasing initial dye concentration form 5 to 50 mg L−1 as shown in Fig. 8. An increase in the initial ARS concentration may increase the driving force, which facilitates more ARS adsorption onto adsorbent fibers by minimization of the mass transfer resistance of the ARS molecules between the solid phase fibers and aqueous solution.27 The PVC/CNT nanocomposite fibers had almost double the adsorption capacity for ARS compared to PVC fibers. The higher adsorption capacity of PVC/CNT nanocomposite fibers than PVC was due mainly to the following two reasons: (i) the addition of CNTs to PVC may provide more open surface to the PVC matrix, which allows easy excess for ARS molecules to the adsorbent surface; (ii) the surface area of PVC/CNT was around 81% higher than PVC fibers which facilitated the higher adsorption; and (iii) the PVC/CNT nanocomposite fibers had more adsorption sites for ARS with an interaction through π–π interaction between CNTs and dye molecules.28 For a better understating of surface property and interactions between ARS and fiber adsorbents, isotherm models, i.e., Langmuir, Freundlich and Temkin isotherm models were applied.The Langmuir isotherm model29 is based on the assumption of monolayer adsorption onto an adsorbent surface that contains an finite number of identical active sites and no transmigration of the adsorbate in the plane of the surface. The linear equation for Langmuir isotherm is as follows:
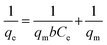 | (4) |
The Freundlich isotherm model30 assumes that adsorption of adsorbate species occurs through a multilayer and heterogeneous system, characterized by the heterogeneity factor 1/n. The linear equation for Freundlich isotherm is as follows:
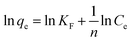 | (5) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe versus ln
qe versus ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce.
Ce.
The Temkin isotherm model31 is based on the adsorbent–adsorbate interactions and along with the saturation of adsorbent active sites, the adsorption energy decreases linearly rather than exponentially. The linear equation for Temkin isotherm model is a follows:
qe = B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A + B A + B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce Ce
| (6) |
Table 3 lists the adsorption isotherm parameters calculated from their respective plots. The value of correlation coefficient (R2) for Langmuir equation is higher for the PVC fibers while for PVC/CNT nanocomposite fibers, Temkin equation shows the highest R2 value, suggesting that the Langmuir and Temkin isotherm models were found to be well representative of the adsorption equilibrium data.32,33
| Adsorbent | Langmuir isotherm model | Freundlich isotherm model | Temkin isotherm model | ||||||
|---|---|---|---|---|---|---|---|---|---|
| qm (mg g−1) | b (L mg−1) | R2 | KF (mg1−1/n L1/n g−1) | n | R2 | A (L mg−1) | B | R2 | |
| PVC | 8.47 | 0.468 | 0.987 | 3.472 | 4.000 | 0.926 | 9.396 | 1.427 | 0.951 |
| PVC/CNT | 14.952 | 0.413 | 0.991 | 4.702 | 2.824 | 0.967 | 4.197 | 3.072 | 0.995 |
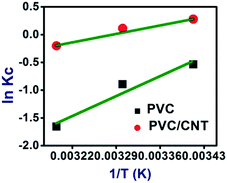
Furthermore, thermodynamic studies were performed to determine the adsorption mechanism of ARS onto PVC and PVC/CNT nanocomposite fibers. The values of thermodynamic parameters such as the standard free energy change (ΔG°), entropy change (ΔS°) and enthalpy change (ΔH°) associated with the adsorption processes were calculated using the following equations:
ΔG0 = −RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kc Kc
| (7) |
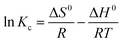 | (8) |
| Adsorbent | ΔG° (kJ mol−1) | ΔH° (kJ mol−1) | ΔS° (J mol−1 K−1) | R2 | ||
|---|---|---|---|---|---|---|
| 30 °C | 40 °C | 50 °C | ||||
| PVC | 1.306 | 2.255 | 4.311 | −42.501 | −148.90 | 0.963 |
| PVC/CNT | −0.676 | −0.277 | 0.527 | −18.249 | −59.752 | 0.950 |
Conclusions
PVC/CNT nanocomposite fibers were synthesized using a simple deposition technique. The adsorption, thermal, structural, and optical behaviors of the nanocomposite fibers was examined. The PVC/CNT nanocomposite fibers showed 81% enhancement in the surface area over the PVC fibers. The PVC/CNT nanocomposite fibers compared to the PVC fibers showed higher glass transition temperature. This also showed a lower optical band gap than PVC, which might be due to the formation of polarons within the nanocomposite. Moreover, the PVC/CNT nanocomposite fibers compared to PVC showed two times higher adsorption capacity for the ARS. Owing to its enhanced adsorption capacity, surface area and better thermo-optical properties, this nanocomposite material is expected to find a suitable role in various practical applications.Acknowledgements
This work was supported by the 2014 Yeungnam University Research Grant. This work was also supported by Priority Research Centers Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2014R1A6A1031189).Notes and references
- A. Guha, W. Lu, T. Zawodzinski and D. Schiraldi, Carbon, 2007, 45, 1506–1517 CrossRef CAS PubMed.
- H. Kong, C. Gao and D. Y. Yan, Macromolecule, 2004, 37, 4022–4030 CrossRef CAS.
- C. Park, Z. Ounaies, K. A. Watson, R. E. Crooks, J. J. Smith and S. E. Lowther, Chem. Phys. Lett., 2002, 364, 303–308 CrossRef CAS.
- Z. Jin, K. P. Pramoda, G. Xu and S. H. Goh, Chem. Phys. Lett., 2001, 337, 43–47 CrossRef CAS.
- Z. Zhang, G. Chen, H. Wang and X. Li, Chem.–Asian J., 2015, 10(1), 149–153 CrossRef CAS PubMed.
- G. Sun, G. Chen, Z. Liu and M. Chen, Carbon, 2010, 48, 1434 CrossRef CAS PubMed.
- G. Sun, G. Chen, J. Liu, J. Yang, J. Xie, Z. Liu, R. Li and X. Li, Polymer, 2009, 50, 5787 CrossRef CAS PubMed.
- C. E. Wilkes, J. W. Summers, C. A. Daniels and M. T. Berard, PVC handbook, Hanser Verlag, Germany, First edn, 2005, pp. 414–415 Search PubMed.
- B. Ivan, T. Kelen and F. Tudos, Degradation and stabilization of polymers, Elsevier Science, Amsterdam, Netherlands, 1989, pp. 483–714 Search PubMed.
- T. Peprnicek, A. Kalendova, E. Pavlova, J. Simonik, J. Duchet and J. F. Gerard, Polym. Degrad. Stab., 2006, 91, 3322 CrossRef CAS PubMed.
- J. Liu, G. Chen and J. Yang, Polymer, 2008, 49, 3923 CrossRef CAS PubMed.
- S. S. Sun, C. Z. Li, L. Zhang, H. L. Du and J. S. Burnell-Gray, Polym. Int., 2006, 55, 158 CrossRef CAS.
- V. J. Mkhabela, A. K. Mishra and X. Y. Mbianda, Carbon, 2011, 49, 610 CrossRef CAS PubMed.
- V. Sajini, P. Jinu, M. Narahari and V. Suresh, Carbon, 2011, 49, 198 CrossRef PubMed.
- T. Sterzynski, J. Tomaszewska, K. Piszczek and K. Skorczewska, Compos. Sci. Technol., 2010, 70, 966–969 CrossRef CAS PubMed.
- M. Hasan, A. N. Banerjee and M. Y. Lee, J. Ind. Eng. Chem., 2015, 21, 828 CrossRef CAS PubMed.
- X. L. Wu, P. Liu, T. Ramanathan and A. A. Abdala, eXPRESS Polym Lett., 2010, 4, 723–728 CrossRef CAS.
- G. W. Becker, Kolloid-Z., 1955, 140, 1 CrossRef CAS.
- M. Kawasumi, N. Hasegawa, M. Kato, A. Usuki and A. Okada, Macromolecule, 1997, 30, 6333 CrossRef CAS.
- M. Hasan and M. Y. Lee, Prog. Nat. Sci., 2014, 24, 579 CrossRef PubMed.
- J. D. Lindberg and D. G. Synder, Appl. Opt., 1973, 12, 573 CrossRef CAS PubMed.
- J. I. Pankove, Optical Process in Semiconductors, Printice-Hall, New Jersey, 1971 Search PubMed.
- E. Yousif, M. Abdullah, H. Hashim, N. Salih, J. Salimon and B. M. Abdullah, Int. J. Ind. Chem., 2013, 4, 4 CrossRef.
- B. A. Hasan, M. A. Saeed and A. A. Hasan, Br. J. Sci., 2012, 7, 14 Search PubMed.
- M. lamabla, in second Mediterranean school on science and technology of advanced polymer-based materials, Capri, 26 may, 1991 Search PubMed.
- S. J. Gregg and K. S. W. Sing, Adsorption, Surface Area and Porosity, Academic Press, London, 2nd edn, 1982 Search PubMed.
- Y. Wu, M. Zhang, H. Zhao, S. Yang and A. Arkin, RSC Adv., 2014, 4, 61256 RSC.
- V. K. Gupta, R. Kumar, A. Nayak, M. A. Barakat and T. A. Saleh, Adv. Colloid Interface Sci., 2013, 191, 24–34 CrossRef PubMed.
- I. Langmuir, J. Am. Chem. Soc., 1918, 40, 1361–1403 CrossRef CAS.
- H. Freundlich, J. Phys. Chem., 1907, 57, 385–470 CAS.
- M. I. Temkin and V. Pyzhev, Acta Physicochim. URSS, 1940, 12, 217–222 Search PubMed.
- M. Ghaedi, A. Najibi, H. Hossainian, A. Shokrollahi and M. Soylak, Toxicol. Environ. Chem., 2012, 94, 40–48 CrossRef CAS.
- M. J. Iqbal and M. N. Ashiq, J. Hazard. Mater., 2007, 139, 57–66 CrossRef CAS PubMed.
- M. J. Jaycock and G. D. Parfitt, Chemistry of Interfaces, Ellis Horwood Ltd, Onichester, 1981 Search PubMed.
- R. Kumar, M. O. Ansari and M. A. Barakat, Ind. Eng. Chem. Res., 2014, 53, 7167 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Sample preparation table, band gap calculation table, direct and indirect optical band gap plots, XPS survey scans for PVC/CNT nanocomposite fibers. See DOI: 10.1039/c4ra16043f |
| This journal is © The Royal Society of Chemistry 2015 |